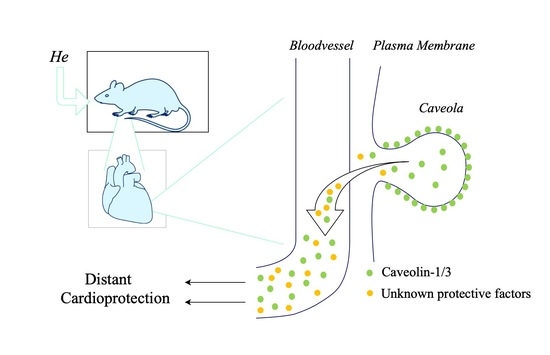
Helium-Induced Changes in Circulating Caveolin in Mice Suggest a Novel Mechanism of Cardiac Protection
Abstract
1. Introduction
2. Results
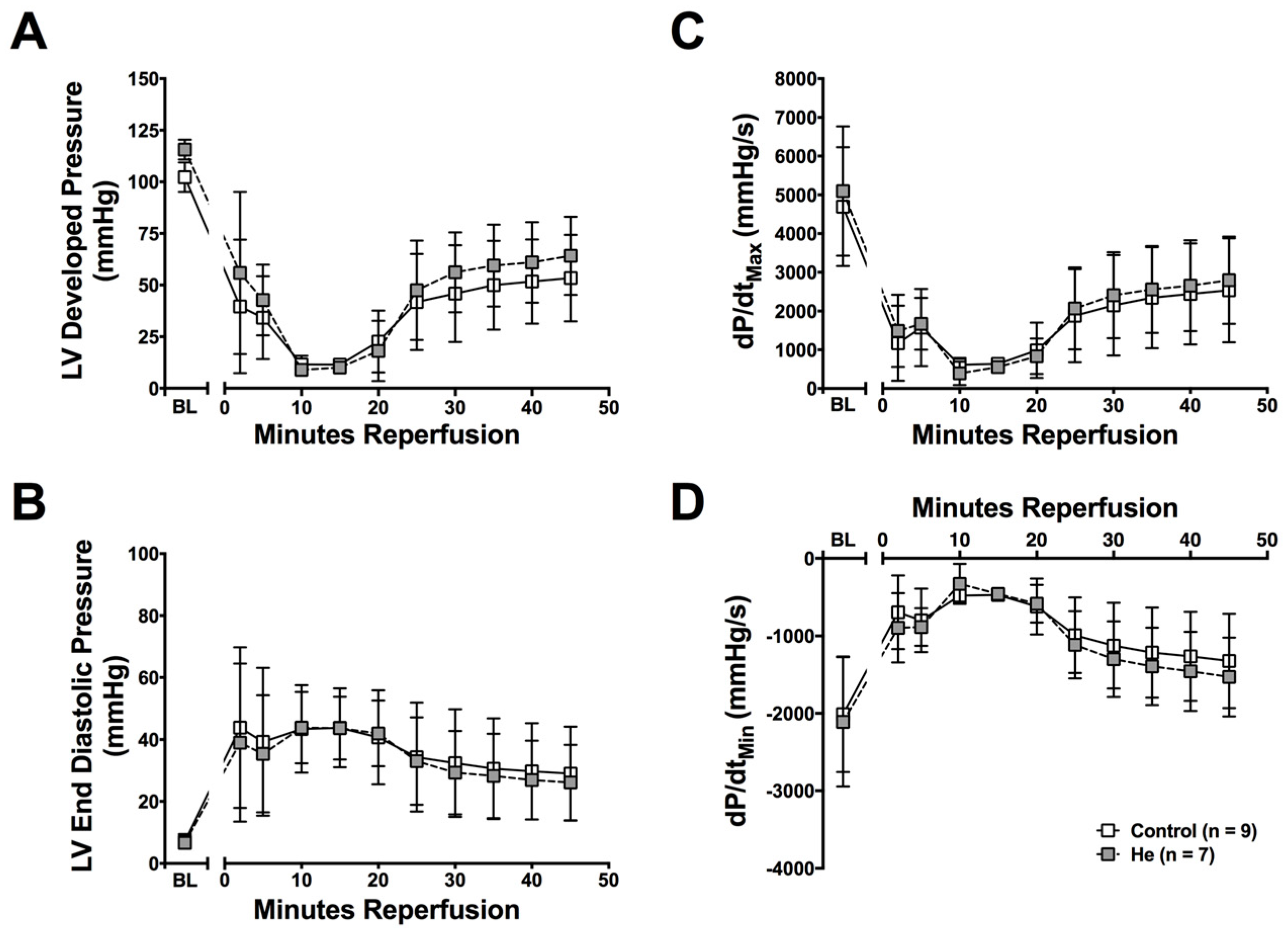
2.1. Cardioprotection Ex Vivo
2.2. Circulating Protective Factors
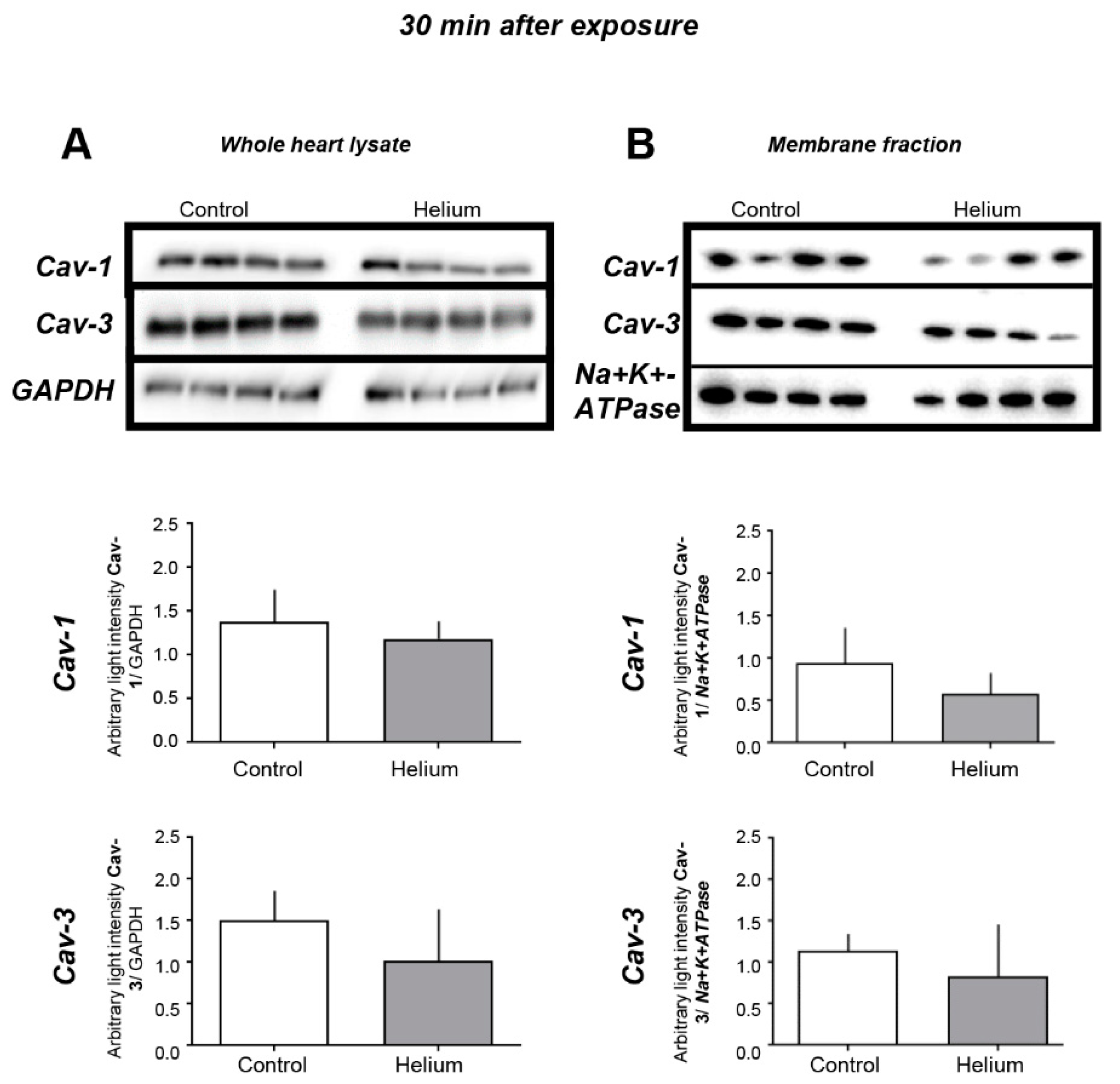
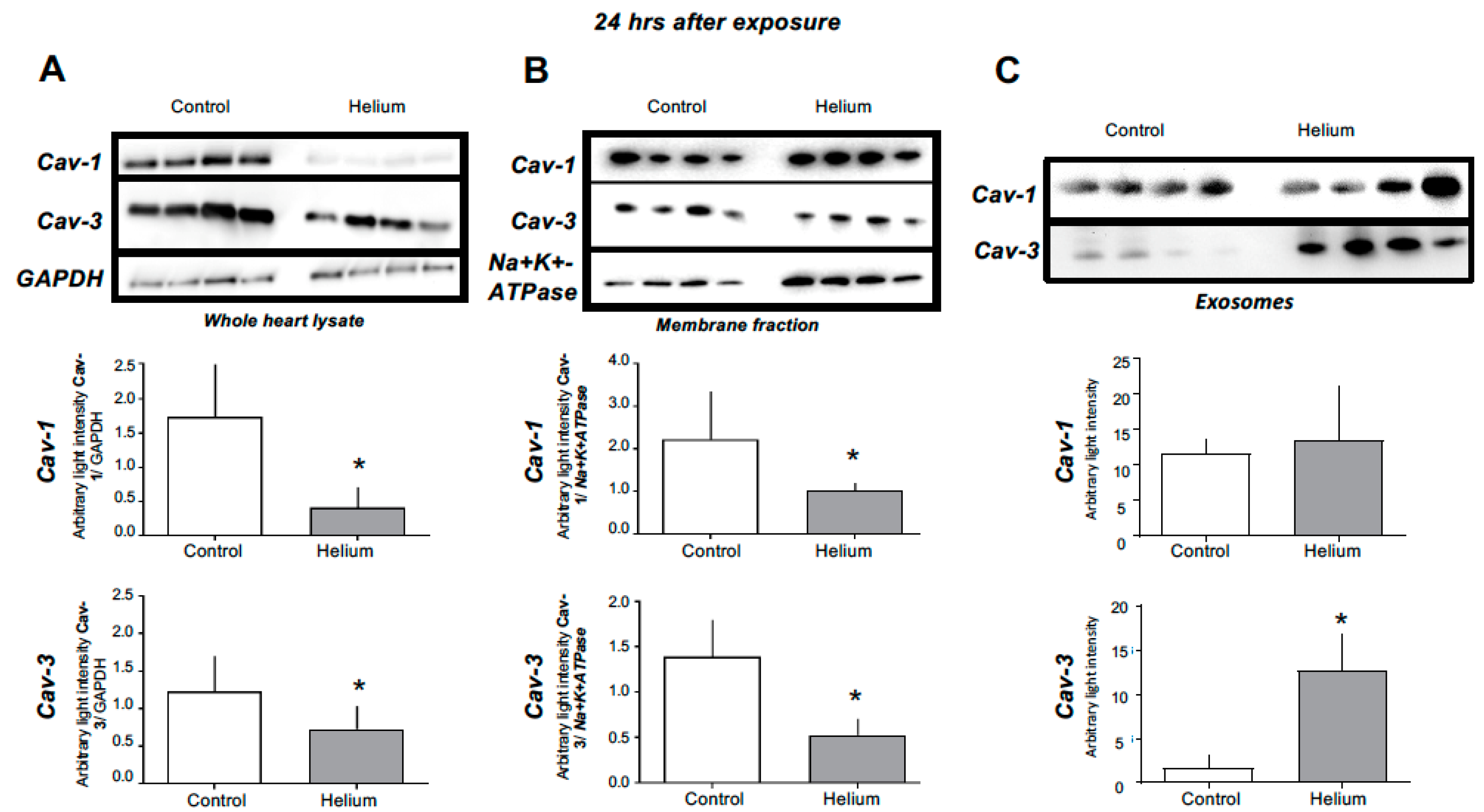
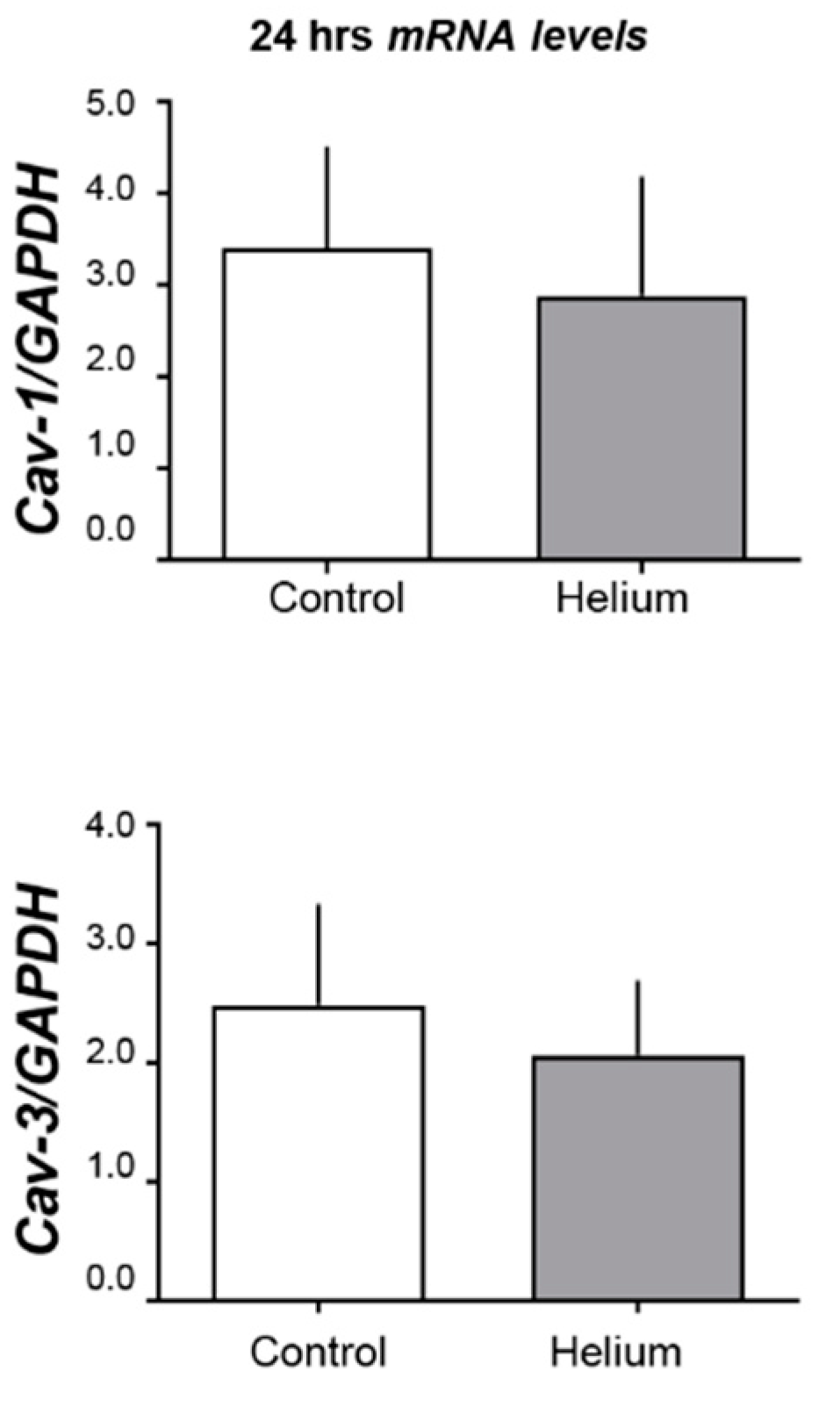
2.3. Cav-1 and -3 Expression
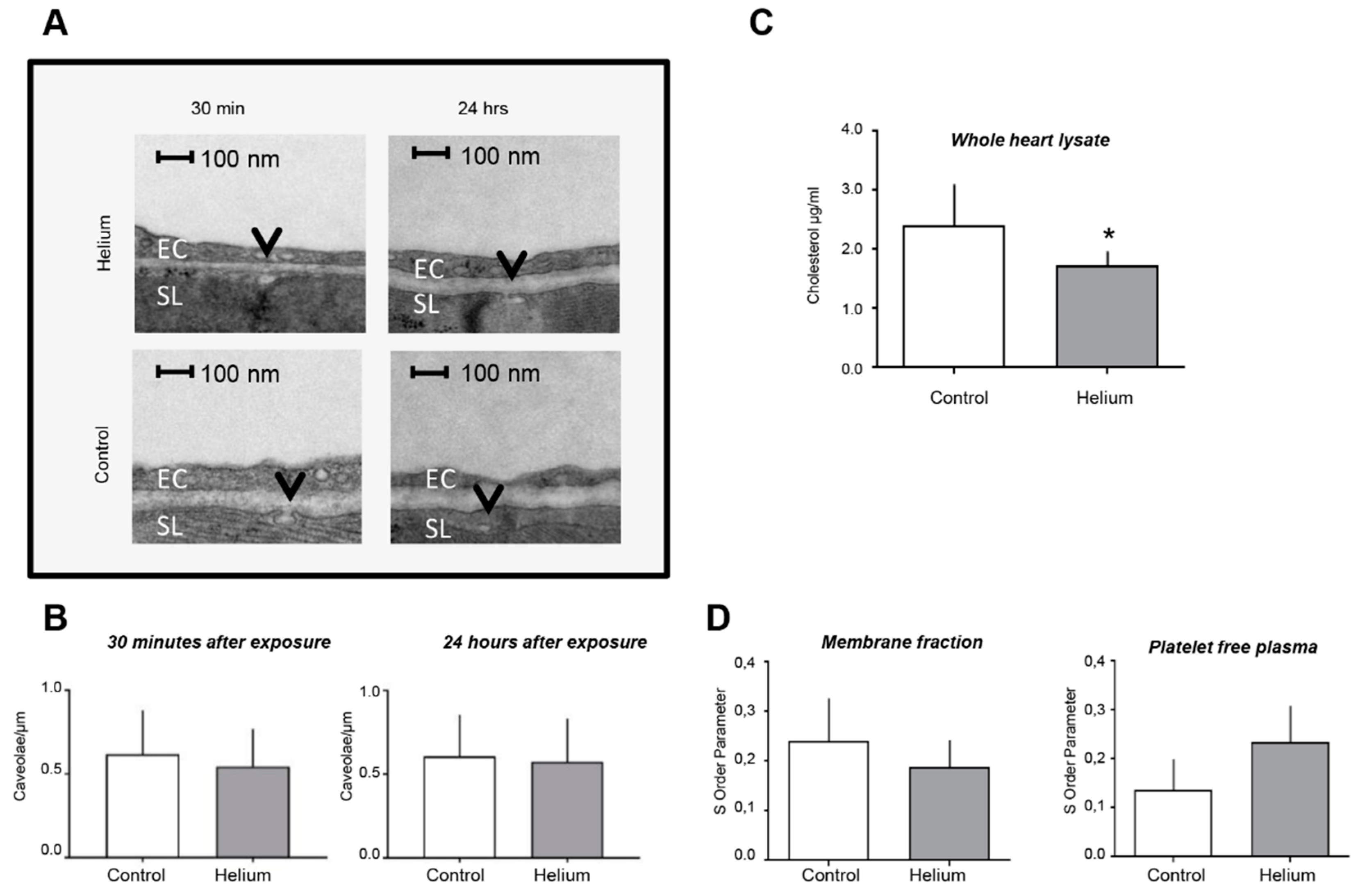
2.4. Cardiac Ultrastructure, Cholesterol Quantity and Blood Plasma Fluidity
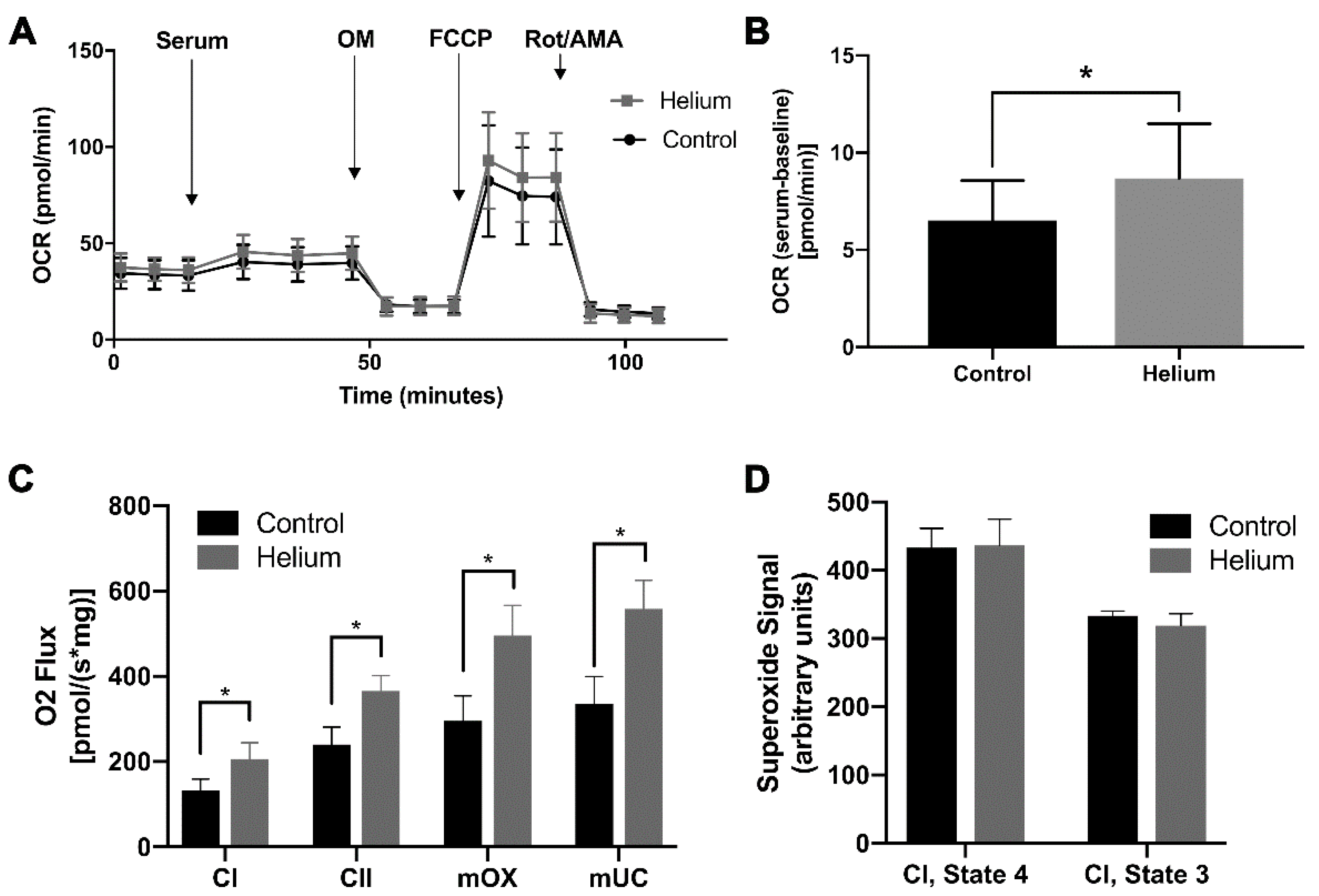
2.5. Biological Effect of Helium-Conditioned Serum on Mitochondrial Function
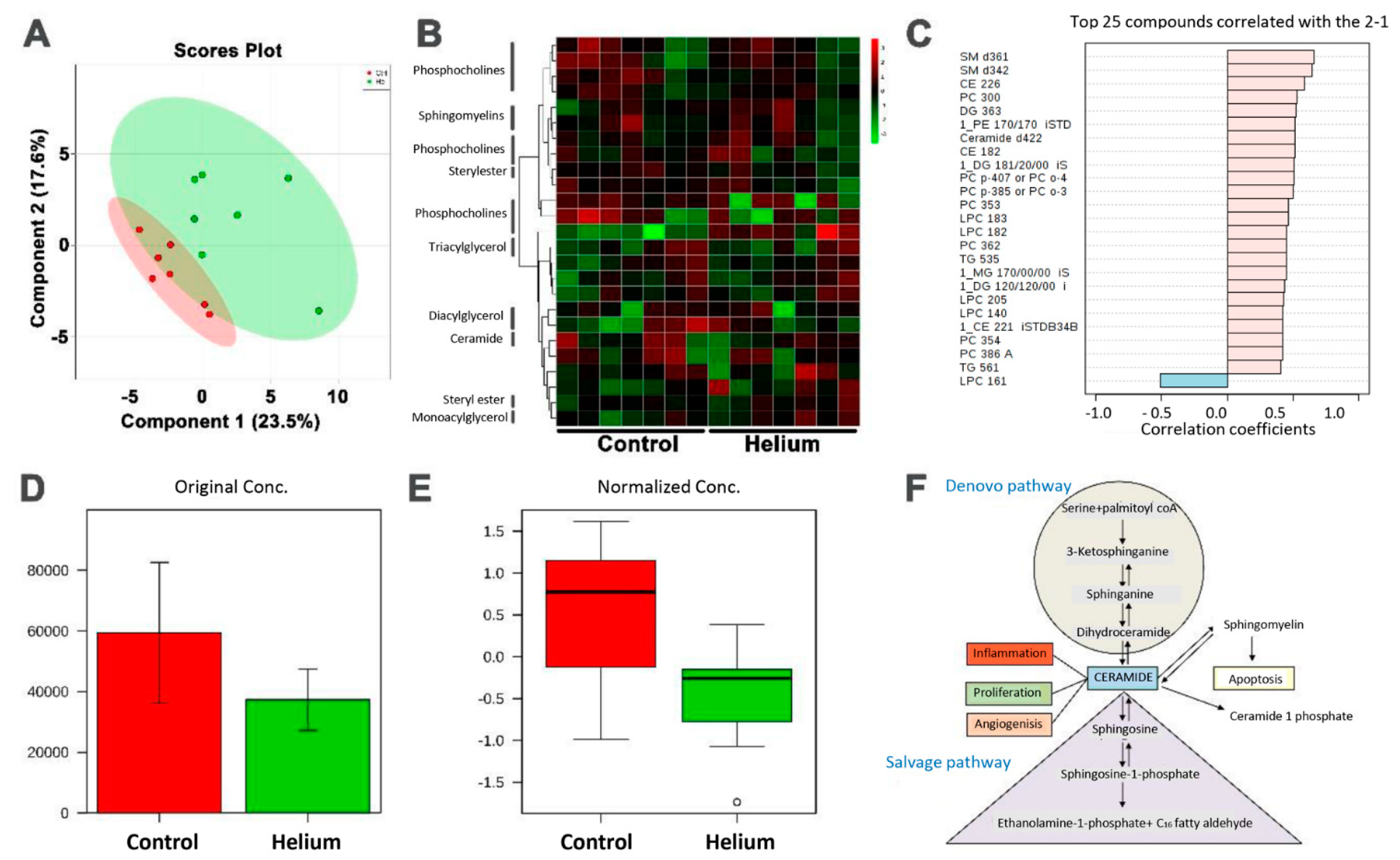
2.6. Primary and Complex Lipid Metabolites in Serum
3. Discussion
3.1. Circulating Factors
3.2. Remote Protection
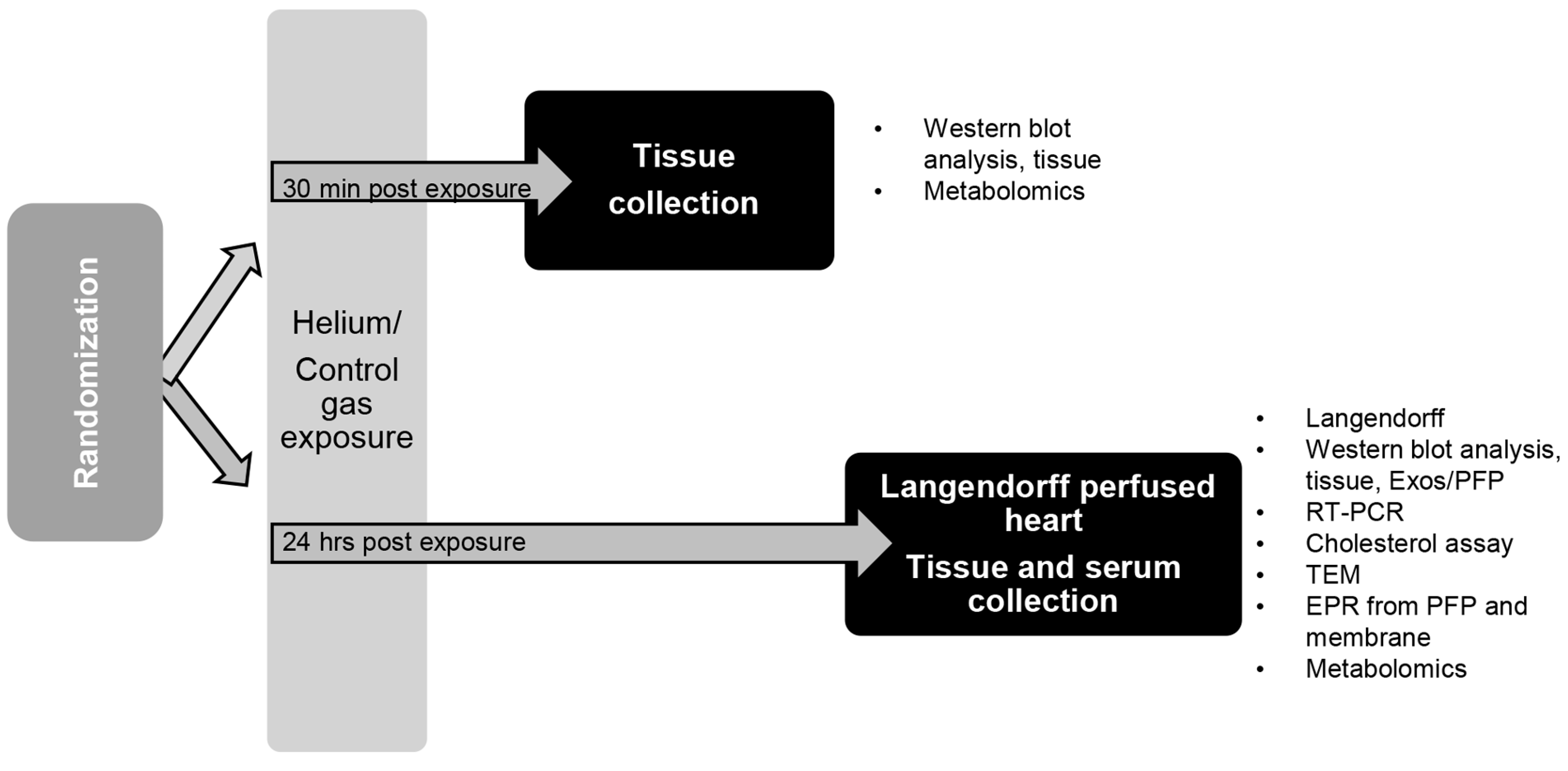
4. Materials and Methods
4.1. Animal Handling
4.2. General Chemicals and Solutions
4.3. Anesthesia Exposure Protocol and Tissue Collection
4.4. Langendorff Perfused Heart Model
4.5. Immunoblot Analysis
4.6. Cholesterol Assay
4.7. Quantitative Real-Time Polymerase Chain Reaction (RT-PCR)
4.8. Isolation of Membrane and Cytosolic Fractions
4.9. Isolation of Serum, Platelet Free Plasma and Exosomes
4.10. Electron Paramagnetic Resonance Spectrometry (EPR)
4.11. Transmission Electron Microscopy (TEM)
4.12. Seahorse Mitochondrial Respiration Assay
4.13. High-Resolution Mitochondrial Respirometry
4.14. Metabolomics
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Cav-1 | Caveolin-1 |
| Cav-2 | Caveolin-2 |
| Cav-3 | Caveolin-3 |
| He | Helium |
| h | Hours |
| min | Minutes |
| Ctrl | Control |
| PFP | Platelet free plasma |
| RISK | Reperfusion Injury Salvage Kinase |
References
- Ding, Y.P.; Zhang, J.Y.; Feng, D.X.; Kong, Y.; Xu, Z.; Chen, G. Advances in molecular mechanism of cardioprotection induced by helium. Med. Gas. Res. 2017, 7, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Smit, K.F.; Weber, N.C.; Hollmann, M.W.; Preckel, B. Noble gases as cardioprotectants—Translatability and mechanism. Br. J. Pharmacol. 2015, 172, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Smit, K.F.; Oei, G.T.; Brevoord, D.; Stroes, E.S.; Nieuwland, R.; Schlack, W.S.; Hollmann, M.W.; Weber, N.C.; Preckel, B. Helium induces preconditioning in human endothelium in vivo. Anesthesiology 2013, 118, 95–104. [Google Scholar] [CrossRef]
- Huhn, R.; Heinen, A.; Weber, N.C.; Hieber, S.; Hollmann, M.W.; Schlack, W.; Preckel, B. Helium-induced late preconditioning in the rat heart in vivo. Br. J. Anaesth 2009, 102, 614–619. [Google Scholar] [CrossRef]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef] [PubMed]
- Heinen, A.; Huhn, R.; Smeele, K.M.; Zuurbier, C.J.; Schlack, W.; Preckel, B.; Weber, N.C.; Hollmann, M.W. Helium-induced preconditioning in young and old rat heart: Impact of mitochondrial Ca(2+) -sensitive potassium channel activation. Anesthesiology 2008, 109, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Huhn, R.; Heinen, A.; Hollmann, M.W.; Schlack, W.; Preckel, B.; Weber, N.C. Cyclosporine A administered during reperfusion fails to restore cardioprotection in prediabetic Zucker obese rats in vivo. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 706–712. [Google Scholar] [CrossRef]
- Huhn, R.; Heinen, A.; Weber, N.C.; Kerindongo, R.P.; Oei, G.T.; Hollmann, M.W.; Schlack, W.; Preckel, B. Helium-induced early preconditioning and postconditioning are abolished in obese Zucker rats in vivo. J. Pharmacol. Exp. Ther. 2009, 329, 600–607. [Google Scholar] [CrossRef]
- Pagel, P.S.; Krolikowski, J.G. Transient metabolic alkalosis during early reperfusion abolishes helium preconditioning against myocardial infarction: Restoration of cardioprotection by cyclosporin A in rabbits. Anesth. Analg. 2009, 108, 1076–1082. [Google Scholar] [CrossRef]
- Fridolfsson, H.N.; Kawaraguchi, Y.; Ali, S.S.; Panneerselvam, M.; Niesman, I.R.; Finley, J.C.; Kellerhals, S.E.; Migita, M.Y.; Okada, H.; Moreno, A.L.; et al. Mitochondria-localized caveolin in adaptation to cellular stress and injury. FASEB J. 2012, 26, 4637–4649. [Google Scholar] [CrossRef]
- Tsutsumi, Y.M.; Horikawa, Y.T.; Jennings, M.M.; Kidd, M.W.; Niesman, I.R.; Yokoyama, U.; Head, B.P.; Hagiwara, Y.; Ishikawa, Y.; Miyanohara, A.; et al. Cardiac-specific overexpression of caveolin-3 induces endogenous cardiac protection by mimicking ischemic preconditioning. Circulation 2008, 118, 1979–1988. [Google Scholar] [CrossRef]
- Wang, J.; Schilling, J.M.; Niesman, I.R.; Headrick, J.P.; Finley, J.C.; Kwan, E.; Patel, P.M.; Head, B.P.; Roth, D.M.; Yue, Y.; et al. Cardioprotective trafficking of caveolin to mitochondria is Gi-protein dependent. Anesthesiology 2014, 121, 538–548. [Google Scholar] [CrossRef]
- Pike, L.J. Lipid rafts: Bringing order to chaos. J. Lipid Res. 2003, 44, 655–667. [Google Scholar] [CrossRef]
- Chun, M.; Liyanage, U.K.; Lisanti, M.P.; Lodish, H.F. Signal transduction of a G protein-coupled receptor in caveolae: Colocalization of endothelin and its receptor with caveolin. Proc. Natl. Acad. Sci. USA 1994, 91, 11728–11732. [Google Scholar] [CrossRef]
- Parton, R.G.; Way, M.; Zorzi, N.; Stang, E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J. Cell. Biol. 1997, 136, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Scherer, P.E.; Tang, Z.; Okamoto, T.; Li, S.; Chafel, M.; Chu, C.; Kohtz, D.S.; Lisanti, M.P. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 1996, 271, 15160–15165. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, Y.T.; Panneerselvam, M.; Kawaraguchi, Y.; Tsutsumi, Y.M.; Ali, S.S.; Balijepalli, R.C.; Murray, F.; Head, B.P.; Niesman, I.R.; Rieg, T.; et al. Cardiac-specific overexpression of caveolin-3 attenuates cardiac hypertrophy and increases natriuretic peptide expression and signaling. J. Am. Coll. Cardiol. 2011, 57, 2273–2283. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, Y.T.; Patel, H.H.; Tsutsumi, Y.M.; Jennings, M.M.; Kidd, M.W.; Hagiwara, Y.; Ishikawa, Y.; Insel, P.A.; Roth, D.M. Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2008, 44, 123–130. [Google Scholar] [CrossRef]
- Patel, H.H.; Tsutsumi, Y.M.; Head, B.P.; Niesman, I.R.; Jennings, M.; Horikawa, Y.; Huang, D.; Moreno, A.L.; Patel, P.M.; Insel, P.A.; et al. Mechanisms of cardiac protection from ischemia/reperfusion injury: A role for caveolae and caveolin-1. FASEB J. 2007, 21, 1565–1574. [Google Scholar] [CrossRef]
- Tsutsumi, Y.M.; Kawaraguchi, Y.; Horikawa, Y.T.; Niesman, I.R.; Kidd, M.W.; Chin-Lee, B.; Head, B.P.; Patel, P.M.; Roth, D.M.; Patel, H.H. Role of caveolin-3 and glucose transporter-4 in isoflurane-induced delayed cardiac protection. Anesthesiology 2010, 112, 1136–1145. [Google Scholar] [CrossRef]
- Li, W.P.; Liu, P.; Pilcher, B.K.; Anderson, R.G. Cell-specific targeting of caveolin-1 to caveolae, secretory vesicles, cytoplasm or mitochondria. J. Cell. Sci. 2001, 114, 1397–1408. [Google Scholar]
- Bosch, M.; Mari, M.; Herms, A.; Fernandez, A.; Fajardo, A.; Kassan, A.; Giralt, A.; Colell, A.; Balgoma, D.; Barbero, E.; et al. Caveolin-1 deficiency causes cholesterol-dependent mitochondrial dysfunction and apoptotic susceptibility. Curr. Biol. 2011, 21, 681–686. [Google Scholar] [CrossRef]
- Pavlides, S.; Tsirigos, A.; Vera, I.; Flomenberg, N.; Frank, P.G.; Casimiro, M.C.; Wang, C.; Fortina, P.; Addya, S.; Pestell, R.G.; et al. Loss of stromal caveolin-1 leads to oxidative stress, mimics hypoxia and drives inflammation in the tumor microenvironment, conferring the “reverse Warburg effect”: A transcriptional informatics analysis with validation. Cell. Cycle 2010, 9, 2201–2219. [Google Scholar] [CrossRef]
- Aehling, C.; Weber, N.C.; Zuurbier, C.J.; Preckel, B.; Galmbacher, R.; Stefan, K.; Hollmann, M.W.; Popp, E.; Knapp, J. Effects of combined helium pre/post-conditioning on the brain and heart in a rat resuscitation model. Acta Anaesthesiol. Scand. 2018, 62, 63–74. [Google Scholar] [CrossRef]
- Flick, M.; Albrecht, M.; Oei, G.T.; Steenstra, R.; Kerindongo, R.P.; Zuurbier, C.J.; Patel, H.H.; Hollmann, M.W.; Preckel, B.; Weber, N.C. Helium postconditioning regulates expression of caveolin-1 and -3 and induces RISK pathway activation after ischaemia/reperfusion in cardiac tissue of rats. Eur. J. Pharmacol. 2016, 791, 718–725. [Google Scholar] [CrossRef]
- Smit, K.F.; Konkel, M.; Kerindongo, R.; Landau, M.A.; Zuurbier, C.J.; Hollmann, M.W.; Preckel, B.; Nieuwland, R.; Albrecht, M.; Weber, N.C. Helium alters the cytoskeleton and decreases permeability in endothelial cells cultured in vitro through a pathway involving Caveolin-1. Sci. Rep. 2018, 8, 4768. [Google Scholar] [CrossRef]
- Pagel, P.S.; Krolikowski, J.G.; Shim, Y.H.; Venkatapuram, S.; Kersten, J.R.; Weihrauch, D.; Warltier, D.C.; Pratt, P.F., Jr. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth. Analg. 2007, 105, 562–569. [Google Scholar] [CrossRef]
- Schilling, J.M.; Roth, D.M.; Patel, H.H. Caveolins in cardioprotection—Translatability and mechanisms. Br. J. Pharmacol. 2015, 172, 2114–2125. [Google Scholar] [CrossRef]
- Oei, G.T.; Weber, N.C.; Hollmann, M.W.; Preckel, B. Cellular effects of helium in different organs. Anesthesiology 2010, 112, 1503–1510. [Google Scholar] [CrossRef]
- Weber, N.C.; Smit, K.F.; Hollmann, M.W.; Preckel, B. Targets Involved in Cardioprotection by the Non-Anesthetic Noble Gas Helium. Curr. Drug Targets 2015, 16, 786–792. [Google Scholar] [CrossRef]
- Oei, G.T.; Heger, M.; Van Golen, R.F.; Alles, L.K.; Flick, M.; van der Wal, A.C.; Van Gulik, T.M.; Hollmann, M.W.; Preckel, B.; Weber, N.C. Reduction of cardiac cell death after helium postconditioning in rats: Transcriptional analysis of cell death and survival pathways. Mol. Med. 2014, 20, 516. [Google Scholar] [CrossRef]
- Shen, J.; Lee, W.S.; Chen, J.; Yang, D. Caveolin-3 Peptide Protects Cardiomyocytes from Apoptotic Cell Death via Preserving Superoxide Dismutase Activity and Inhibiting Caspase-3 Activation under Hypoxia-reoxygenation. Am. J. Biomed. Sci. 2011, 3, 126–144. [Google Scholar] [CrossRef][Green Version]
- Rassaf, T.; Totzeck, M.; Hendgen-Cotta, U.B.; Shiva, S.; Heusch, G.; Kelm, M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ. Res. 2014, 114, 1601–1610. [Google Scholar] [CrossRef]
- Hildebrandt, H.A.; Kreienkamp, V.; Gent, S.; Kahlert, P.; Heusch, G.; Kleinbongard, P. Kinetics and Signal Activation Properties of Circulating Factor(s) From Healthy Volunteers Undergoing Remote Ischemic Pre-Conditioning. JACC Basic Transl. Sci. 2016, 1, 3–13. [Google Scholar] [CrossRef]
- Schmidt, M.R.; Redington, A.; Botker, H.E. Remote conditioning the heart overview: Translatability and mechanism. Br. J. Pharmacol. 2015, 172, 1947–1960. [Google Scholar] [CrossRef]
- Ciardiello, C.; Cavallini, L.; Spinelli, C.; Yang, J.; Reis-Sobreiro, M.; de Candia, P.; Minciacchi, V.R.; Di Vizio, D. Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer. Int. J. Mol. Sci. 2016, 17, 175. [Google Scholar] [CrossRef]
- Mirzapoiazova, T.; Lennon, F.E.; Mambetsariev, B.; Allen, M.; Riehm, J.; Poroyko, V.A.; Singleton, P.A. Extracellular Vesicles from Caveolin-Enriched Microdomains Regulate Hyaluronan-Mediated Sustained Vascular Integrity. Int. J. Cell. Biol. 2015, 2015, 481493. [Google Scholar] [CrossRef]
- Quest, A.F.; Gutierrez-Pajares, J.L.; Torres, V.A. Caveolin-1: An ambiguous partner in cell signalling and cancer. J. Cell. Mol. Med. 2008, 12, 1130–1150. [Google Scholar] [CrossRef]
- Tahir, S.A.; Yang, G.; Ebara, S.; Timme, T.L.; Satoh, T.; Li, L.; Goltsov, A.; Ittmann, M.; Morrisett, J.D.; Thompson, T.C. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001, 61, 3882–3885. [Google Scholar]
- Tahir, S.A.; Yang, G.; Goltsov, A.A.; Watanabe, M.; Tabata, K.; Addai, J.; Fattah el, M.A.; Kadmon, D.; Thompson, T.C. Tumor cell-secreted caveolin-1 has proangiogenic activities in prostate cancer. Cancer Res. 2008, 68, 731–739. [Google Scholar] [CrossRef]
- Thompson, T.C.; Tahir, S.A.; Li, L.; Watanabe, M.; Naruishi, K.; Yang, G.; Kadmon, D.; Logothetis, C.J.; Troncoso, P.; Ren, C.; et al. The role of caveolin-1 in prostate cancer: Clinical implications. Prostate Cancer Prostatic Dis. 2010, 13, 6–11. [Google Scholar] [CrossRef]
- Watanabe, M.; Yang, G.; Cao, G.; Tahir, S.A.; Naruishi, K.; Tabata, K.; Fattah, E.A.; Rajagopalan, K.; Timme, T.L.; Park, S.; et al. Functional analysis of secreted caveolin-1 in mouse models of prostate cancer progression. Mol. Cancer Res. 2009, 7, 1446–1455. [Google Scholar] [CrossRef]
- Shatz, M.; Liscovitch, M. Caveolin-1 and cancer multidrug resistance: Coordinate regulation of pro-survival proteins? Leuk. Res. 2004, 28, 907–908. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, N.; Liu, P.; Peng, F.; Tang, H.; Chen, Q.; Xu, R.; Dai, Y.; Lin, Y.; Xie, X.; et al. Caveolin-1, a stress-related oncotarget, in drug resistance. Oncotarget 2015, 6, 37135–37150. [Google Scholar] [CrossRef]
- Ong, S.B.; Lee, W.H.; Shao, N.Y.; Ismail, N.I.; Katwadi, K.; Lim, M.M.; Kwek, X.Y.; Michel, N.A.; Li, J.; Newson, J.; et al. Calpain Inhibition Restores Autophagy and Prevents Mitochondrial Fragmentation in a Human iPSC Model of Diabetic Endotheliopathy. Stem Cell. Rep. 2019, 12, 597–610. [Google Scholar] [CrossRef]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef]
- Cremesti, A.E.; Goni, F.M.; Kolesnick, R. Role of sphingomyelinase and ceramide in modulating rafts: Do biophysical properties determine biologic outcome? FEBS Lett. 2002, 531, 47–53. [Google Scholar] [CrossRef]
- Headrick, J.P.; Willems, L.; Ashton, K.J.; Holmgren, K.; Peart, J.; Matherne, G.P. Ischaemic tolerance in aged mouse myocardium: The role of adenosine and effects of A1 adenosine receptor overexpression. J. Physiol. (Lond.) 2003, 549, 823–833. [Google Scholar] [CrossRef]
- Peart, J.N.; Gross, E.R.; Headrick, J.P.; Gross, G.J. Impaired p38 MAPK/HSP27 signaling underlies aging-related failure in opioid-mediated cardioprotection. J. Mol. Cell. Cardiol. 2007, 42, 972–980. [Google Scholar] [CrossRef]
- Kawaraguchi, Y.; Horikawa, Y.T.; Murphy, A.N.; Murray, F.; Miyanohara, A.; Ali, S.S.; Head, B.P.; Patel, P.M.; Roth, D.M.; Patel, H.H. Volatile anesthetics protect cancer cells against tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via caveolins. Anesthesiology 2011, 115, 499–508. [Google Scholar] [CrossRef]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Gabbita, S.P.; Butterfield, D.A.; Hensley, K.; Shaw, W.; Carney, J.M. Aging and caloric restriction affect mitochondrial respiration and lipid membrane status: An electron paramagnetic resonance investigation. Free Radic. Biol. Med. 1997, 23, 191–201. [Google Scholar] [CrossRef]
- Gabbita, S.P.; Subramaniam, R.; Allouch, F.; Carney, J.M.; Butterfield, D.A. Effects of mitochondrial respiratory stimulation on membrane lipids and proteins: An electron paramagnetic resonance investigation. Biochim. Biophys. Acta 1998, 1372, 163–173. [Google Scholar] [CrossRef]
- Panneerselvam, M.; Ali, S.S.; Finley, J.C.; Kellerhals, S.E.; Migita, M.Y.; Head, B.P.; Patel, P.M.; Roth, D.M.; Patel, H.H. Epicatechin regulation of mitochondrial structure and function is opioid receptor dependent. Mol. Nutr. Food Res. 2013, 57, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, E.; Mahmoud, A.M.; Khalifa, A.M.; Ali, S.S. Physiological and pathophysiological ROS as probed by EPR spectroscopy: The underutilized research window on muscle aging. J. Physiol. (Lond.) 2016. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, T.; Mahata, S.; Bandyopadhyay, G.K.; Biswas, A.; Perkins, G.A.; Sinha-Hikim, A.P.; Goldstein, D.S.; Eiden, L.E.; Mahata, S.K. Impact of Chromogranin A deficiency on catecholamine storage, catecholamine granule morphology and chromaffin cell energy metabolism in vivo. Cell. Tissue Res. 2016, 363, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, 10–1126. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, N.C.; Schilling, J.M.; Warmbrunn, M.V.; Dhanani, M.; Kerindongo, R.; Siamwala, J.; Song, Y.; Zemljic-Harpf, A.E.; Fannon, M.J.; Hollmann, M.W.; et al. Helium-Induced Changes in Circulating Caveolin in Mice Suggest a Novel Mechanism of Cardiac Protection. Int. J. Mol. Sci. 2019, 20, 2640. https://doi.org/10.3390/ijms20112640
Weber NC, Schilling JM, Warmbrunn MV, Dhanani M, Kerindongo R, Siamwala J, Song Y, Zemljic-Harpf AE, Fannon MJ, Hollmann MW, et al. Helium-Induced Changes in Circulating Caveolin in Mice Suggest a Novel Mechanism of Cardiac Protection. International Journal of Molecular Sciences. 2019; 20(11):2640. https://doi.org/10.3390/ijms20112640
Chicago/Turabian StyleWeber, Nina C., Jan M. Schilling, Moritz V. Warmbrunn, Mehul Dhanani, Raphaela Kerindongo, Jamila Siamwala, Young Song, Alice E. Zemljic-Harpf, McKenzie J. Fannon, Markus W. Hollmann, and et al. 2019. "Helium-Induced Changes in Circulating Caveolin in Mice Suggest a Novel Mechanism of Cardiac Protection" International Journal of Molecular Sciences 20, no. 11: 2640. https://doi.org/10.3390/ijms20112640
APA StyleWeber, N. C., Schilling, J. M., Warmbrunn, M. V., Dhanani, M., Kerindongo, R., Siamwala, J., Song, Y., Zemljic-Harpf, A. E., Fannon, M. J., Hollmann, M. W., Preckel, B., Roth, D. M., & Patel, H. H. (2019). Helium-Induced Changes in Circulating Caveolin in Mice Suggest a Novel Mechanism of Cardiac Protection. International Journal of Molecular Sciences, 20(11), 2640. https://doi.org/10.3390/ijms20112640