Mechanistic and Kinetic Study on Self-/Cross- Condensation of PCTA/DT Formation Mechanisms from Three Types of Radicals of 2,4-Dichlorothiophenol
Abstract
1. Introduction
2. Results
2.1. Formation of 2,4-Dichlorothiophenoxy Radical R1, 2-Sulfydryl-3,5-Dichlorophenyl Radical R2 and 3,5-Dichlorothiophenoxyl Diradical DR
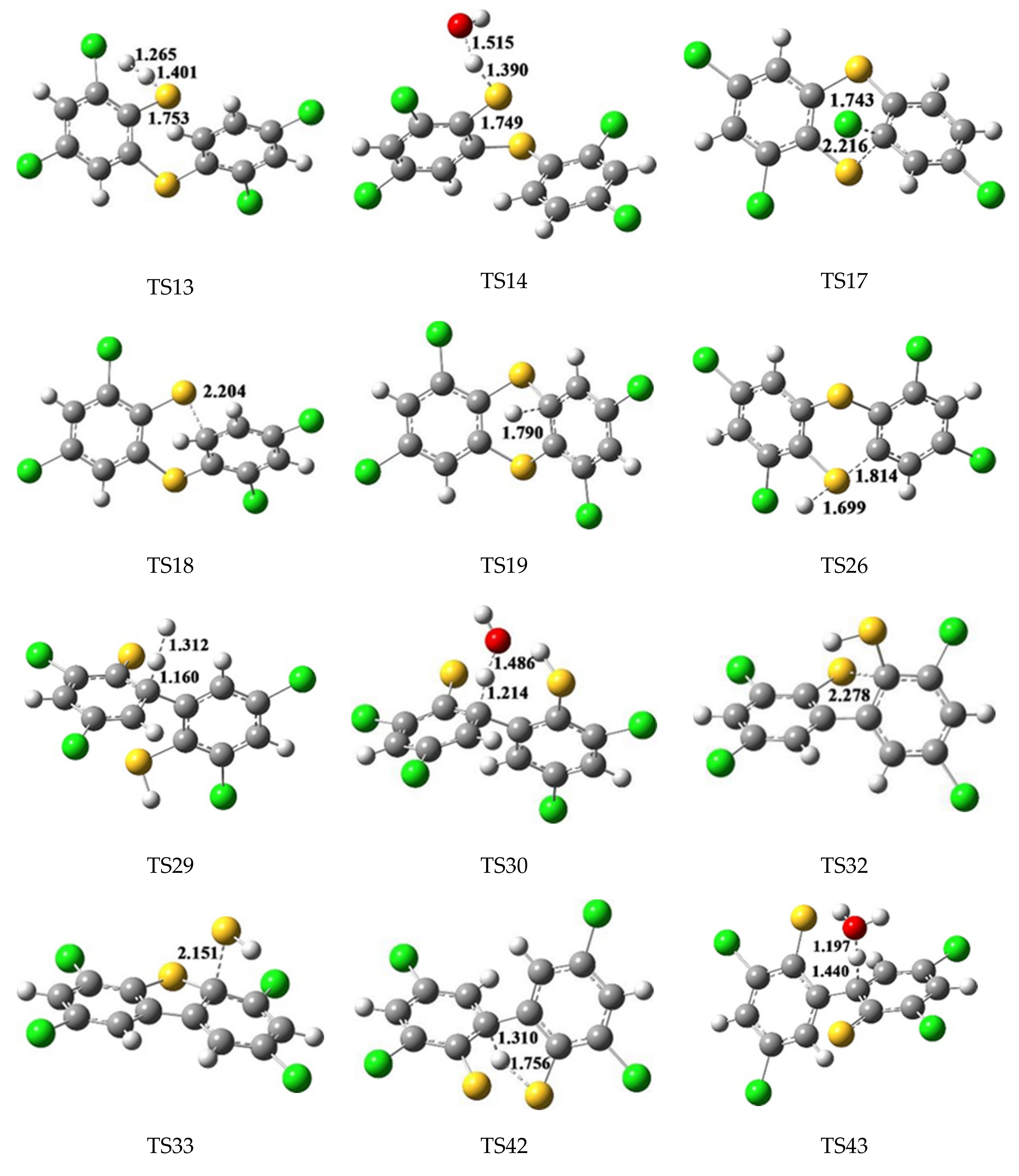
2.2. Formation of PCTAs from R1, R2 and DR
2.3. Formation of PCDTs from R1, R2 and DR
2.4. Rate Constant Calculations
3. Discussion
3.1. Formation of 2,4-Dichlorothiophenoxy Radical R1, 2-Sulfydryl-3,5-Dichlorophenyl Radical R2 and 3,5-Dichlorothiophenoxyl Diradical DR
3.2. Formation of PCTAs from R1, R2 and DR
3.3. Formation of PCDTs from R1, R2 and DR
3.4. Rate Constant Calculations
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PCDTs | polychlorinated dibenzothiophenes |
| PCTAs | polychlorinated thianthrenes |
| PCDDs | polychlorinated dibenzo-p-dioxins |
| PCDFs | polychlorinated dibenzofurans |
| R1 | 2,4-dichlorothiophenoxy radical |
| R2 | 2-sulfydryl-3,5-dichlorophenyl radical |
| DR | 3,5-dichlorothiophenoxyl diradical |
| 1,3,8-TCTA | 1,3,8-trichlorothianthrene |
| 1,3,6,8-TeCTA | 1,3,6,8-tetrachlorothianthrene |
| 1,3,7,9-TeCTA | 1,3,7,9-tetrachlorothianthrene |
| 1,3,7-TCTA | 1,3,7-trichlorothianthrene |
| 2,4,7,9-TeCTA | 2,4,7,9-tetrachlorothianthrene |
| 2,4,6,8-TeCDT | 2,4,6,8-tetrachlorodibenzothiophene |
| 2,6,8-TeCDT | 2,6,8-trichlorodibenzothiophene |
| DFT | density functional theory |
| TST | transition state theory |
| IRC | intrinsic reaction coordinate |
| ZPE | zero-point energy |
| MEPs | minimum energy paths |
| CVT | canonical variational transition-state theory |
| SCT | small curvature tunneling |
References
- Sinkkonen, S.; Kolehmainen, E.; Paasivirta, J.; Koistinen, J.; Lahtipera, M.; Lammi, R. Identification and level estimation of chlorinated neutral aromatic sulfur compounds and their alkylated derivatives in pulp mill effluents and sediments. Chemosphere 1994, 28, 2049–2066. [Google Scholar] [CrossRef]
- Sinkkonen, S. New Types of Persistent Halogenated Compounds; Springer: Berlin, Germany, 2000; Volume 3, pp. 289–314. [Google Scholar]
- Kopponen, P.; Krenlampi, S.; Sinkkonen, S. Sulphur analogues of polychlorinated dioxins, furans and diphenylethers as inducers of aryl hydrocarbon hydroxylase. Organohalogen Compd. 1993, 13, 229–232. [Google Scholar]
- Nakai, S.; Kishita, S.; Nomura, Y.; Hosomi, M. Polychlorinated dibenzothiophenes in Japanese environmental samples and their photodegradability and dioxin-like endocrine-disruption potential. Chemosphere 2007, 67, 1852–1857. [Google Scholar] [CrossRef]
- Weber, R.; Hagenmaier, H.; Schrenk, D. Elimination kinetics and toxicity of 2,3,7,8-tetrachlorothiantheren, a thio analogue of 2, 3, 7, 8-TCDD. Chemosphere 1998, 36, 2635–2641. [Google Scholar] [CrossRef]
- Buser, H.R.; Rappe, C. Determination of polychlorodibenzothiophenes, the sulfur analogs of polychlorodibenzofurans, using various gas chromatographic/mass spectrometric techniques. Anal. Chem. 1991, 63, 1210–1217. [Google Scholar] [CrossRef]
- Sinkkonen, S.; Vattulainen, A.; Aittola, J.-P.; Paasivirta, J.; Tarhanen, J.; Lahtiperä, M. Metal reclamation produces sulphur analogues of toxic dioxins and furans. Chemosphere 1994, 28, 1279–1288. [Google Scholar] [CrossRef]
- Rappe, C. Sources of exposure, environmental concentrations and exposure assessment of PCDDs and PCDFs. Chemosphere 1993, 27, 211–225. [Google Scholar] [CrossRef]
- Sinkkonen, S.; Kolehmainen, E.; Koistinen, J.; Lahtiperä, M. High-resolution gas chromatographic-mass spectrometric determination of neutral chlorinated aromatic sulphur compounds in stack gas samples. J. Chromatogr. A 1993, 641, 309–317. [Google Scholar] [CrossRef]
- Pruell, R.; Taplin, B.; McGovern, D.; McKinney, R.; Norton, S. Organic contaminant distributions in sediments, polychaetes (Nereis virens) and American lobster (Homarus americanus) from a laboratory food chain experiment. Mar. Environ Res. 2000, 49, 19–36. [Google Scholar] [CrossRef]
- Pruell, R.; Rubinstein, N.; Taplin, B.; LiVolsi, J.; Bowen, R. Accumulation of polychlorinated organic contaminants from sediment by three benthic marine species. Arch. Environ. Contam. Toxicol. 1993, 24, 290–297. [Google Scholar] [CrossRef]
- Huntley, S.; Wenning, R.; Paustenbach, D.; Wong, A.; Luksemburg, W. Potential sources of polychlorinated dibenzothiophenes in the Passaic River, New Jersey. Chemosphere 1994, 29, 257–272. [Google Scholar] [CrossRef]
- Klasmeier, J.; Matthies, M.; Macleod, M.; Fenner, K.; Scheringer, M.; Stroebe, M.; Le Gall, A.C.; Mckone, T.; Van De Meent, D.; Wania, F. Application of multimedia models for screening assessment of long-range transport potential and overall persistence. Environ. Sci. Technol. 2006, 40, 53–60. [Google Scholar] [CrossRef]
- Sinkkonen, S.; Paasivirta, J.; Lahtiperä, M. Chlorinated and methylated dibenzothiophenes in sediment samples from a river contaminated by organochlorine wastes. J. Soils Sediments 2001, 1, 9–14. [Google Scholar] [CrossRef]
- Buser, H. Identification and sources of dioxin-like compounds: I. Polychlorodibenzothiophenes and polychlorothianthrenes, the sulfur-analogues of the polychlorodibenzofurans and polychlorodibenzodioxins. Chemosphere 1992, 25, 45–48. [Google Scholar] [CrossRef]
- Sinkkonen, S.; Paasivirta, J.; Koistinen, J.; Tarhanen, J. Tetra-and pentachlorodibenzothiophenes are formed in waste combustion. Chemosphere 1991, 23, 583–587. [Google Scholar] [CrossRef]
- Sato, S.; Matsumura, A.; Urushigawa, Y.; Metwally, M.; Al-Muzaini, S. Structural analysis of weathered oil from Kuwait’s environment. Environ. Int. 1998, 24, 77–87. [Google Scholar] [CrossRef]
- Atlas, R. Fate of oil from two major oil spills: Role of microbial degradation in removing oil from the Amoco Cadiz and IXTOC I spills. Environ. Int. 1981, 5, 33–38. [Google Scholar] [CrossRef]
- Cai, Z.W.; Giblin, D.E.; Ramanujam, V.S.; Gross, M.L.; Cristini, A. Mass-profile monitoring in trace analysis: Identification of polychlorodibenzothiophenes in crab tissues collected from the Newark/Raritan Bay system. Environ. Sci. Technol. 1994, 28, 1535–1538. [Google Scholar] [CrossRef] [PubMed]
- Parette, R.; Pearson, W.N. 2,4,6,8-Tetrachlorodibenzothiophene in the Newark Bay Estuary: The likely source and reaction pathways. Chemosphere 2014, 111, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Dar, T.; Altarawneh, M.; Dlugogorski, B. Theoretical study in the dimerisation of 2-chlrothiophenol/2-chlorothiophenoxy: Precursors to PCDT/TA. Organohalogen Compd. 2012, 74, 657–660. [Google Scholar]
- Dar, T.; Altarawneh, M.; Dlugogorski, B.Z. Quantum chemical study on formation of PCDT/TA from 2-chlorothiophenol precursor. Environ. Sci. Technol. 2013, 47, 11040–11047. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.; Bierbrauer, K.; Mijangos, C.; Goiti, E.; Reinecke, H. Modification of poly (vinyl chloride) with new aromatic thiol compounds. Synthesis and characterization. Polym. Degrad. Stab. 2008, 93, 585–591. [Google Scholar] [CrossRef]
- Dar, T.; Shah, K.; Moghtaderi, B.; Page, A.J. Formation of persistent organic pollutants from 2, 4, 5-trichlorothiophenol combustion: a density functional theory investigation. J. Mol. Model. 2016, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.S.; Dellinger, B. Mechanisms of dioxin formation from the high-temperature pyrolysis of 2-chlorophenol. Environ. Sci. Technol. 2003, 37, 1325–1330. [Google Scholar] [CrossRef]
- Evans, C.S.; Dellinger, B. Mechanisms of dioxin formation from the high-temperature oxidation of 2-chlorophenol. Environ. Sci. Technol. 2005, 39, 122–127. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Li, S.Q.; Qu, X.H.; Shi, X.Y.; Wang, W.X. A quantum mechanical study on the formation of PCDD/Fs from 2-chlorophenol as precursor. Environ. Sci. Technol. 2008, 42, 7301–7308. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.H.; Wang, H.; Zhang, Q.Z.; Shi, X.Y.; Xu, F.; Wang, W.X. Mechanistic and kinetic studies on the homogeneous gas-phase formation of PCDD/Fs from 2,4,5-trichlorophenol. Environ. Sci. Technol. 2009, 43, 4068–4075. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shi, X.L.; Li, Y.F.; Zhang, Q.Z. Mechanistic and kinetic studies on the homogeneous gas-phase formation of PCTA/DTs from 2,4-dichlorothiophenol and 2,4,6-trichlorothiophenol. Int. J. Mol. Sci. 2015, 16, 20449–20467. [Google Scholar] [CrossRef]
- Yu, X.Q.; Chang, J.M.; Liu, X.; Pan, W.X.; Zhang, A.Q. Theoretical study on the formation mechanism of polychlorinated dibenzothiophenes/thianthrenes from 2-chlorothiophenol molecules. J. Environ. Sci. 2018, 66, 318–327. [Google Scholar] [CrossRef]
- Briois, C.; Visez, N.; Baillet, C.; Sawerysyn, J.-P. Experimental study on the thermal oxidation of 2-chlorophenol in air over the temperature range 450–900 °C. Chemosphere 2006, 62, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.; Parker, D.; Zhang, F.; Landera, A.; Kislov, V.; Mebel, A. PAH formation under single collision conditions: reaction of phenyl radical and 1,3-butadiene to form 1,4-dihydronaphthalene. J. Phys. Chem. A 2012, 116, 4248–4258. [Google Scholar] [CrossRef]
- Shukla, B.; Susa, A.; Miyoshi, A.; Koshi, M. Role of phenyl radicals in the growth of polycyclic aromatic hydrocarbons. J. Phys. Chem. A 2008, 112, 2362–2369. [Google Scholar] [CrossRef]
- Bonnichon, F.; Richard, C.; Grabner, G. Formation of an α-ketocarbene by photolysis of aqueous 2-bromophenol. Chem. Commun. 2001, 1, 73–74. [Google Scholar] [CrossRef]
- Lomnicki, S.; Truong, H.; Dellinger, B. Mechanisms of product formation from the pyrolytic thermal degradation of catechol. Chemosphere 2008, 73, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.X.; Zhang, D.J.; Han, Z.; Zhan, J.H.; Liu, C.B. New insight into the formation mechanism of PCDD/Fs from 2-chlorophenol precursor. Environ. Sci. Technol. 2013, 47, 8489–8498. [Google Scholar] [CrossRef]
- Sinkkonen, S. Sources and environmental fate of PCDTs. Toxicol Environ. Chem. 1998, 66, 105–112. [Google Scholar] [CrossRef]
- Czerwiński, J. Pathways of polychlorinated dibenzothiophenes (PCDTs) in the environment. Arch. Environ. Prot. 2008, 34, 169–181. [Google Scholar]
- Xu, F.; Shi, X.L.; Zhang, Q.Z.; Wang, W.X. Formation of chlorotriophenoxy radicals from complete series reactions of chlorotriophenols with H and OH radicals. Int. J. Mol. Sci. 2015, 16, 18714–18731. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Qu, X.H.; Wang, W.X. Mechanism of OH-initiated atmospheric photooxidation of dichlorvos: a quantum mechanical study. Environ. Sci. Technol. 2007, 41, 6109–6116. [Google Scholar] [CrossRef]
- Baldridge, K.K.; Gordon, M.S.; Steckler, R.; Truhlar, D.G. Ab initio reaction paths and direct dynamics calculations. J. Phys. Chem. 1989, 93, 5107–5119. [Google Scholar] [CrossRef]
- Gonzalez-Lafont, A.; Truong, T.N.; Truhlar, D.G. Interpolated variational transition-state theory: Practical methods for estimating variational transition-state properties and tunneling contributions to chemical reaction rates from electronic structure calculations. J. Chem. Phys. 1991, 95, 8875–8894. [Google Scholar] [CrossRef]
- Garrett, B.C.; Truhlar, D.G. Generalized transition state theory. Classical mechanical theory and applications to collinear reactions of hydrogen molecules. J. Phys. Chem. 1979, 83, 1052–1079. [Google Scholar] [CrossRef]
- Fernandez-Ramos, A.; Ellingson, B.A.; Garret, B.C.; Truhlar, D.G. Variational transition state theory with multidimensional tunneling. In Reviews in Computational Chemistry; Lipkowitz, K.B., Cundari, T.R., Eds.; Wiley-VCH: Hoboken, NJ, USA, 2007. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: The MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J. Phys. Chem. A 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions-the IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Corchado, J.C.; Chuang, Y.Y.; Fast, P.L.; Villa, J.; Hu, W.P.; Liu, Y.P.; Lynch, G.C.; Nguyen, K.A.; Jackels, C.F.; Melissas, V.S.; et al. POLYRATE Version 9.7; University of Minnesota: Minneapolis, MN, USA, 2007. [Google Scholar]
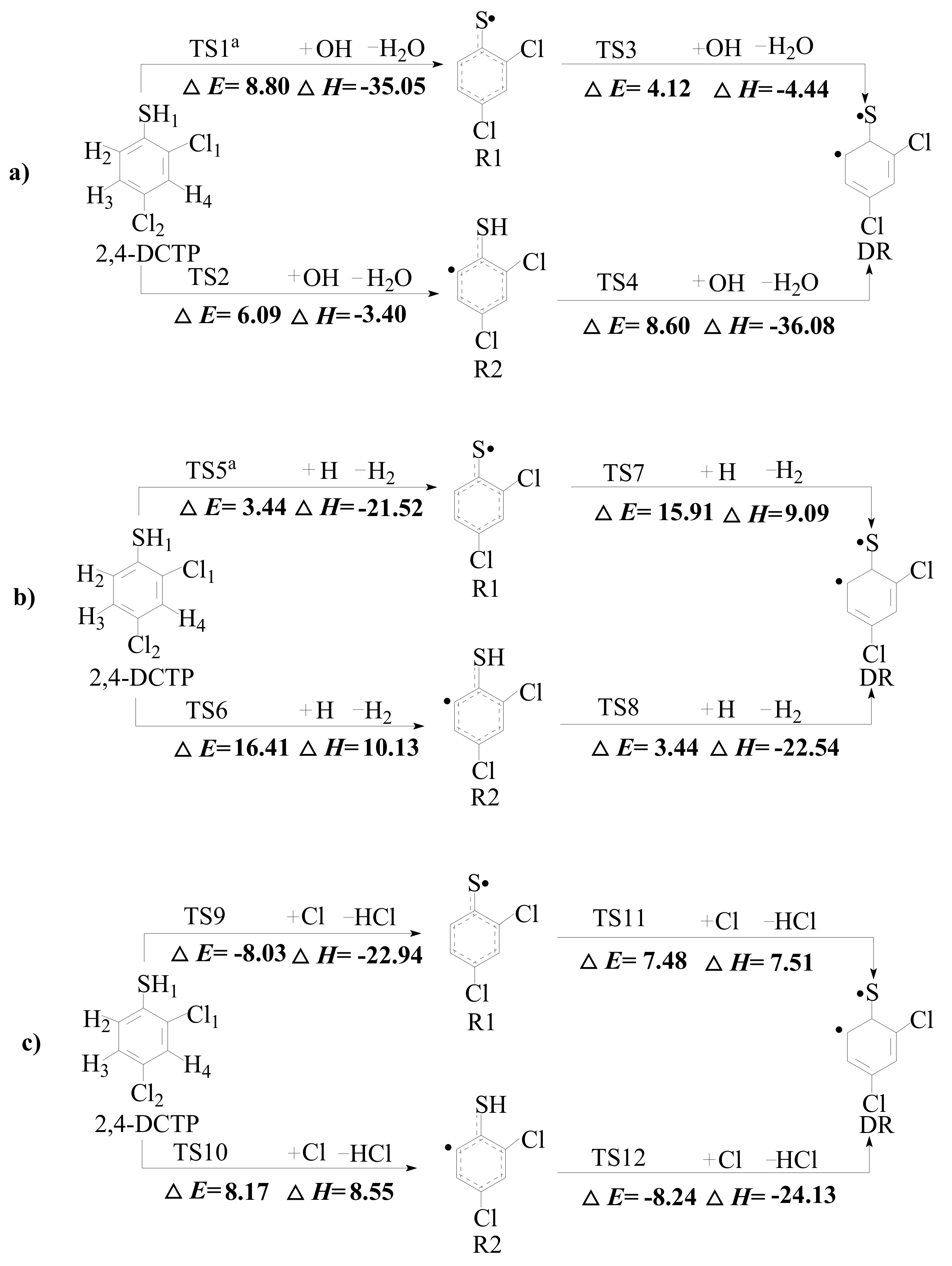
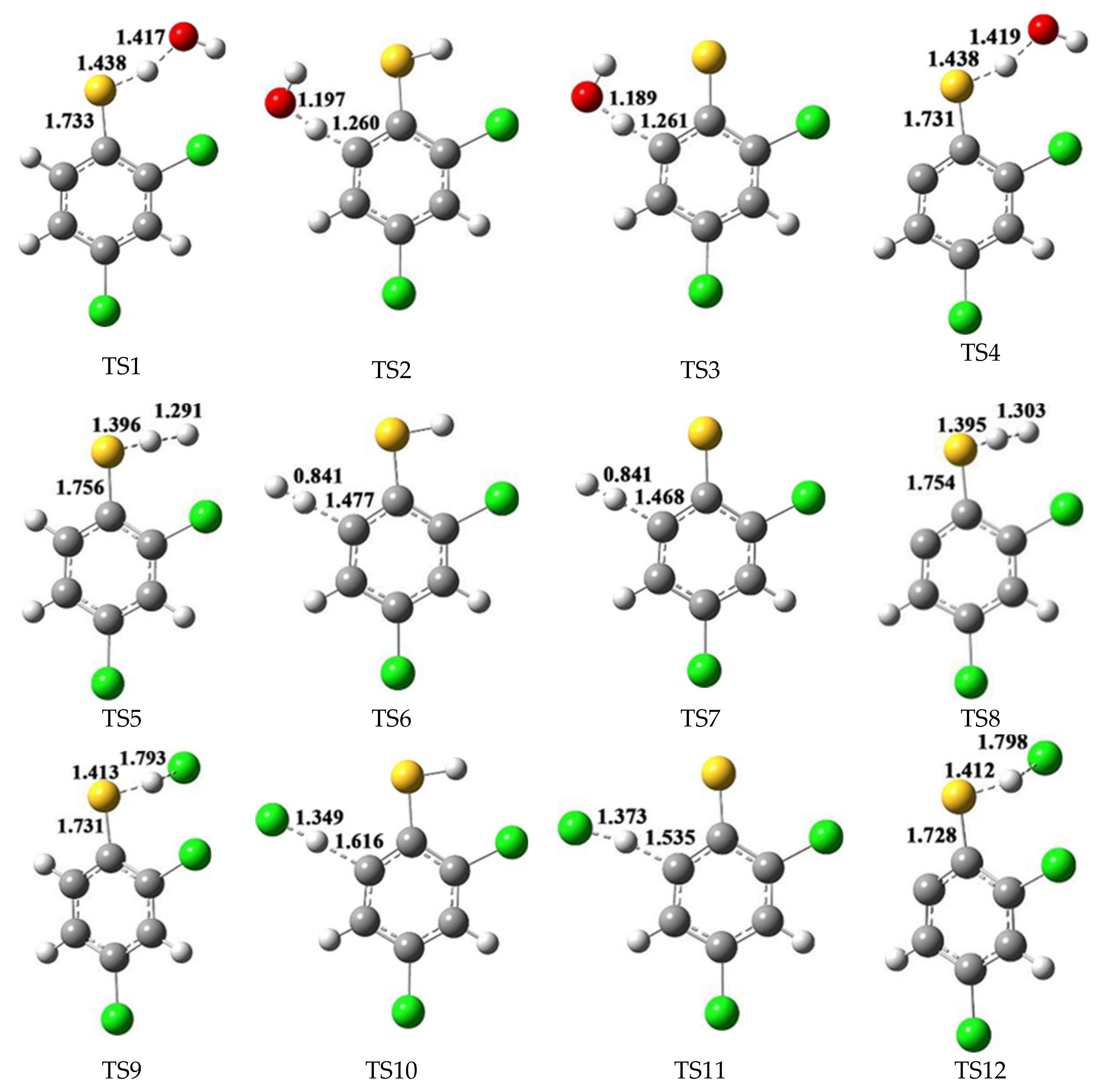
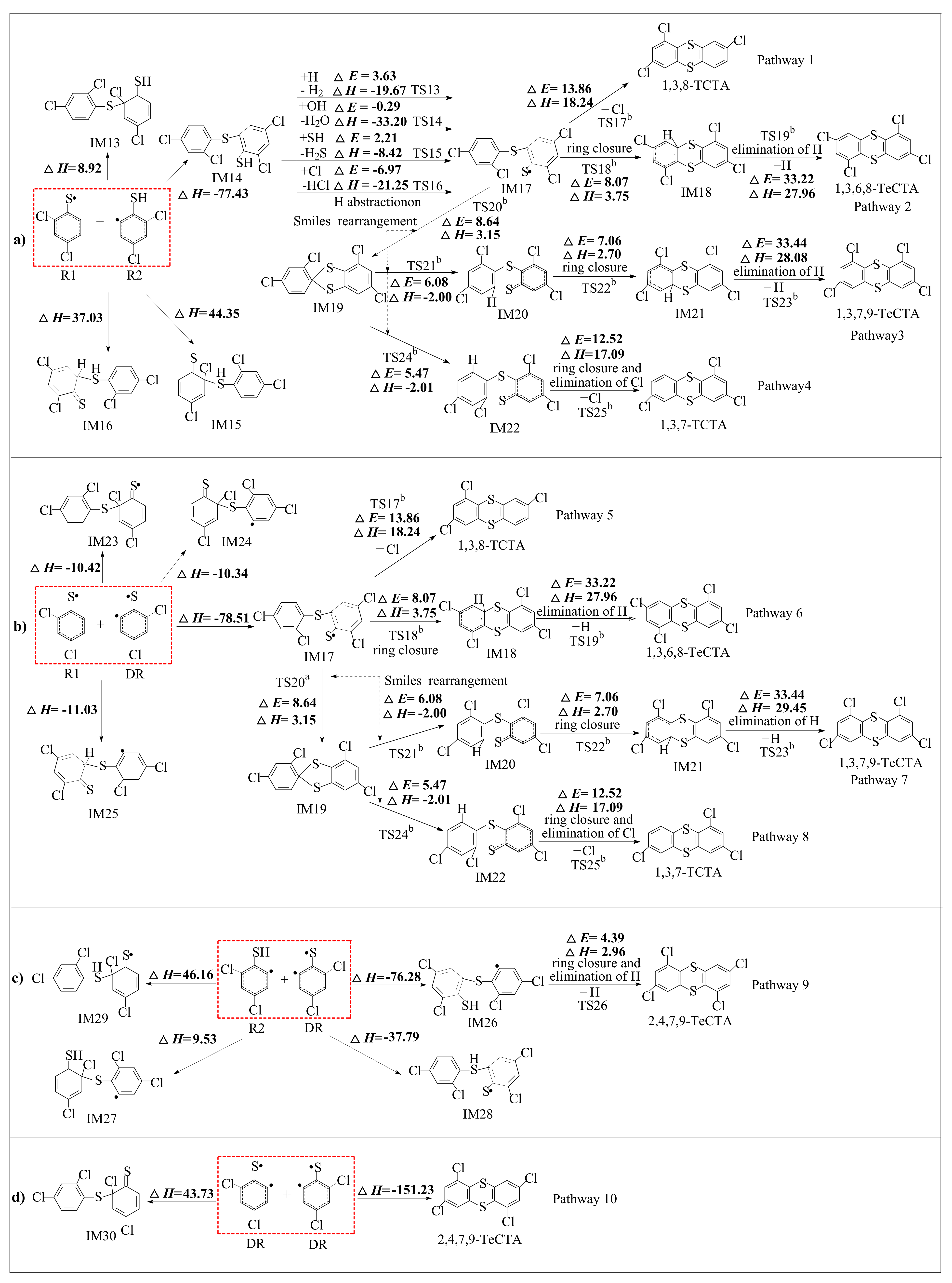
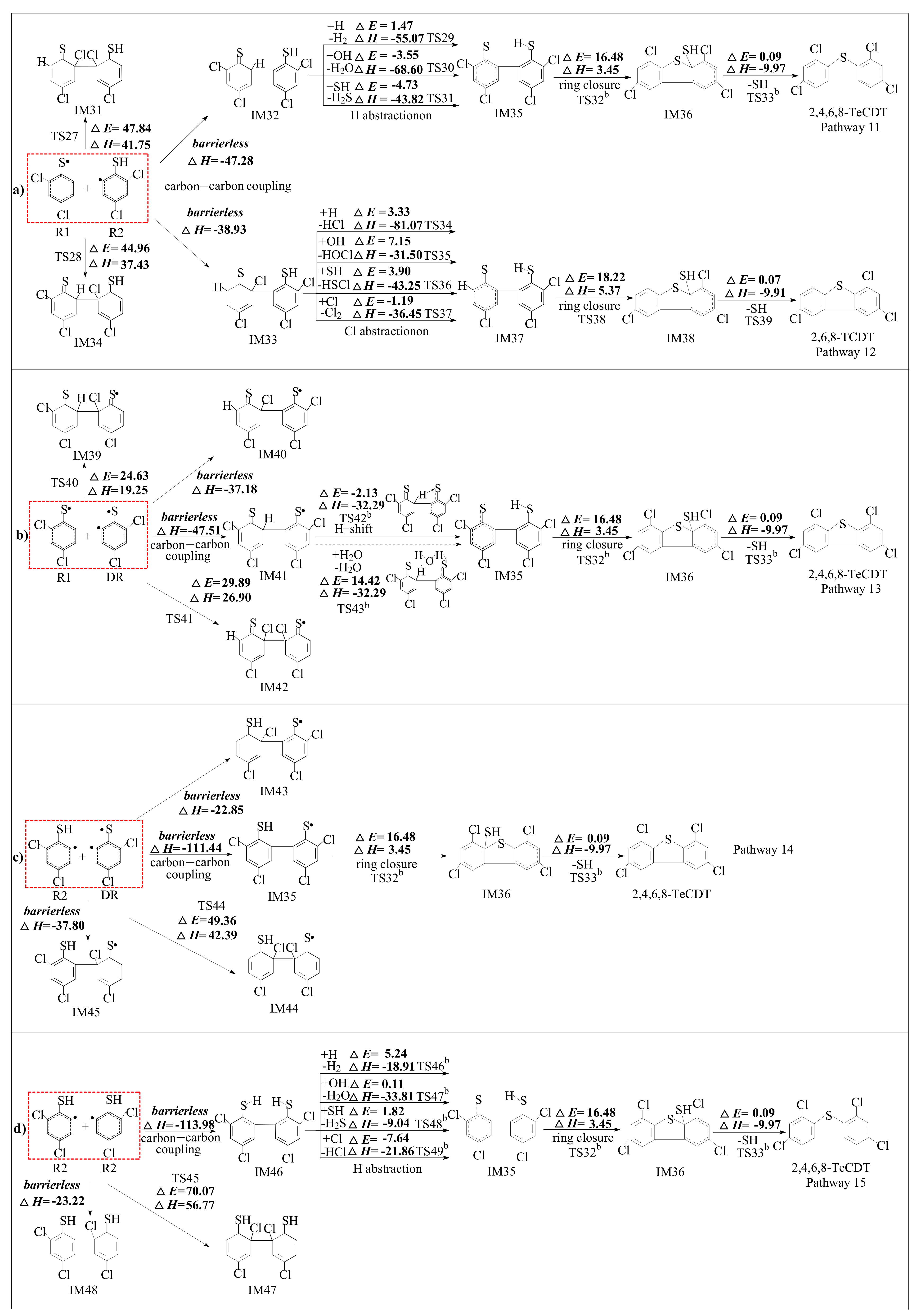
| Reactions Arrhenius Formulas | Arrhenius Formulas |
|---|---|
| 2,4-DCTP + OH → R1 + H2O via TS1 a | k(T) = (6.69 × 10−14) exp (−5861/T) |
| 2,4-DCTP + OH → R2 + H2O via TS2 | k(T) = (1.07 × 10−15) exp (−12857/T) |
| R1 + OH → DR + H2O via TS3 | k(T) = (7.18 × 10−12) exp (−3795/T) |
| R2 + OH → DR + H2O via TS4 | k(T) = (3.19 × 10−13) exp (−3630/T) |
| 2,4-DCTP + H → R1 + H2 via TS5 a | k(T) = (2.84 × 10−11) exp (−2229/T) |
| 2,4-DCTP + H → R2 + H2 via TS6 | k(T) = (9.24 × 10−11) exp (−8977/T) |
| R1 + H → DR + H2 via TS7 | k(T) = (6.55 × 10−11) exp (−8600/T) |
| R2 + H → DR + H2 via TS8 | k(T) = (3.99 × 10−12) exp (−1331/T) |
| 2,4-DCTP + Cl → R2 + HCl via TS10 | k(T) = (1.52 × 10−10) exp (−5852/T) |
| R1 + Cl → DR + HCl via TS11 | k(T) = (1.47 × 10−10) exp (−5978/T) |
| IM2 + H → IM5 + H2 via TS13 | k(T) = (3.53 × 10−11) exp (−2623/T) |
| IM2 + SH → IM5 + H2S via TS15 | k(T) = (8.24 × 10−13) exp (−3080/T) |
| IM5 → 1,3,8-TCTA + Cl via TS17 b | k(T) = (4.52 × 1011) exp (−7140/T) |
| IM5 → IM6 via TS18 b | k(T) = (6.15 × 1011) exp (−4332/T) |
| IM6 → 1,3,6,8-TeCTA + H via TS19 b | k(T) = (2.84 × 1013) exp (−17667/T) |
| IM14 → 2,4,7,9-TeCTA + H via TS26 | k(T) = (4.52 × 1011) exp (−2395/T) |
| IM20 + H → IM23 + H2 via TS29 | k(T) = (1.00 × 10−11) exp (−1531/T) |
| IM23 → IM24 via TS32 b | k(T) = (3.07 × 1011) exp (−8241/T) |
| IM24 → 2,4,6,8-TeCDT + SH via TS33 b | k(T) = (5.86 × 1012) exp (−351/T) |
| IM21 + H → IM25 + HCl via TS34 | k(T) = (9.78 × 10−11) exp (−2502/T) |
| IM21 + OH → IM25 + HOCl via TS35 | k(T) = (1.13 × 10−11) exp (−12954/T) |
| IM21 + SH → IM25 + HSCl via TS36 | k(T) = (4.82 × 10−12) exp (−3861/T) |
| IM25 → IM26 via TS38 | k(T) = (7.61 × 1011) exp (−9275/T) |
| IM26 → 2,6,8-TCDT + SH via TS39 | k(T) = (8.96 × 1012) exp (−275/T) |
| IM29 + H2O → IM23 + H2O via TS43 b | k(T) = (3.20 × 10−15) exp (−3310/T) |
| IM34 + H → IM23 + H2 via TS46 b | k(T) = (5.71 × 10−9) exp (−1148/T) |
| IM34 + OH → IM23 + H2O via TS47 b | k(T) = (7.66 × 10−12) exp (−1889/T) |
| IM34 + SH → IM23 + H2S via TS48 b | k(T) = (1.10 × 10−12) exp (−2808/T) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zuo, C.; Zheng, S.; Sun, Y.; Xu, F.; Zhang, Q. Mechanistic and Kinetic Study on Self-/Cross- Condensation of PCTA/DT Formation Mechanisms from Three Types of Radicals of 2,4-Dichlorothiophenol. Int. J. Mol. Sci. 2019, 20, 2623. https://doi.org/10.3390/ijms20112623
Wang H, Zuo C, Zheng S, Sun Y, Xu F, Zhang Q. Mechanistic and Kinetic Study on Self-/Cross- Condensation of PCTA/DT Formation Mechanisms from Three Types of Radicals of 2,4-Dichlorothiophenol. International Journal of Molecular Sciences. 2019; 20(11):2623. https://doi.org/10.3390/ijms20112623
Chicago/Turabian StyleWang, Hetong, Chenpeng Zuo, Siyuan Zheng, Yanhui Sun, Fei Xu, and Qingzhu Zhang. 2019. "Mechanistic and Kinetic Study on Self-/Cross- Condensation of PCTA/DT Formation Mechanisms from Three Types of Radicals of 2,4-Dichlorothiophenol" International Journal of Molecular Sciences 20, no. 11: 2623. https://doi.org/10.3390/ijms20112623
APA StyleWang, H., Zuo, C., Zheng, S., Sun, Y., Xu, F., & Zhang, Q. (2019). Mechanistic and Kinetic Study on Self-/Cross- Condensation of PCTA/DT Formation Mechanisms from Three Types of Radicals of 2,4-Dichlorothiophenol. International Journal of Molecular Sciences, 20(11), 2623. https://doi.org/10.3390/ijms20112623





