Role of Hyaluronan in Human Adipogenesis: Evidence from in-Vitro and in-Vivo Studies
Abstract
1. Introduction
2. Results
2.1. Subsection
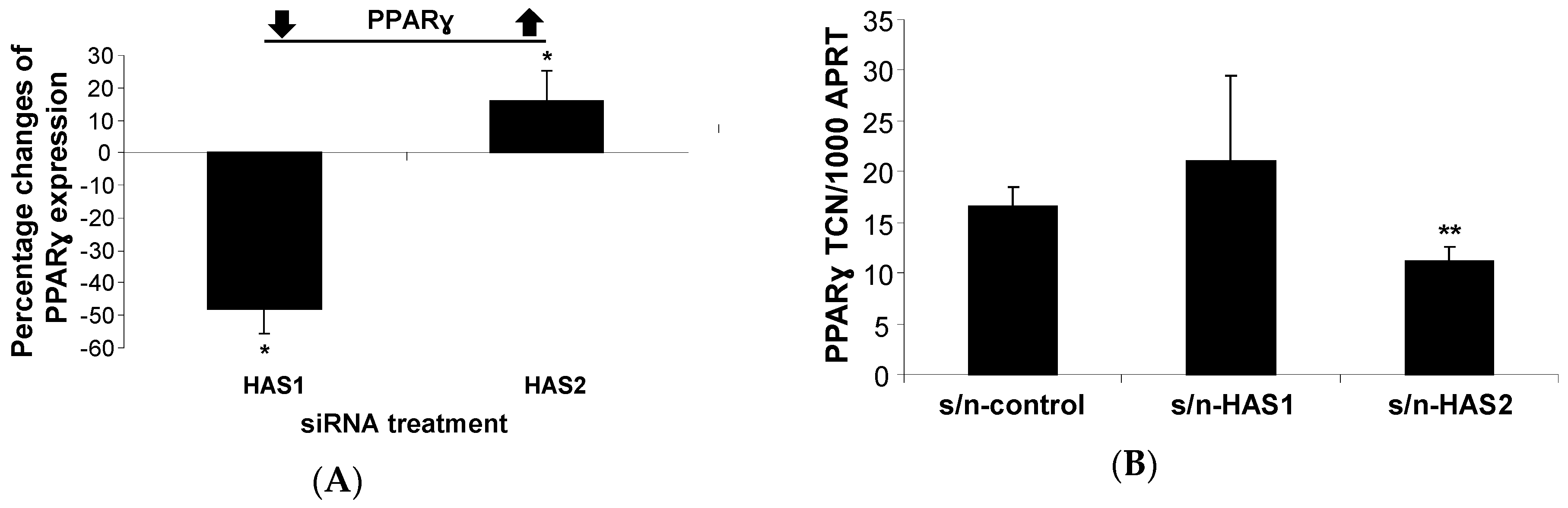
2.1.1. HA Affects PPARγ Expression in Human PFs
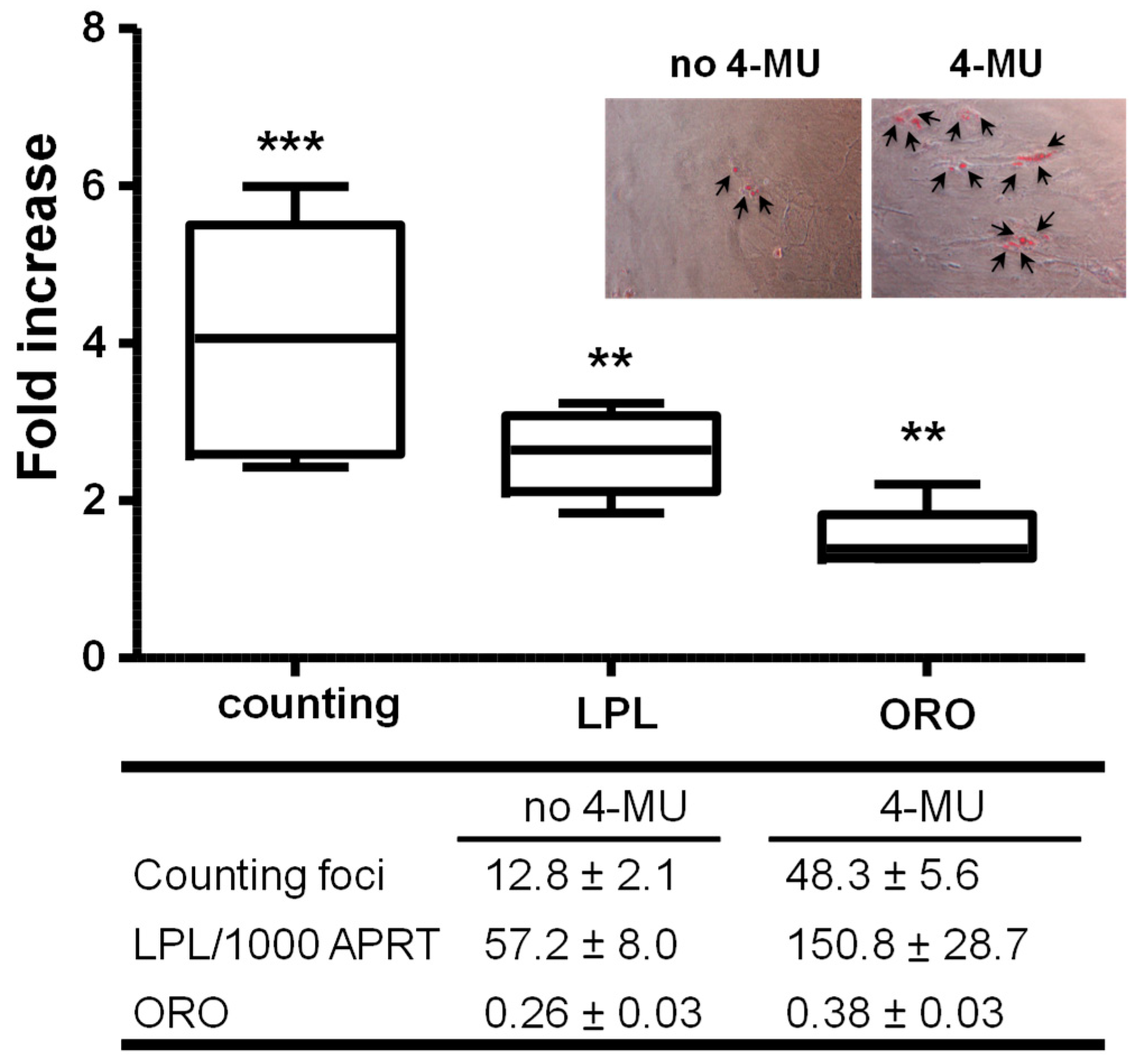
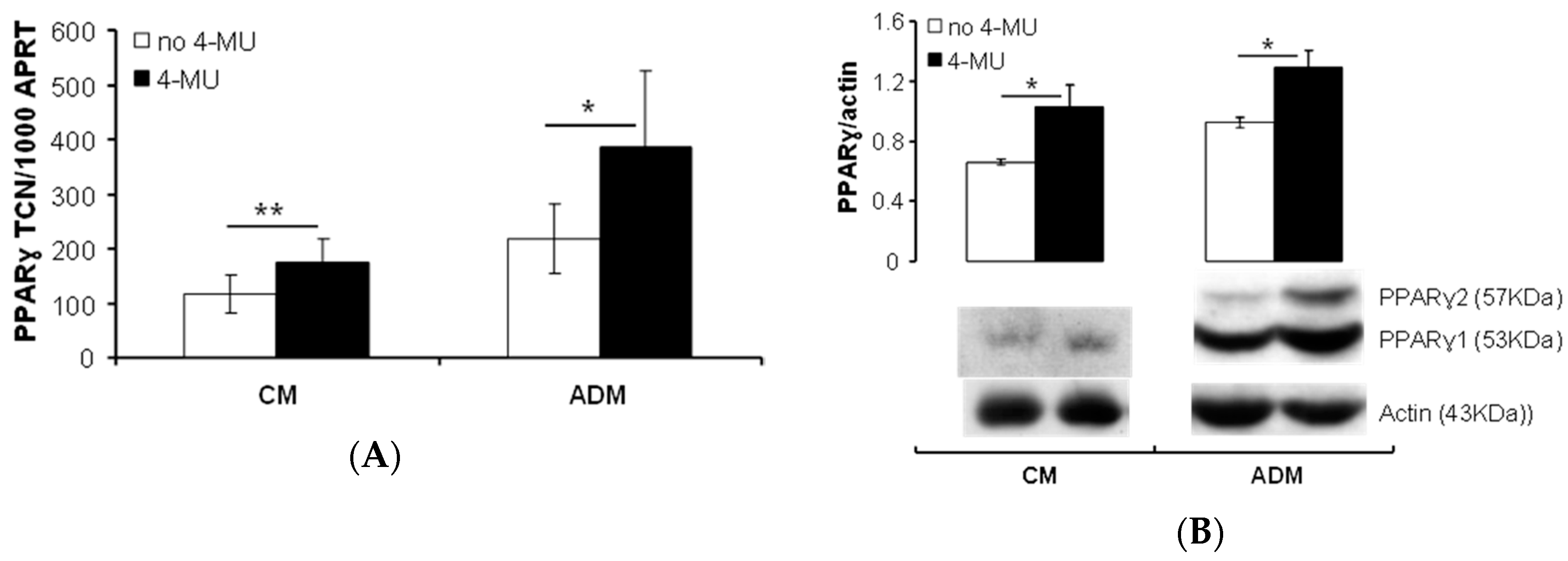
2.1.2. Inhibiting HA Production Enhances Adipogenesis
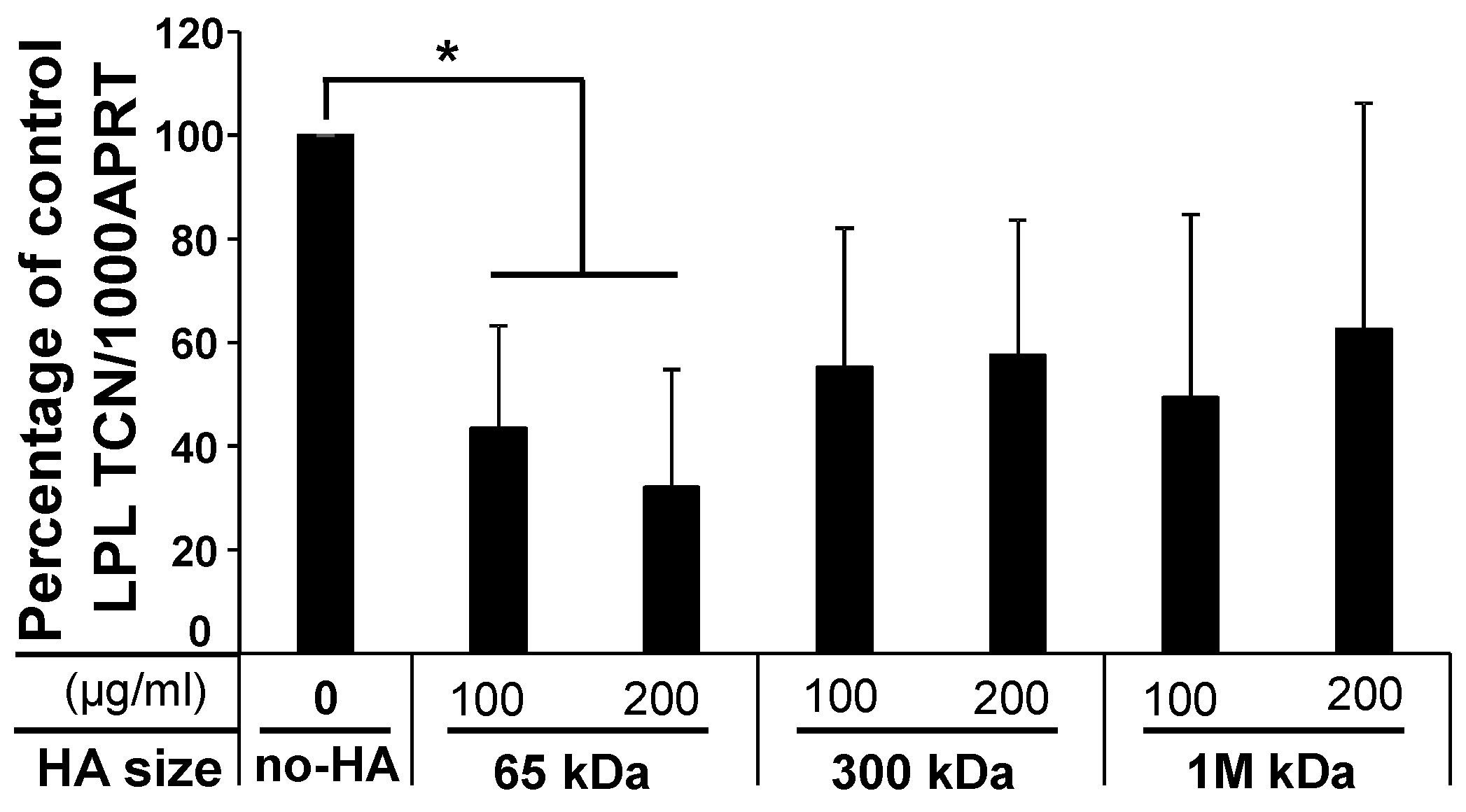
2.1.3. Low-Molecular-Weight (LMW) HA Exerts an Inhibitory Effect upon Adipogenesis
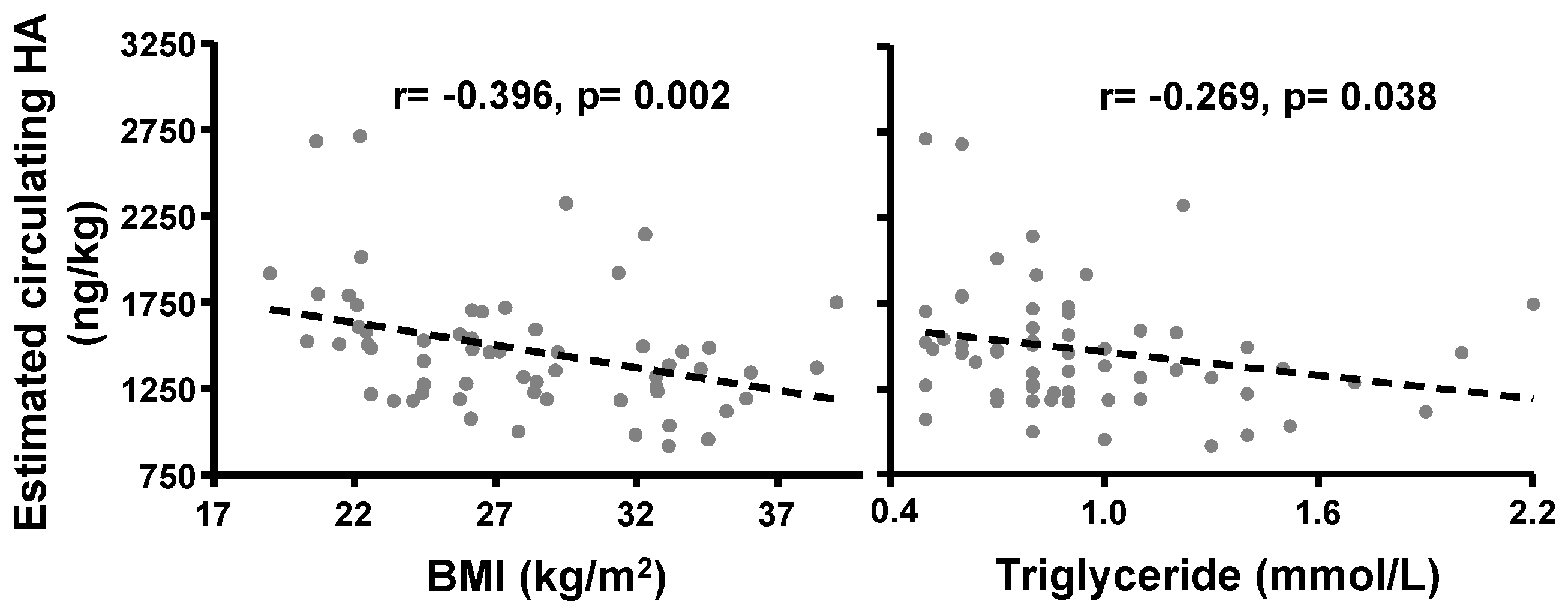
2.1.4. Correlation of BMI with Circulating HA Concentrations
3. Discussion
4. Materials and Methods
4.1. Adipose Tissue Collection and Preparation
4.2. Knockdown of HAS1 and HAS2 using siRNA
4.3. HAS1, HAS2 Overexpression
4.4. Effect of the Inhibition of HA Synthesis on Induced Adipogenesis
4.5. QPCR of PPARγ, LPL Genes
4.6. Western Blotting
4.7. HA Treatment
4.8. Clinical Study Examining the Relationship between Serum HA Concentrations and BMI
4.9. Measurement of HA
4.10. Blood Volume-Adjusted Circulating HA Concentrations
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | Hyaluronan |
| MSC | Mesenchymal stem cell |
| PFs | Preadipocytes/fibroblasts |
| 4-MU | 4-methylumbelliferone |
References
- Chun, T.H.; Kang, L. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem. Pharmacol. 2016, 119, 8–16. [Google Scholar]
- Lowe, C.E.; O’Rahilly, S.; Rochford, J.J. Adipogenesis at a glance. J. Cell Sci. 2011, 124, 2681–2686. [Google Scholar] [CrossRef]
- Garantziotis, S.; Savani, R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019, 78–79, 1–10. [Google Scholar] [CrossRef]
- Zhu, Y.; Kruglikov, I.L.; Akgul, Y.; Scherer, P.E. Hyaluronan in adipogenesis, adipose tissue physiology and systemic metabolism. Matrix Biol. 2019, 78–79, 284–291. [Google Scholar] [CrossRef]
- Itano, N.; Sawai, T.; Yoshida, M.; Lenas, P.; Yamada, Y.; Imagawa, M.; Shinomura, T.; Hamaguchi, M.; Yoshida, Y.; Ohnuki, Y.; et al. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 1999, 274, 25085–25092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bowen, T.; Grennan-Jones, F.; Paddon, C.; Giles, P.; Webber, J.; Steadman, R.; Ludgate, M. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: Contributory role in hyaluronan accumulation in thyroid dysfunction. J. Biol. Chem. 2009, 284, 26447–26455. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, S.M.; Hawn, T.R.; Arrigoni, A.; Wight, T.N.; Bollyky, P.L. Tissue integrity signals communicated by high-molecular weight hyaluronan and the resolution of inflammation. Immunol. Res. 2014, 58, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Lantier, L.; Kennedy, A.; Bonner, J.S.; Mayes, W.H.; Bracy, D.P.; Bookbinder, L.H.; Hasty, A.H.; Thompson, C.B.; Wasserman, D.H. Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes 2013, 62, 1888–1896. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Kuipers, H.F.; Marshall, P.L.; Wang, E.; Kaber, G.; Bollyky, P.L. Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol. 2019, 78–79, 292–313. [Google Scholar] [CrossRef]
- Rotter Sopasakis, V.; Wickelgren, R.; Sukonina, V.; Brantsing, C.; Svala, E.; Hansson, E.; Enerback, S.; Lindahl, A.; Skioldebrand, E. Elevated Glucose Levels Preserve Glucose Uptake, Hyaluronan Production, and Low Glutamate Release Following Interleukin-1beta Stimulation of Differentiated Chondrocytes. Cartilage 2018. [Google Scholar] [CrossRef]
- Kakizaki, I.; Kojima, K.; Takagaki, K.; Endo, M.; Kannagi, R.; Ito, M.; Maruo, Y.; Sato, H.; Yasuda, T.; Mita, S.; et al. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J. Biol. Chem. 2004, 279, 33281–33289. [Google Scholar] [CrossRef] [PubMed]
- Kudo, D.; Suto, A.; Hakamada, K. The Development of a Novel Therapeutic Strategy to Target Hyaluronan in the Extracellular Matrix of Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2017, 18, 3. [Google Scholar] [CrossRef]
- Nagy, N.; Kaber, G.; Johnson, P.Y.; Gebe, J.A.; Preisinger, A.; Falk, B.A.; Sunkari, V.G.; Gooden, M.D.; Vernon, R.B.; Bogdani, M.; et al. Inhibition of hyaluronan synthesis restores immune tolerance during autoimmune insulitis. J. Clin. Invest. 2015, 125, 3928–3940. [Google Scholar] [CrossRef]
- Nagy, N.; Kuipers, H.F.; Frymoyer, A.R.; Ishak, H.D.; Bollyky, J.B.; Wight, T.N.; Bollyky, P.L. 4-methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front. Immunol. 2015, 6, 123. [Google Scholar] [CrossRef] [PubMed]
- Ji, E.; Jung, M.Y.; Park, J.H.; Kim, S.; Seo, C.R.; Park, K.W.; Lee, E.K.; Yeom, C.H.; Lee, S. Inhibition of adipogenesis in 3T3-L1 cells and suppression of abdominal fat accumulation in high-fat diet-feeding C57BL/6J mice after downregulation of hyaluronic acid. Int. J. Obes. (Lond) 2014, 38, 1035–1043. [Google Scholar] [CrossRef]
- Park, B.G.; Lee, C.W.; Park, J.W.; Cui, Y.; Park, Y.S.; Shin, W.S. Enzymatic fragments of hyaluronan inhibit adipocyte differentiation in 3T3-L1 pre-adipocytes. Biochem. Biophys. Res. Commun. 2015, 467, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Park, B.G.; Park, Y.S.; Park, J.W.; Shin, E.; Shin, W.S. Anti-obesity potential of enzymatic fragments of hyaluronan on high-fat diet-induced obesity in C57BL/6 mice. Biochem. Biophys. Res. Commun. 2016, 473, 290–295. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Haakonsson, A.K.; Lazar, M.A.; Mandrup, S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 2014, 25, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Grennan-Jones, F.; Lane, C.; Rees, D.A.; Dayan, C.M.; Ludgate, M. Adipose tissue depot-specific differences in the regulation of hyaluronan production of relevance to Graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2012, 97, 653–662. [Google Scholar] [CrossRef]
- Hales, C.; Taylor, P.N.; Channon, S.; Paradice, R.; McEwan, K.; Zhang, L.; Gyedu, M.; Bakhsh, A.; Okosieme, O.; Muller, I.; et al. Controlled Antenatal Thyroid Screening II: Effect of Treating Maternal Suboptimal Thyroid Function on Child Cognition. J. Clin. Endocrinol. Metab. 2018, 103, 1583–1591. [Google Scholar] [CrossRef]
- Zhang, L.; Baker, G.; Janus, D.; Paddon, C.A.; Fuhrer, D.; Ludgate, M. Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Invest. Ophthalmol. Visual Sci. 2006, 47, 5197–5203. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Noble, P.W.; Lake, F.R.; Henson, P.M.; Riches, D.W. Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alpha-dependent mechanism in murine macrophages. J. Clin. Invest. 1993, 91, 2368–2377. [Google Scholar] [CrossRef]
- Zhang, L.; Paddon, C.; Lewis, M.D.; Grennan-Jones, F.; Ludgate, M. Gsalpha signalling suppresses PPARgamma2 generation and inhibits 3T3L1 adipogenesis. J. Endocrinol. 2009, 202, 207–215. [Google Scholar] [CrossRef]
- De la Motte, C.; Nigro, J.; Vasanji, A.; Rho, H.; Kessler, S.; Bandyopadhyay, S.; Danese, S.; Fiocchi, C.; Stern, R. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am. J. Pathol. 2009, 174, 2254–2264. [Google Scholar] [CrossRef]
- Cyphert, J.M.; Trempus, C.S.; Garantziotis, S. Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol. 2015, 2015, 563818. [Google Scholar] [CrossRef]
- Solis, M.A.; Chen, Y.H.; Wong, T.Y.; Bittencourt, V.Z.; Lin, Y.C.; Huang, L.L. Hyaluronan regulates cell behavior: A potential niche matrix for stem cells. Biochem. Res. Int. 2012, 2012, 346972. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Ibrahim, A.E.; Saad, D.A. Stem cell facelift: Between reality and fiction. Aesthet. Surg. J. 2013, 33, 334–338. [Google Scholar] [CrossRef]
- Kruglikov, I.; Trujillo, O.; Kristen, Q.; Isac, K.; Zorko, J.; Fam, M.; Okonkwo, K.; Mian, A.; Thanh, H.; Koban, K.; et al. The Facial Adipose Tissue: A Revision. Facial Plast Surg. 2016, 32, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Rohrich, R.J.; Ghavami, A.; Crosby, M.A. The role of hyaluronic acid fillers (Restylane) in facial cosmetic surgery: Review and technical considerations. Plast. Reconstr. Surg. 2007, 120, 41S–54S. [Google Scholar] [CrossRef]
- Karpe, F.; Pinnick, K.E. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat. Rev. Endocrinol. 2015, 11, 90–100. [Google Scholar] [CrossRef]
- Lemmens, H.J.; Bernstein, D.P.; Brodsky, J.B. Estimating blood volume in obese and morbidly obese patients. Obes. Surg. 2006, 16, 773–776. [Google Scholar] [CrossRef]
- Fuhrer, D.; Lewis, M.D.; Alkhafaji, F.; Starkey, K.; Paschke, R.; Wynford-Thomas, D.; Eggo, M.; Ludgate, M. Biological activity of activating thyroid-stimulating hormone receptor mutants depends on the cellular context. Endocrinology 2003, 144, 4018–4030. [Google Scholar] [CrossRef] [PubMed]
- Draman, M.S.; Stechman, M.; Scott-Coombes, D.; Dayan, C.M.; Rees, D.A.; Ludgate, M.; Zhang, L. The Role of Thyrotropin Receptor Activation in Adipogenesis and Modulation of Fat Phenotype. Front. Endocrinol. (Lausanne) 2017, 8, 83. [Google Scholar] [CrossRef]
- Rice, S.P.; Zhang, L.; Grennan-Jones, F.; Agarwal, N.; Lewis, M.D.; Rees, D.A.; Ludgate, M. Dehydroepiandrosterone (DHEA) treatment in vitro inhibits adipogenesis in human omental but not subcutaneous adipose tissue. Mol. Cell Endocrinol. 2010, 320, 51–57. [Google Scholar] [CrossRef]
- Lazarus, J.H.; Bestwick, J.P.; Channon, S.; Paradice, R.; Maina, A.; Rees, R.; Chiusano, E.; John, R.; Guaraldo, V.; George, L.M.; et al. Antenatal thyroid screening and childhood cognitive function. N. Engl. J. Med. 2012, 366, 493–501. [Google Scholar] [CrossRef]
- Adams, J.P.; Murphy, P.G. Obesity in anaesthesia and intensive care. Br. J. Anaesth 2000, 85, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Feldschuh, J.; Enson, Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation 1977, 56, 605–612. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, N.; Steadman, R.; Muller, I.; Draman, M.; Rees, D.A.; Taylor, P.; Dayan, C.M.; Ludgate, M.; Zhang, L. Role of Hyaluronan in Human Adipogenesis: Evidence from in-Vitro and in-Vivo Studies. Int. J. Mol. Sci. 2019, 20, 2675. https://doi.org/10.3390/ijms20112675
Wilson N, Steadman R, Muller I, Draman M, Rees DA, Taylor P, Dayan CM, Ludgate M, Zhang L. Role of Hyaluronan in Human Adipogenesis: Evidence from in-Vitro and in-Vivo Studies. International Journal of Molecular Sciences. 2019; 20(11):2675. https://doi.org/10.3390/ijms20112675
Chicago/Turabian StyleWilson, Nicholas, Robert Steadman, Ilaria Muller, Mohd Draman, D. Aled Rees, Peter Taylor, Colin M. Dayan, Marian Ludgate, and Lei Zhang. 2019. "Role of Hyaluronan in Human Adipogenesis: Evidence from in-Vitro and in-Vivo Studies" International Journal of Molecular Sciences 20, no. 11: 2675. https://doi.org/10.3390/ijms20112675
APA StyleWilson, N., Steadman, R., Muller, I., Draman, M., Rees, D. A., Taylor, P., Dayan, C. M., Ludgate, M., & Zhang, L. (2019). Role of Hyaluronan in Human Adipogenesis: Evidence from in-Vitro and in-Vivo Studies. International Journal of Molecular Sciences, 20(11), 2675. https://doi.org/10.3390/ijms20112675





