Peptide TNIIIA2 Derived from Tenascin-C Contributes to Malignant Progression in Colitis-Associated Colorectal Cancer via β1-Integrin Activation in Fibroblasts
Abstract
:1. Introduction
2. Results
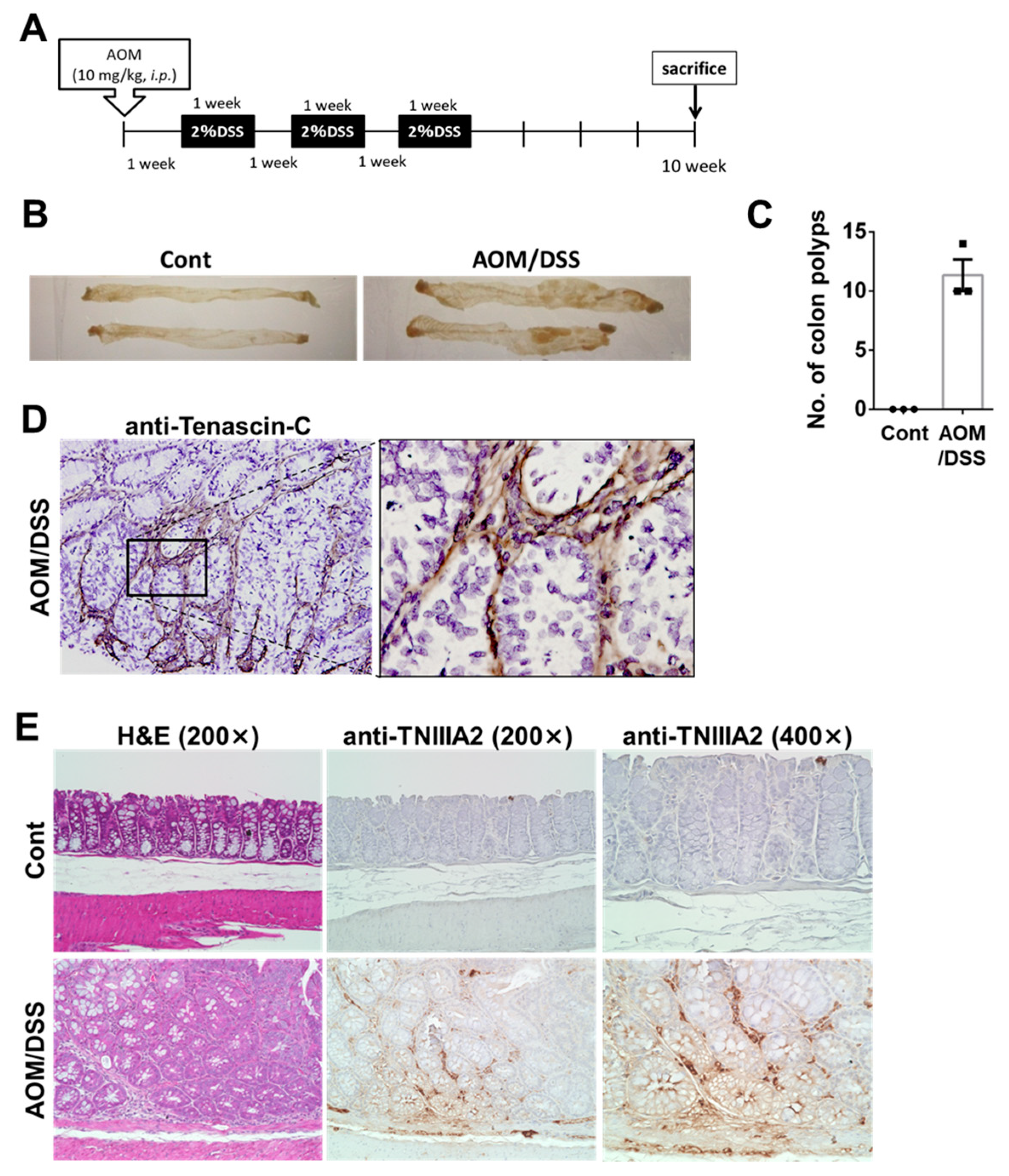
2.1. TNC/TNIIIA2 Expression in a Murine AOM/DSS Model
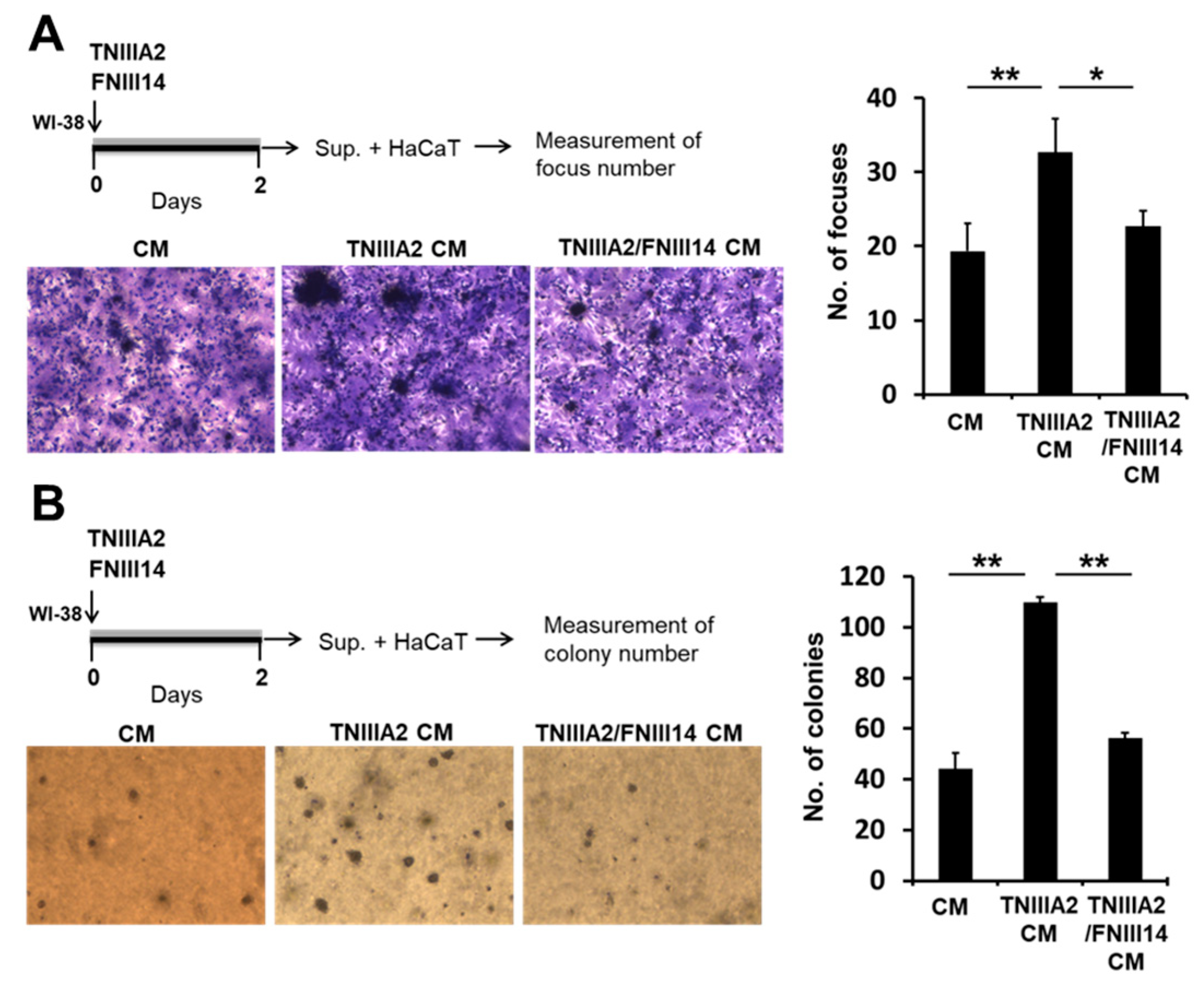
2.2. Paracrine Factor(s) Released from Fibroblasts in Response to Stimulation of Peptide TNIIIA2 Induces Dysregulated Proliferation in Premalignant and Malignant Cells
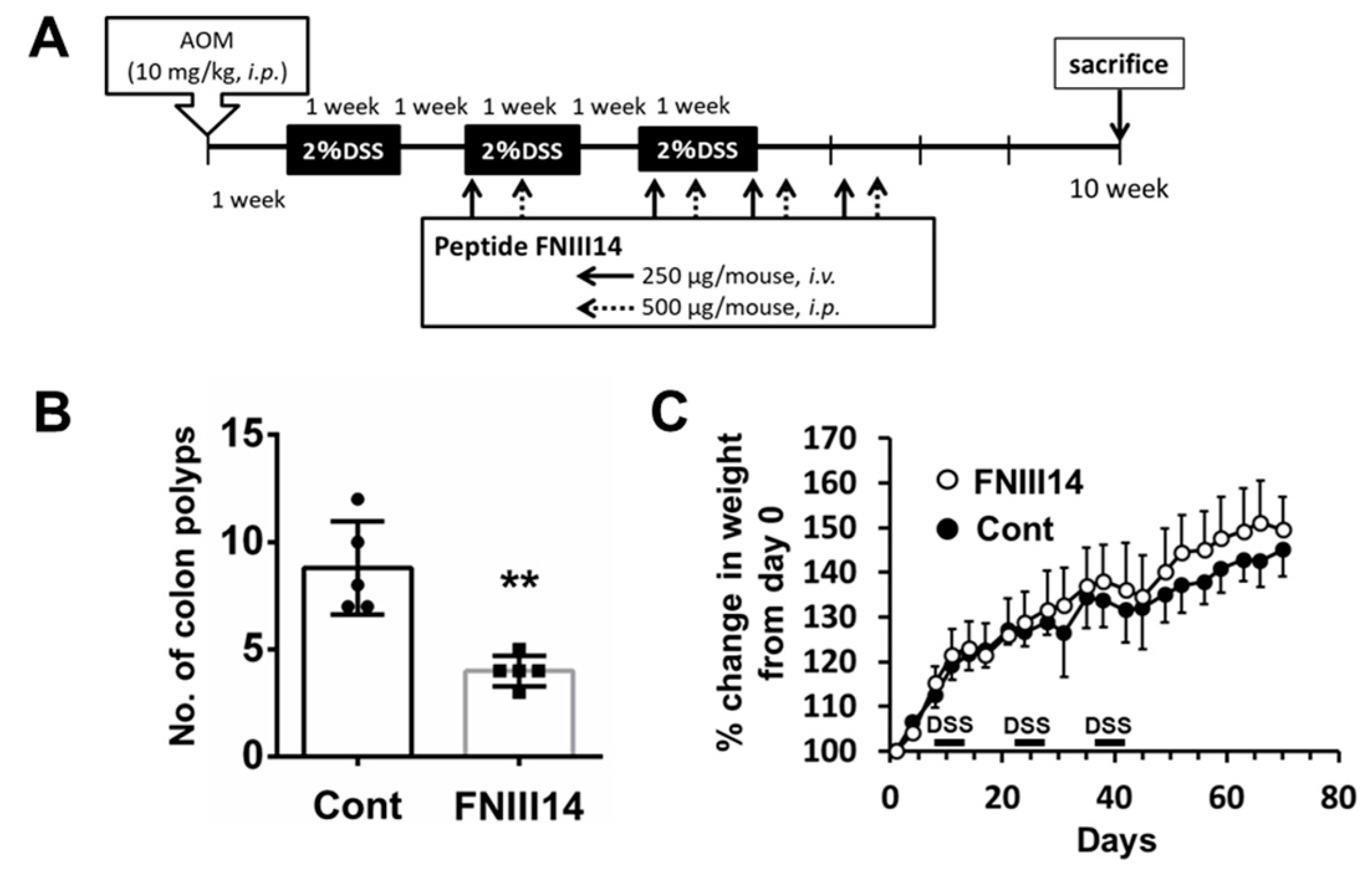
2.3. Peptide FNIII14 Suppresses Polyp Formation in the AOM/DSS Model
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Murine AOM/DSS Colitis-Associated Carcinoma Model
4.3. Cell Culture
4.4. Immunohistochemistry
4.5. Cell Adhesion Assay
4.6. Flow Cytometry
4.7. 2D Co-Culture Experiments
4.8. Preparation of Conditioned Medium from WI-38 Cells
4.9. Cell Proliferation Assay
4.10. Colony Formation Assay
4.11. Focus Formation Assay
4.12. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IBD | inflammatory bowel disease |
| UC | ulcerative colitis |
| CD | Crohn’s disease |
| CAC | colitis-associated colorectal cancer |
| AOM | azoxymethane |
| DSS | dextran sodium sulfate |
| i.p. | intraperitoneal injection |
| EGF | epidermal growth factor |
| CM | conditioned medium |
| MMP | matrix metalloproteinase |
| ECM | extracellular matrix |
| HB-EGF | heparin-binding EGF-like growth factor |
| ABS | absorbance |
| Sup. | supernatant |
References
- Ekbom, A.; Helmick, C.; Zack, M.; Adami, H.O. Ulcerative colitis and colorectal cancer. A population-based study. N. Engl. J. Med. 1990, 323, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jess, T.; Loftus, E.V.; Velayos, F.S.; Harmsen, W.S.; Zinsmeister, A.R.; Smyrk, T.C.; Schleck, C.D.; Tremaine, W.J.; Melton, L.J.; Munkholm, P.; et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology 2006, 130, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Ullman, T.A.; Itzkowitz, S.H. Intestinal inflammation and cancer. Gastroenterology 2011, 140, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Zisman, T.L.; Rubin, D.T. Colorectal cancer and dysplasia in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 2662–2669. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Hussenet, T.; Langlois, B.; Orend, G. Advances in tenascin-C biology. Cell. Mol. Life Sci. CMLS 2011, 68, 3175–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giblin, S.P.; Midwood, K.S. Tenascin-C: Form versus function. Cell Adhes. Migr. 2015, 9, 48–82. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Tandara, A.; Reinshagen, M.; Hinz, U.; Faissner, A.; Bodenmüller, H.; Buhr, H.J.; Herfarth, C.; Möller, P. Serum tenascin-C is an indicator of inflammatory bowel disease activity. Int. J. Colorectal Dis. 2001, 16, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Spenlé, C.; Lefebvre, O.; Lacroute, J.; Méchine-Neuville, A.; Barreau, F.; Blottière, H.M.; Duclos, B.; Arnold, C.; Hussenet, T.; Hemmerlé, J.; et al. The Laminin Response in Inflammatory Bowel Disease: Protection or Malignancy? PLOS ONE 2014, 9, e111336. [Google Scholar] [CrossRef]
- Li, M.; Peng, F.; Li, G.; Fu, Y.; Huang, Y.; Chen, Z.; Chen, Y. Proteomic analysis of stromal proteins in different stages of colorectal cancer establishes Tenascin-C as a stromal biomarker for colorectal cancer metastasis. Oncotarget 2016, 7, 37226–37237. [Google Scholar] [CrossRef] [Green Version]
- Takeda, A.; Otani, Y.; Iseki, H.; Takeuchi, H.; Aikawa, K.; Tabuchi, S.; Shinozuka, N.; Saeki, T.; Okazaki, Y.; Koyama, I. Clinical significance of large tenascin-C spliced variant as a potential biomarker for colorectal cancer. World J. Surg. 2007, 31, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Imazeki, H.; Miura, S.; Yoshimura, T.; Okutsu, H.; Harada, Y.; Ohwaki, T.; Nagao, O.; Kamiya, S.; Hayashi, R.; et al. A peptide derived from tenascin-C induces beta1 integrin activation through syndecan-4. J. Biol. Chem. 2007, 282, 34929–34937. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Seki, Y.; Saito, Y.; Kamiya, S.; Fujita, M.; Okutsu, H.; Iyoda, T.; Takai, T.; Owaki, T.; Yajima, H.; et al. Tenascin-C-derived peptide TNIIIA2 highly enhances cell survival and platelet-derived growth factor (PDGF)-dependent cell proliferation through potentiated and sustained activation of integrin α5β1. J. Biol. Chem. 2014, 289, 17699–17708. [Google Scholar] [CrossRef] [PubMed]
- Dueck, M.; Riedl, S.; Hinz, U.; Tandara, A.; Möller, P.; Herfarth, C.; Faissner, A. Detection of tenascin-C isoforms in colorectal mucosa, ulcerative colitis, carcinomas and liver metastases. Int. J. Cancer 1999, 82, 477–483. [Google Scholar] [CrossRef]
- Suzuki, H.; Sasada, M.; Kamiya, S.; Ito, Y.; Watanabe, H.; Okada, Y.; Ishibashi, K.; Iyoda, T.; Yanaka, A.; Fukai, F. The Promoting Effect of the Extracellular Matrix Peptide TNIIIA2 Derived from Tenascin-C in Colon Cancer Cell Infiltration. Int. J. Mol. Sci. 2017, 18, 181. [Google Scholar] [CrossRef]
- Kato, R.; Ishikawa, T.; Kamiya, S.; Oguma, F.; Ueki, M.; Goto, S.; Nakamura, H.; Katayama, T.; Fukai, F. A new type of antimetastatic peptide derived from fibronectin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 2455–2462. [Google Scholar]
- Tanaka, R.; Owaki, T.; Kamiya, S.; Matsunaga, T.; Shimoda, K.; Kodama, H.; Hayashi, R.; Abe, T.; Harada, Y.P.; Shimonaka, M.; et al. VLA-5-mediated adhesion to fibronectin accelerates hemin-stimulated erythroid differentiation of K562 cells through induction of VLA-4 expression. J. Biol. Chem. 2009, 284, 19817–19825. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003, 94, 965–973. [Google Scholar] [CrossRef]
- Kawamura, T.; Yamamoto, M.; Suzuki, K.; Suzuki, Y.; Kamishima, M.; Sakata, M.; Kurachi, K.; Setoh, M.; Konno, H.; Takeuchi, H. Tenascin-C Produced by Intestinal Myofibroblasts Promotes Colitis-associated Cancer Development Through Angiogenesis. Inflamm. Bowel Dis. 2018, 25, 732–741. [Google Scholar] [CrossRef]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef]
- Mukaida, N.; Sasaki, S. Fibroblasts, an inconspicuous but essential player in colon cancer development and progression. World J. Gastroenterol. 2016, 22, 5301–5316. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kikuchi, H.; Ishimatsu, H.; Iino, I.; Hirotsu, A.; Matsumoto, T.; Ozaki, Y.; Kawabata, T.; Hiramatsu, Y.; Ohta, M.; et al. Tenascin C in colorectal cancer stroma is a predictive marker for liver metastasis and is a potent target of miR-198 as identified by microRNA analysis. Br. J. Cancer 2017, 117, 1360–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geboes, K.; El-Zine, M.Y.; Dalle, I.; El-Haddad, S.; Rutgeerts, P.; Van Eyken, P. Tenascin and strictures in inflammatory bowel disease: an immunohistochemical study. Int. J. Surg. Pathol. 2001, 9, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Stamenkovic, I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J. Pathol. 2003, 200, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Davis, G.E.; Bayless, K.J.; Davis, M.J.; Meininger, G.A. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am. J. Pathol. 2000, 156, 1489–1498. [Google Scholar] [CrossRef]
- Shimshoni, E.; Yablecovitch, D.; Baram, L.; Dotan, I.; Sagi, I. ECM remodelling in IBD: innocent bystander or partner in crime? The emerging role of extracellular molecular events in sustaining intestinal inflammation. Gut 2015, 64, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Kofla-Dlubacz, A.; Matusiewicz, M.; Krzystek-Korpacka, M.; Iwanczak, B. Correlation of MMP-3 and MMP-9 with Crohn’s disease activity in children. Dig. Dis. Sci. 2012, 57, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Baba, T.; Shinagawa, K.; Matsushima, K.; Mukaida, N. Crucial involvement of the CCL3-CCR5 axis-mediated fibroblast accumulation in colitis-associated carcinogenesis in mice. Int. J. Cancer 2014, 135, 1297–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neufert, C.; Becker, C.; Türeci, Ö.; Waldner, M.J.; Backert, I.; Floh, K.; Atreya, I.; Leppkes, M.; Jefremow, A.; Vieth, M.; et al. Tumor fibroblast-derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J. Clin. Invest. 2013, 123, 1428–1443. [Google Scholar] [CrossRef]
- Yang, Z.-T.; Yeo, S.-Y.; Yin, Y.-X.; Lin, Z.-H.; Lee, H.-M.; Xuan, Y.-H.; Cui, Y.; Kim, S.-H. Tenascin-C, a Prognostic Determinant of Esophageal Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0145807. [Google Scholar] [CrossRef]
- Yang, Z.; Ni, W.; Cui, C.; Fang, L.; Xuan, Y. Tenascin C is a prognostic determinant and potential cancer-associated fibroblasts marker for breast ductal carcinoma. Exp. Mol. Pathol. 2017, 102, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.-D.; Yang, Z.-T.; Cui, C.-A.; Cui, Y.; Fang, L.-Y.; Xuan, Y.-H. Tenascin-C is a potential cancer-associated fibroblasts marker and predicts poor prognosis in prostate cancer. Biochem. Biophys. Res. Commun. 2017, 486, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, C.; Qi, W.; Cui, C.; Cui, Y.; Xuan, Y. Tenascin-C as a prognostic determinant of colorectal cancer through induction of epithelial-to-mesenchymal transition and proliferation. Exp. Mol. Pathol. 2018, 105, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikova, Y.A.; Mouw, J.K.; Barnes, J.M.; Pickup, M.W.; Lakins, J.N.; Kim, Y.; Lobo, K.; Persson, A.I.; Reis, G.F.; McKnight, T.R.; et al. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol. 2016, 18, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.M.; Kaushik, S.; Bainer, R.O.; Sa, J.K.; Woods, E.C.; Kai, F.; Przybyla, L.; Lee, M.; Lee, H.W.; Tung, J.C.; et al. A tension-mediated glycocalyx-integrin feedback loop promotes mesenchymal-like glioblastoma. Nat. Cell Biol. 2018, 20, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Rodansky, E.S.; Sauder, K.L.; Horowitz, J.C.; Mih, J.D.; Tschumperlin, D.J.; Higgins, P.D. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm. Bowel Dis. 2013, 19, 891–903. [Google Scholar] [CrossRef]
- Miekka, S.I.; Ingham, K.C.; Menache, D. Rapid methods for isolation of human plasma fibronectin. Thromb. Res. 1982, 27, 1–14. [Google Scholar] [CrossRef]
- Fukai, F.; Hasebe, S.; Ueki, M.; Mutoh, M.; Ohgi, C.; Takahashi, H.; Takeda, K.; Katayama, T. Identification of the anti-adhesive site buried within the heparin-binding domain of fibronectin. J. Biochem. 1997, 121, 189–192. [Google Scholar]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl. Acad. Sci. USA. 2001, 98, 12072–12077. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, M.; Ito-Fujita, Y.; Iyoda, T.; Sasada, M.; Okada, Y.; Ishibashi, K.; Osawa, T.; Kodama, H.; Fukai, F.; Suzuki, H. Peptide TNIIIA2 Derived from Tenascin-C Contributes to Malignant Progression in Colitis-Associated Colorectal Cancer via β1-Integrin Activation in Fibroblasts. Int. J. Mol. Sci. 2019, 20, 2752. https://doi.org/10.3390/ijms20112752
Fujita M, Ito-Fujita Y, Iyoda T, Sasada M, Okada Y, Ishibashi K, Osawa T, Kodama H, Fukai F, Suzuki H. Peptide TNIIIA2 Derived from Tenascin-C Contributes to Malignant Progression in Colitis-Associated Colorectal Cancer via β1-Integrin Activation in Fibroblasts. International Journal of Molecular Sciences. 2019; 20(11):2752. https://doi.org/10.3390/ijms20112752
Chicago/Turabian StyleFujita, Motomichi, Yuka Ito-Fujita, Takuya Iyoda, Manabu Sasada, Yuko Okada, Kazuma Ishibashi, Takuro Osawa, Hiroaki Kodama, Fumio Fukai, and Hideo Suzuki. 2019. "Peptide TNIIIA2 Derived from Tenascin-C Contributes to Malignant Progression in Colitis-Associated Colorectal Cancer via β1-Integrin Activation in Fibroblasts" International Journal of Molecular Sciences 20, no. 11: 2752. https://doi.org/10.3390/ijms20112752
APA StyleFujita, M., Ito-Fujita, Y., Iyoda, T., Sasada, M., Okada, Y., Ishibashi, K., Osawa, T., Kodama, H., Fukai, F., & Suzuki, H. (2019). Peptide TNIIIA2 Derived from Tenascin-C Contributes to Malignant Progression in Colitis-Associated Colorectal Cancer via β1-Integrin Activation in Fibroblasts. International Journal of Molecular Sciences, 20(11), 2752. https://doi.org/10.3390/ijms20112752





