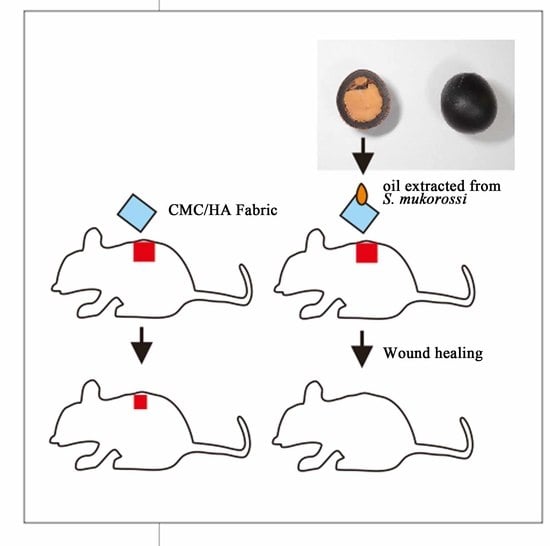
Effects of Sapindus mukorossi Seed Oil on Skin Wound Healing: In Vivo and in Vitro Testing
Abstract
1. Introduction
2. Results
2.1. GC-MS Analysis
2.2. HPLC Analysis
2.3. Antimicrobial Activity Testing
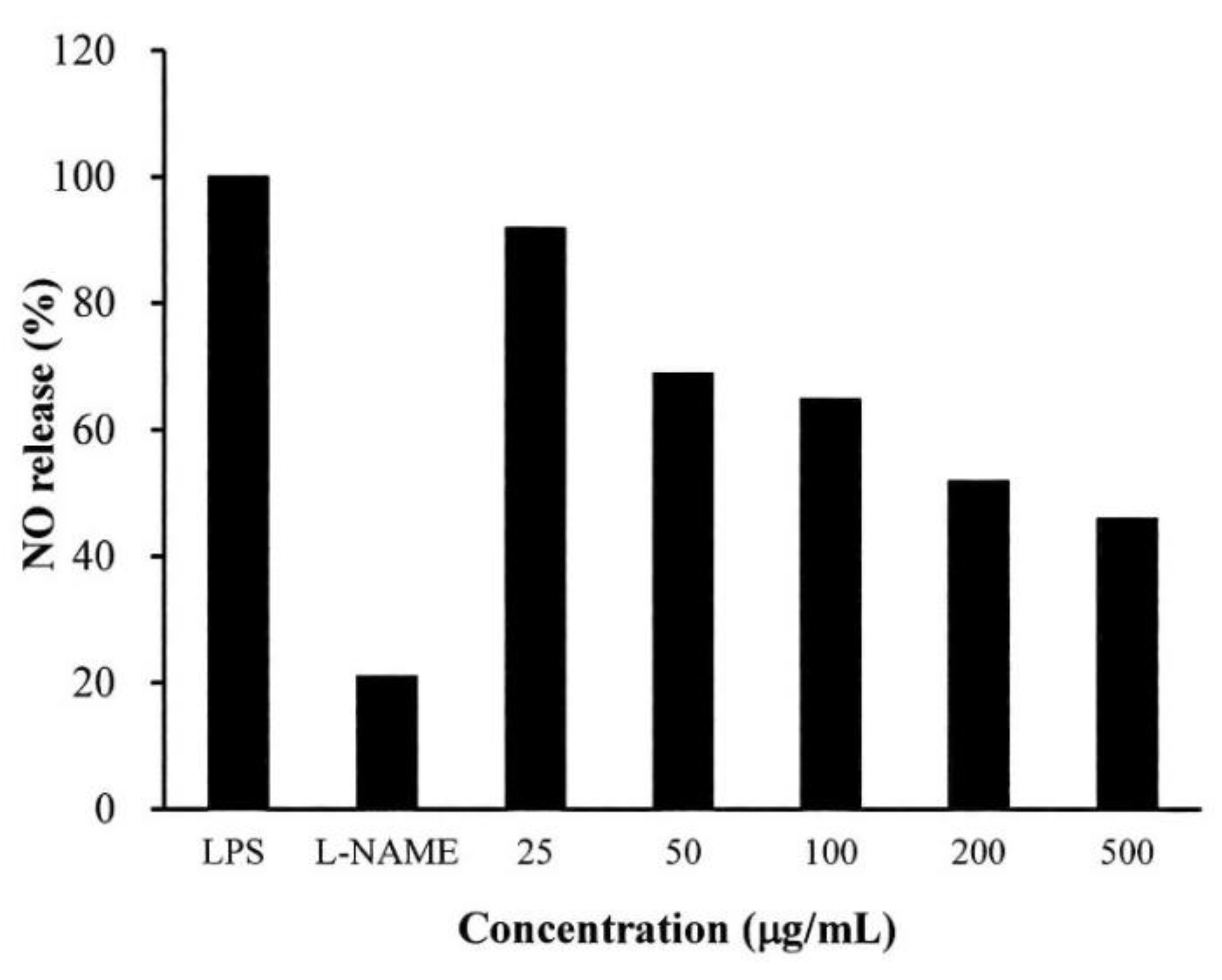
2.4. Anti-Inflammatory Testing
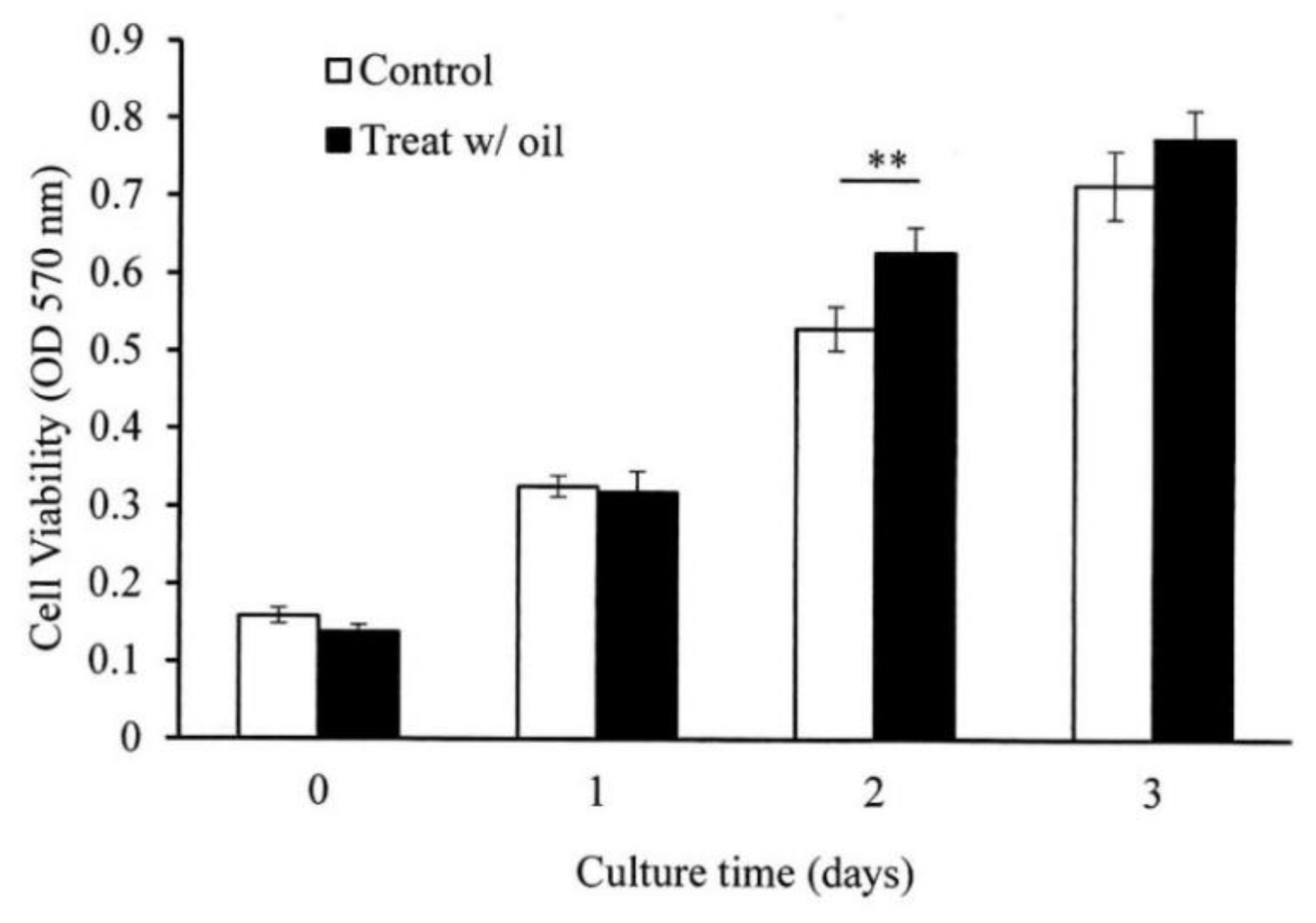
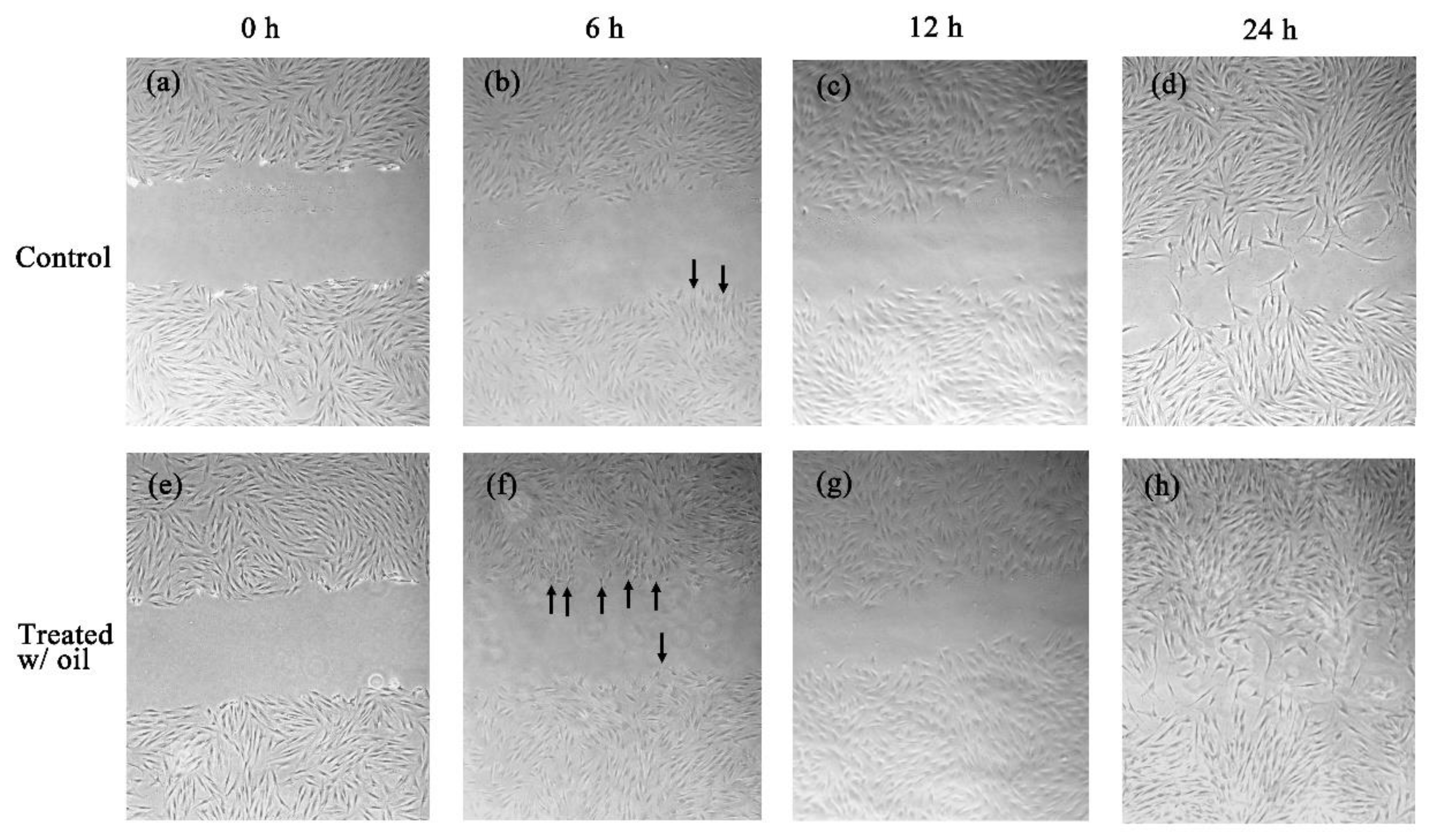
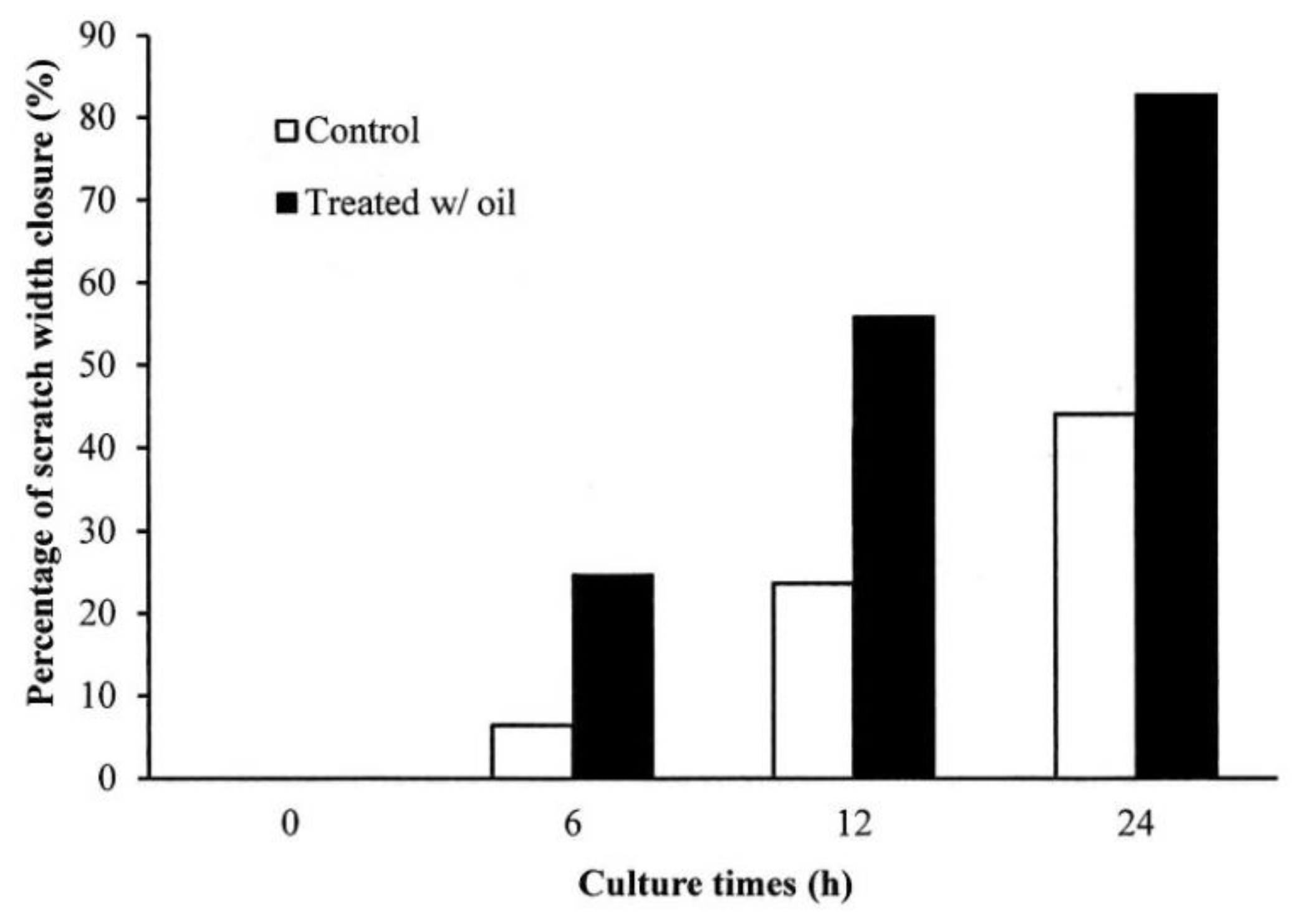
2.5. Cell Proliferation Assay
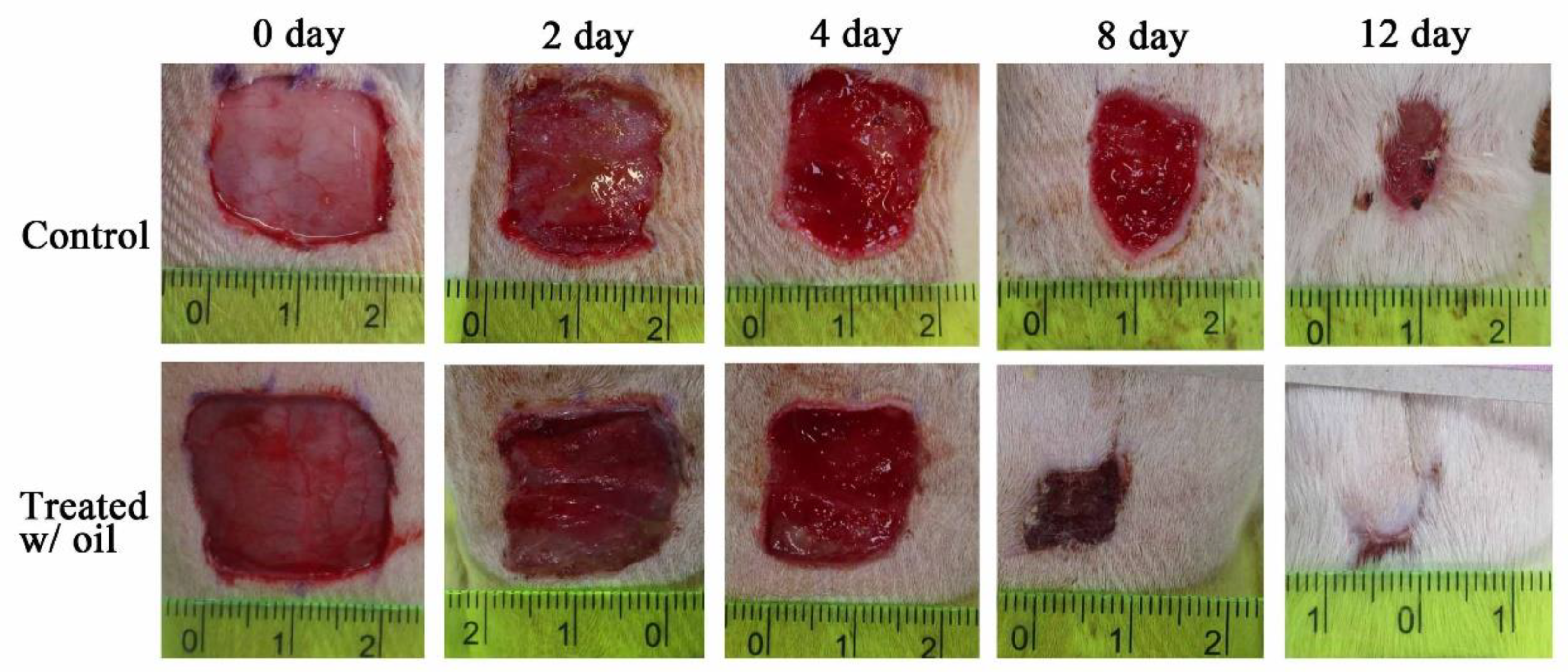
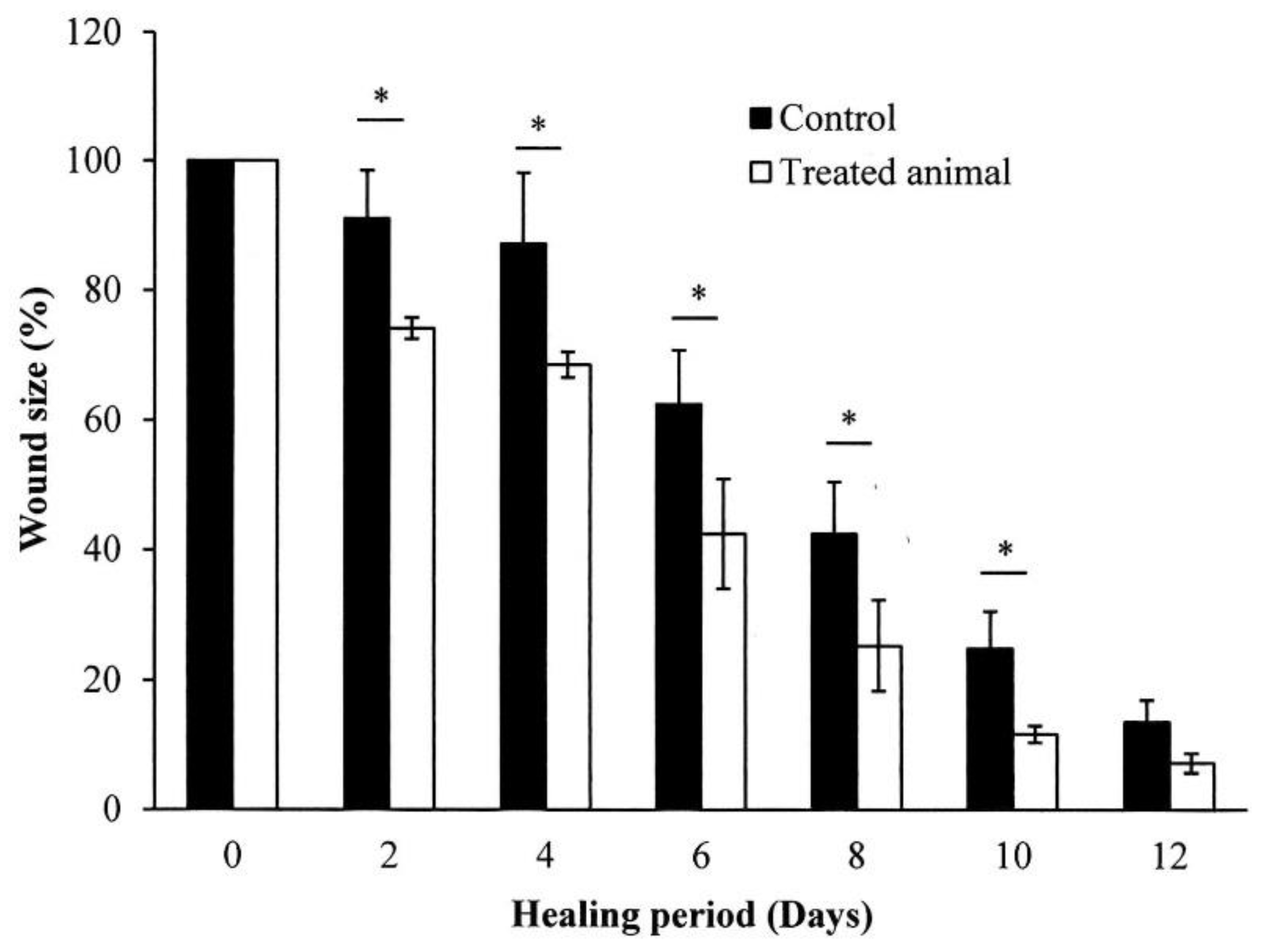
2.6. In Vivo Wound Healing Experiment
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Phytochemical Analysis of Kernel Oil
4.3.1. GC-MS Analysis
4.3.2. HPLC Analysis
4.4. Antimicrobial Assay
4.5. Anti-Inflammatory Test
4.6. In Vitro Skin Cell Analysis
4.6.1. Cell Proliferation Assay
4.6.2. Scratch Wound Healing Test
4.7. Wound Healing Activity Test
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| GC-MS | gas chromatography-mass spectrometry |
| HPLC | high-pressure liquid chromatography |
| CMC | carboxymethyl cellulose |
| HA | hyaluronic acid |
| SA | sodium alginate |
| NO | nitric oxide |
| LPS | lipopolysaccharide |
| l-NAME | N(ω)-nitro-l-arginine methylester hydrochloride |
| NOS | nitric oxide synthase |
| FAME | fatty acid methyl esters |
| MTT | tetrazolium salt |
| DMEM | Dulbecco’s modified Eagle medium |
| FBS NIST | fetal bovine serum National Institute of Standards and Technology |
| EPA | Environmental Protection Agency |
| NIH | National Institutes of Health |
References
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.H.; Islam, M.R. Non-edible plant oils as new sources for biodiesel production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef]
- Sonawane, S.M.; Sonawane, H. A review of recent and current research studies on the biological and pharmalogical activities of Sapindus mukorossi. Int.J. Interdiscip. Res. Innov. 2015, 3, 85–95. [Google Scholar]
- Anjali, R.S.; Divya, J. Sapindus mukorossi: A review article. Pharm. Innov. 2018, 7, 470–472. [Google Scholar]
- Kuo, Y.H.; Huang, H.C.; Kuo, L.M.Y.; Hsu, Y.W.; Lee, K.H.; Chang, F.R.; Wu, Y.C. New dammarane-type saponins from the galls of Sapindus mukorossi. J. Agric. Food Chem. 2005, 53, 4722–4727. [Google Scholar] [CrossRef]
- Yin, S.W.; Chen, J.H.; Sun, S.D.; Tang, C.H.; Yang, X.Q.; Wen, Q.B.; Qi, J.R. Physicochemical and structural characterisation of protein isolate, globulin and albumin from soapnut seeds (Sapindus mukorossi Gaertn.). Food Chem. 2011, 128, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sati, S.C.; Sati, O.P.; Sati, M.D.; Kothiyal, S.K. Triterpenoid Saponins from the Pericarps of Sapindus mukorossi. J. Chem. 2013. Article ID 613190. [Google Scholar]
- Upadhyay, A.; Singh, D.K. Pharmacological effects of Sapindus mukorossi. Rev. Inst. Med. Trop. 2012, 54, 273–280. [Google Scholar] [CrossRef]
- Suhagia, B.N.; Rathod, I.S.; Sindhu, S. Sapindus mukorossi (Areetha): An Overview. Int. J. Pharm. Sci. 2011, 2, 1905–1913. [Google Scholar]
- Shah, M.A.H.; Dutta, K.; Deka, D.C. Fatty acid composition of Sapindus mukorossi seed oil. Adv. Appl. Sci. Res. 2014, 5, 43–50. [Google Scholar]
- Kumar, P.; Vijeth, P.F.; Raju, K. A Study on performance and emission characteristics of cotton seed methyl ester, Sapindous mukorossi seed oil, and diesel blends on CI engine. Energy Power 2015, 5, 10–14. [Google Scholar]
- Mahar, K.S.; Rana, T.S.; Ranade, S.A. Molecular analyses of genetic variability in soap nut (Sapindus mukorossi Gaertn.). Ind. Crop. Prod. 2011, 34, 1111–1118. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chiang, T.H.; Chen, J.H. Properties of soapnut (Sapindus mukorossi) oil biodiesel and its blends with diesel. Biom. Bioen. 2013, 52, 15–21. [Google Scholar] [CrossRef]
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel production from non-edible plant oils. Energ. Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef]
- Rekik, D.M.; Khedir, S.B.; Moalla, K.K.; Kammoun, N.G.; Rebai, T.; Sahnoun, Z. Evaluation of wound healing properties of grape seed, sesame, and fenugreek oils. Evid.-Based Complementary Altern. Med. 2016. Article ID 7965689. [Google Scholar]
- Dakiche, H.; Khali, M.; Boutoumi, H. Phytochemical characterization and in vivo anti-inflammatory and wound-healing activities of Argania spinosa (L.) skeels seed oil. Rec. Nat. Prod. 2017, 11, 171–184. [Google Scholar]
- Lin, T.Z.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef]
- Pai, A.; Rajendra, M.J.; Rao, J.S.; Sudhakar, M. Evaluation of anti microbial, anti oxidant activity and estimation of total flavonoid content in Sapindus trifoliatus seed extracts. Int. J. Pharm. Pharm. Sci. 2014, 6, 550–554. [Google Scholar]
- Rodríguez-Hernández, D.; Demuner, A.J.; Montanari, R.M.; Barbosa, L.C.A. Cyanolipids from Sapindus saponaria L. seeds oil. Bol. Latinoam. Caribe Plantas Med. Aromát. 2016, 15, 364–372. [Google Scholar]
- Jadon, I.S.; Shukla, R.N.; Goshwami, G.C. Biodiesel from Sapindus mukorossi and Jatropha oils by transesterification. Int. J. Pharm. Sci. Invent. 2012, 2, 26–40. [Google Scholar]
- Mikolajczak, K.L. Cyanolipids. Prog. Chm. Fors Orher Lipids. 1977, 15, 97–130. [Google Scholar] [CrossRef]
- Srinivasarao, M.; Lakshminarasu, M.; Anjum, A.; Ibrahim, M. Comparative study on phytochemical, antimicrobial and antioxidant activity of Sapindus mukorossi Gaertn. and Rheum emodi Wall. ex Meissn.: In vitro studies. Ann. Phytomed. 2015, 4, 93–97. [Google Scholar]
- Dweck, A.C. Isoflavones, phytohormones and phytosterols. J. Appl. Cosmetol. 2006, 24, 17–33. [Google Scholar]
- Marques, S.R.; Peixoto, C.A.; Messias, J.B.; de Albuquerque, A.R.; da Silva, V.A., Jr. The effects of topical application of sunflower-seed oil on open wound healing in lambs. Acta Cir. Bras. 2004, 19, 196–209. [Google Scholar] [CrossRef]
- Wang, Y.F.; Que, H.F.; Wang, Y.J.; Cui, X.J. Chinese herbal medicines for treating skin and soft-tissue infections. Cochrane Database Syst. Rev. 2014, 25, CD010619. [Google Scholar] [CrossRef] [PubMed]
- Monsuur, H.N.; Boink, M.A.; Weijers, E.M.; Roffel, S.; Breetveld, M.; Gefen, A.; van den Broek, L.J.; Gibbs, S. Methods to study differences in cell mobility during skin wound healing in vitro. J. Biomech. 2016, 49, 1381–1387. [Google Scholar] [CrossRef]
- De Moura Sperotto, N.D.; Steffens, L.; Veríssimo, R.M.; Henn, J.G.; Péres, V.F.; Vianna, P.; Chies, J.A.B.; Roehe, A.; Saffi, J.; Moura, D.J. Wound healing and anti-inflammatory activities induced by a Plantago australis hydroethanolic extract standardized in verbascoside. J. Ethnopharmacol. 2018, 225, 178–188. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Fatty acids in vegetable oils and their importance in cosmetic industry. Chemik 2014, 68, 103–110. [Google Scholar]
- Israel, M.O. Shea Butter: An opposite replacement for trans fat in margarine. J. Nutr. Food Sci. 2015, S11, 001. [Google Scholar] [CrossRef]
- Alander, J. Shea butter—a multifunctional ingredient for food and cosmetics. Lipid Technol. 2004, 16, 202–205. [Google Scholar]
- Prieto, J.M.; Recio, M.C.; Giner, R.M. Anti-inflammatory activity of β-sitosterol in a model of oxazolone induced contact-delayed-type hypersensitivity. Bol. Latinoam. Caribe Plant. Med. Aromat. 2006, 5, 57–62. [Google Scholar]
- Puglia, C.; Bonina, F. In vivo spectrophotometric evaluation of skin barrier recovery after topical application of soybean phytosterols. J. Cosmet. Sci. 2008, 59, 217–224. [Google Scholar] [PubMed]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of beta-sitosterol–A review. Eur. J. Med. Plants 2014, 4, 590–609. [Google Scholar] [CrossRef]
- Sen, A.; Dhavan, P.; Khukla, K.K.; Singh, S.; Tejovathi, G. Analysis of IR, NMR and antimicrobial activity of β-sitosterol isolated from Momordica charantia. Sci. Secure J. Biotechnol. 2012, 1, 9–13. [Google Scholar]
- Moon, E.J.; Lee, Y.M.; Lee, O.H.; Lee, M.J.; Lee, S.K.; Chung, M.H.; Park, Y.I.; Sung, C.K.; Choi, J.S.; Kim, K.W. A novel angiogenic factor derived from Aloe vera gel: beta-sitosterol, a plant sterol. Angiogenesis 1999, 3, 117–123. [Google Scholar] [CrossRef]
- De Castro Campos Pinto, N.; Cassini-Vieira, P.; Souza-Fagundes, E.M.; Barcelos, L.S.; Castañon, M.C.M.N.; Scio, E. Pereskia aculeata Miller leaves accelerate excisional wound healing in mice. J. Ethnopharmacol. 2016, 194, 131–136. [Google Scholar] [CrossRef]
- Tsai, M.L.; Huang, H.P.; Hsu, J.D.; Lai, Y.R.; Hsiao, Y.P.; Lu, F.J.; Chang, H.R. Topical N-acetylcysteine accelerates wound healing in vitro and in vivo via the PKC/Stat3 Pathway. Int. J. Mol. Sci. 2014, 15, 7563–7578. [Google Scholar] [CrossRef]
- Mwipatayi, B.P.; Angel, D.; Norrish, J.; Hamilton, M.J.; Scott, A.; Sieunarine, K. The use of honey in chronic leg ulcers: a literature review. Primary Intention 2004, 12, 107–112. [Google Scholar]
- O’Sullivan, S.M.; Woods, J.A.; O’Brien, N.M. Use of Tween 40 and Tween 80 to deliver a mixture of phytochemicals to human colonic adenocarcinoma cell (CaCo-2) monolayers. Br. J. Nutr. 2004, 91, 757–764. [Google Scholar] [CrossRef]
- Lew, W.Z.; Feng, S.W.; Lin, C.T.; Huang, H.M. Use of 0.4-Tesla static magnetic field to promote reparative dentin formation of dental pulp stems cells through activation of p38 MAPK signaling pathway. Int. Endod. J. 2019, 52, 28–43. [Google Scholar] [CrossRef]
- Kumar, S.; Lakshmi, P.K.; Sah, C.; Pawar, R.S. Sida cordifolia accelerates wound healing process delayed by dexamethasone in rats: Effect on ROS and probable mechanism of action. J. Ethnopharmacol. 2019, 235, 279–292. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, J.; Peng, C.; Huang, T.; Zhou, H.; Ou, B.; Chen, J.; Liu, Q.; He, S.; Cao, D.; et al. Fabrication and physical properties of gelatin/sodium alginate/hyaluronic acid composite wound dressing hydrogel. J. Macromol. Sci. A 2014, 51, 318–325. [Google Scholar] [CrossRef]
- Huang, Y.C.; Huang, K.U.; Yang, B.Y.; Ko, C.H.; Huang, H.M. Fabrication of novel hydrogel with berberine-enriched carboxymethylcellulose and hyaluronic acid as an anti-inflammatory barrier membrane. BioMed. Res. Int. 2016, 2016, 3640182. [Google Scholar] [CrossRef]

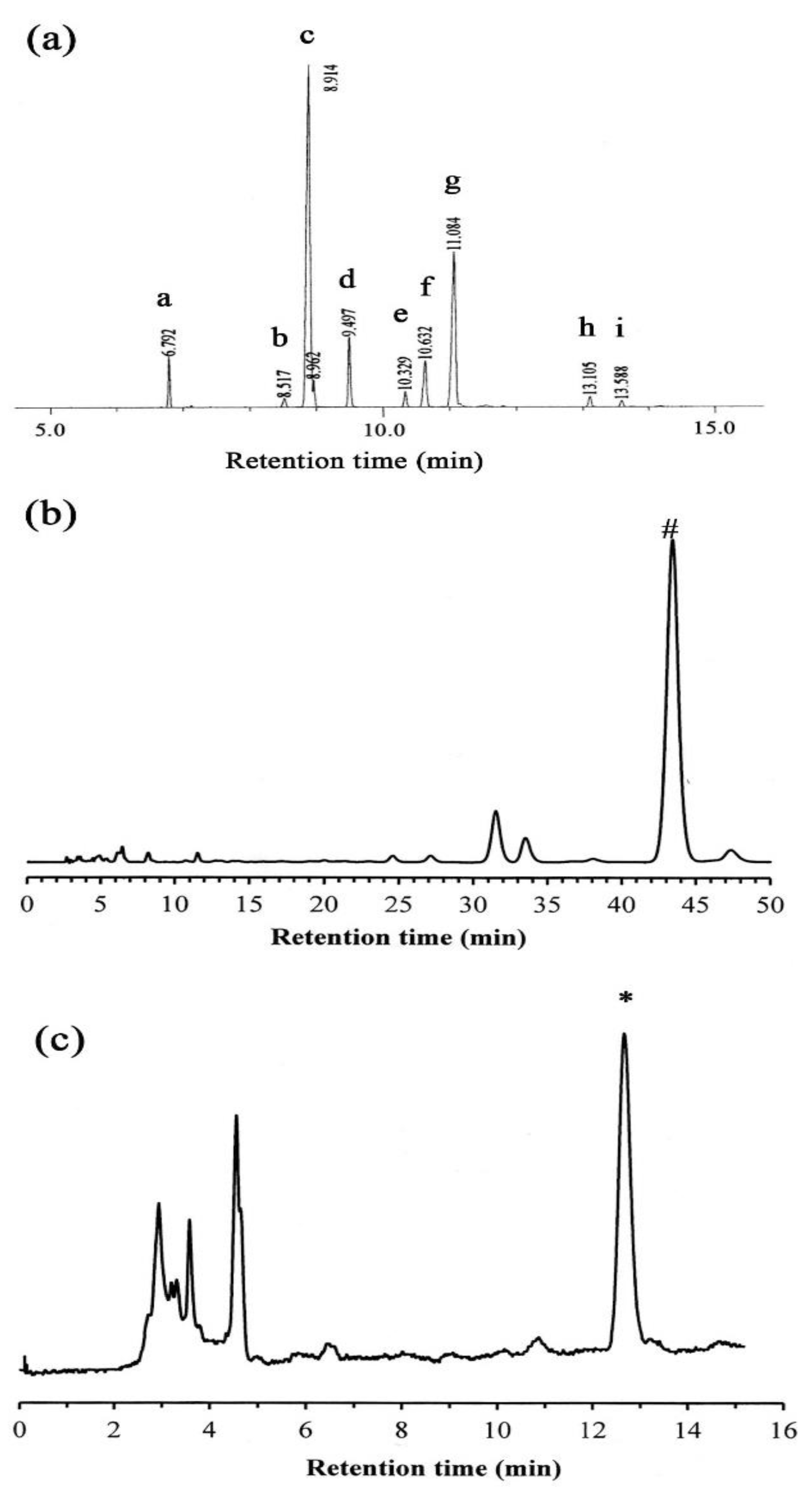
| Peak | Retention Time (min) | Percentage | Fatty Acid |
|---|---|---|---|
| a | 6.792 | 5.35 | Palmitic acid (16:0) |
| b | 8.317 | 0.90 | Stearic acid (18:0) |
| c | 8.914 | 52.46 | Oleic acid (18:1) |
| d | 9.497 | 7.19 | Linoleic acid (18:2) |
| e | 10.329 | 1.61 | Linolenic acid (18:3) |
| f | 10.632 | 6.84 | Arachidic acid (20:0) |
| g | 11.084 | 23.71 | Eicosenic acid (20:1) |
| h | 13.105 | 1.24 | Behenic acid (22:0) |
| i | 13.588 | 0.68 | Erucic acid (22:1) |
| Compound | Calibration Equation a | Retention Time (tr) | Correlation Coefficient (r2) |
|---|---|---|---|
| δ-Tocopherol | Y = 4903.9X ± 27882 | 12.67 | 0.9908 |
| β-Sitosterol | Y = 3480.3X ± 7887.6 | 43.40 | 0.9996 |
| Microorganism | Inactivation Rate a (%) |
|---|---|
| Propionibacterium acnes | >99.99 |
| Staphylococcus aureus | >99.99 |
| Candida albicans | 99.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Nien, C.-J.; Chen, L.-G.; Huang, K.-Y.; Chang, W.-J.; Huang, H.-M. Effects of Sapindus mukorossi Seed Oil on Skin Wound Healing: In Vivo and in Vitro Testing. Int. J. Mol. Sci. 2019, 20, 2579. https://doi.org/10.3390/ijms20102579
Chen C-C, Nien C-J, Chen L-G, Huang K-Y, Chang W-J, Huang H-M. Effects of Sapindus mukorossi Seed Oil on Skin Wound Healing: In Vivo and in Vitro Testing. International Journal of Molecular Sciences. 2019; 20(10):2579. https://doi.org/10.3390/ijms20102579
Chicago/Turabian StyleChen, Chang-Chih, Chia-Jen Nien, Lih-Geeng Chen, Kuen-Yu Huang, Wei-Jen Chang, and Haw-Ming Huang. 2019. "Effects of Sapindus mukorossi Seed Oil on Skin Wound Healing: In Vivo and in Vitro Testing" International Journal of Molecular Sciences 20, no. 10: 2579. https://doi.org/10.3390/ijms20102579
APA StyleChen, C.-C., Nien, C.-J., Chen, L.-G., Huang, K.-Y., Chang, W.-J., & Huang, H.-M. (2019). Effects of Sapindus mukorossi Seed Oil on Skin Wound Healing: In Vivo and in Vitro Testing. International Journal of Molecular Sciences, 20(10), 2579. https://doi.org/10.3390/ijms20102579