Remarkable Alteration of PD-L1 Expression after Immune Checkpoint Therapy in Patients with Non-Small-Cell Lung Cancer: Two Autopsy Case Reports
Abstract
1. Introduction
2. Results
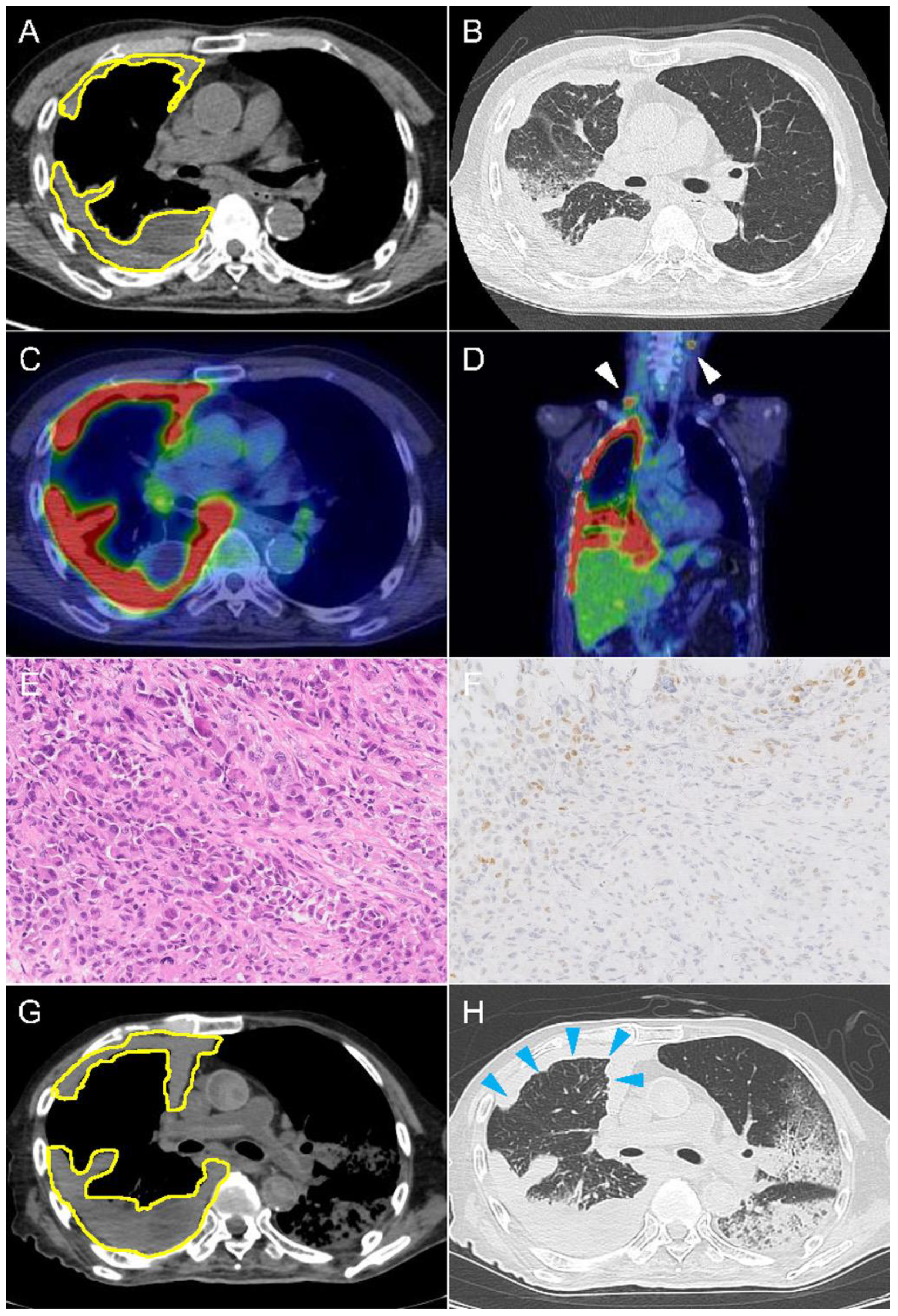
2.1. Case 1
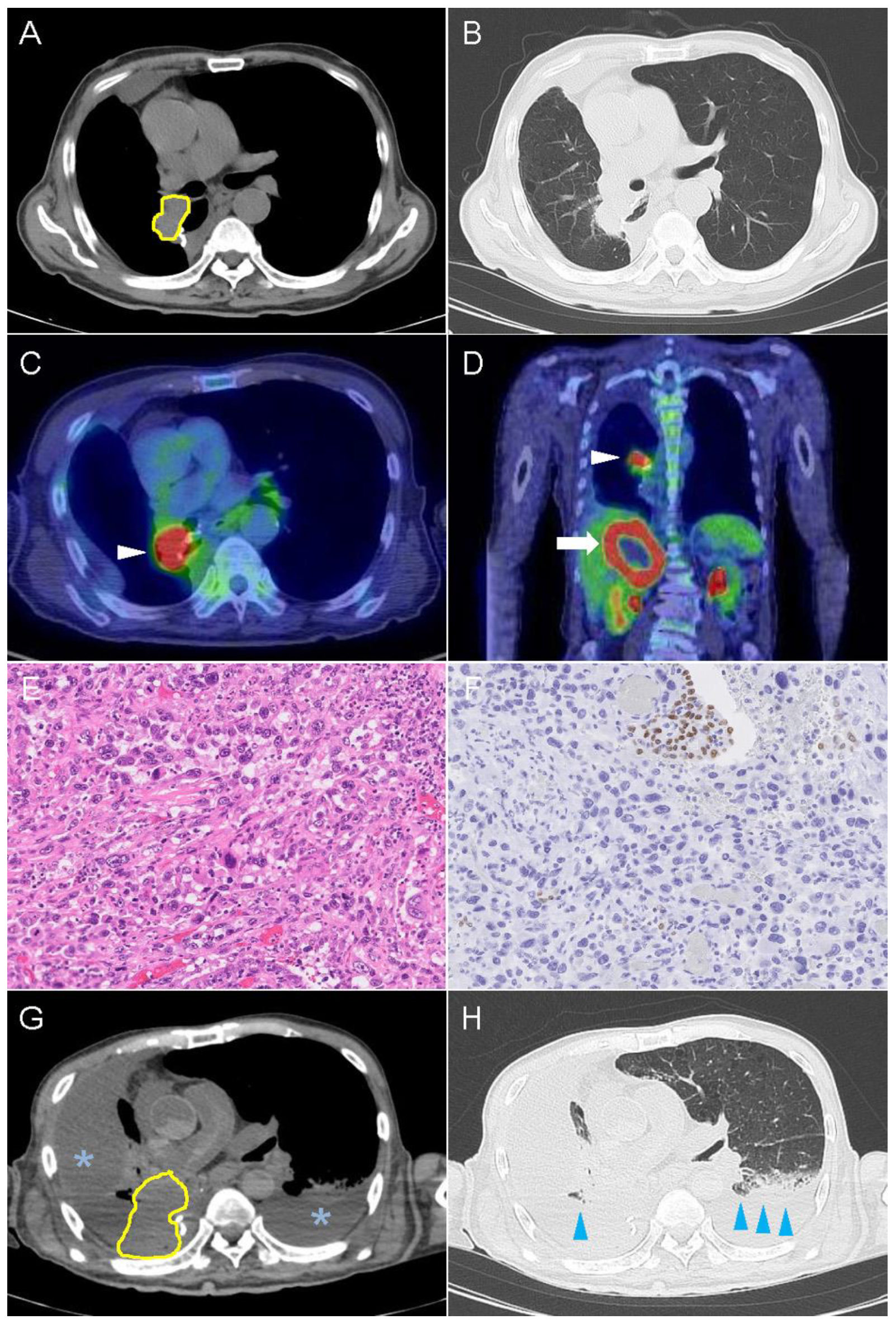
2.2. Case 2
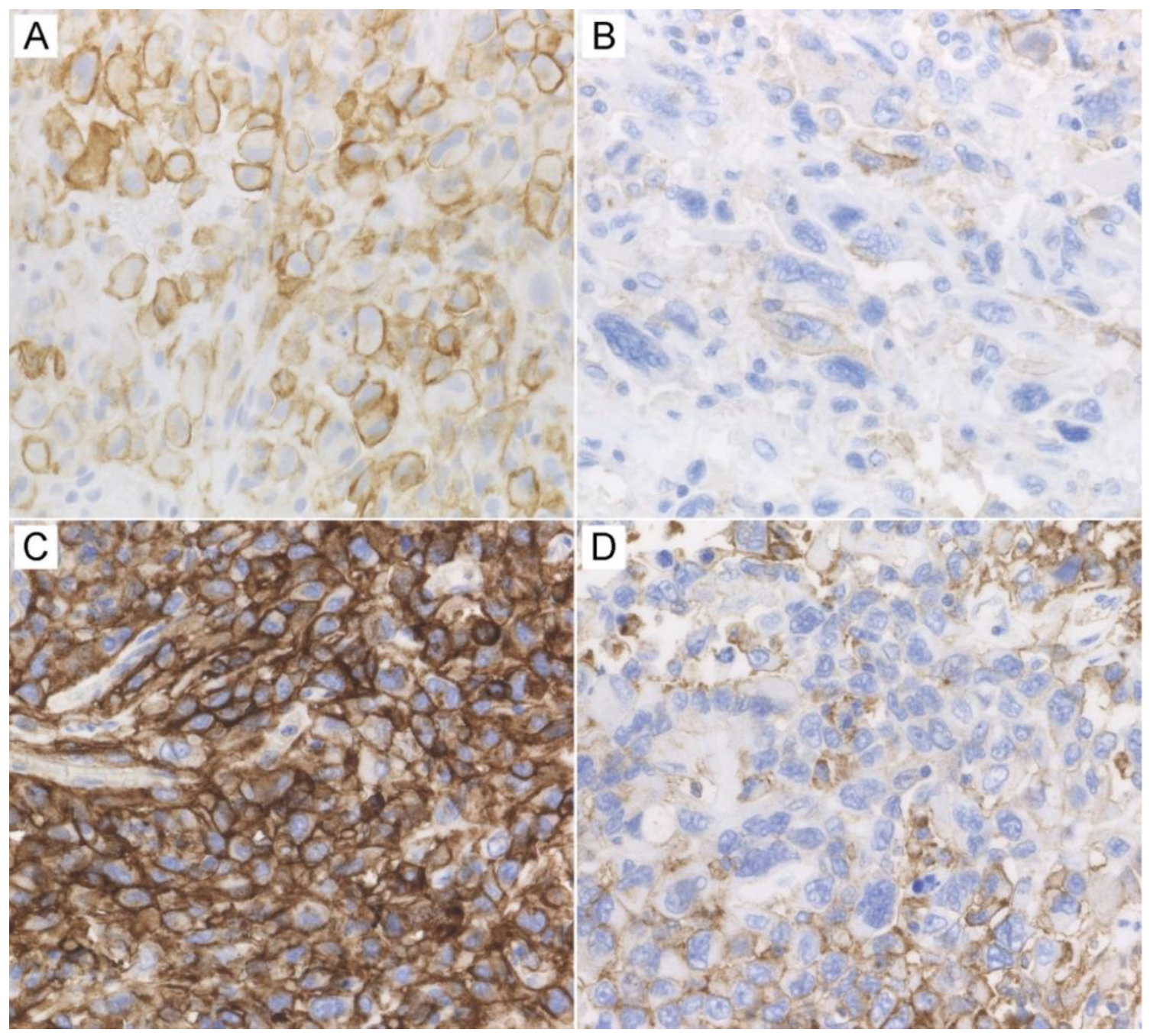
2.3. Immunohistochemical Findings
3. Discussion
4. Materials and Methods
4.1. Immunohistochemistry
4.2. Evaluation of Immunostaining and Digital Image Analysis
5. Conclusions
Statement Confirming Consent
Additional Disclosures
Author Contributions
Funding
Conflicts of Interest
References
- Tokito, T.; Azuma, K.; Kawahara, A.; Ishii, H.; Yamada, K.; Matsuo, N.; Kinoshita, T.; Mizukami, N.; Ono, H.; Kage, M.; et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur. J. Cancer 2016, 55, 7–14. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Vignot, S. NCCN Clinical Practice Guideline: Non-small Cell Lung Cancer. Oncologie 2006, 8, 643–646. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and function of the PD-L1 checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Rojkó, L.; Reiniger, L.; Téglási, V.; Fábián, K.; Pipek, O.; Vágvölgyi, A.; Agócs, L.; Fillinger, J.; Kajdácsi, Z.; Tímár, J.; et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J. Cancer Res. Clin. Oncol. 2018, 144, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, D.; Uehara, K.; Sato, Y.; Sakanoue, I.; Ito, M.; Teraoka, S.; Nagata, K.; Nakagawa, A.; Kosaka, Y.; Otsuka, K.; et al. Alteration of PD-L1 expression and its prognostic impact after concurrent chemoradiation therapy in non-small cell lung cancer patients. Sci. Rep. 2017, 7, 2–11. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Long, G.V.; Scolyer, R.A.; Teng, M.W.; Smyth, M.J. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat. Rev. 2017, 52, 71–81. [Google Scholar] [CrossRef]
- Boes, M. Cancer immunotherapy: Moving beyond checkpoint inhibition. Oncotarget 2018, 9, 1–21. [Google Scholar] [CrossRef]
- Bai, J.; Gao, Z.; Li, X.; Dong, L.; Han, W.; Nie, J. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PDL1 blockade. Oncotarget 2017, 8, 110693–110707. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Song, W.; Li, J.; Zhao, G.; Cao, H. PD-L1 Expression and CD8+ T cell infiltration predict a favorable prognosis in advanced gastric cancer. J. Immunol. Res. 2018, 4180517. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Fang, W.; Yu, J.; Chen, N.; Zhan, J.; Ma, Y.; Yang, Y.; Huang, Y.; Zhao, H.; Zhang, L. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Omori, S.; Kenmotsu, H.; Abe, M.; Watanabe, R.; Sugino, T.; Kobayashi, H.; Nakashima, K.; Wakuda, K.; Ono, A.; Taira, T.; et al. Changes in programmed death ligand 1 expression in non-small cell lung cancer patients who received anticancer treatments. Int. J. Clin. Oncol. 2018, 23, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Takeda, M.; Sakai, K.; Nakamura, Y.; Ito, A.; Hayashi, H.; Tanaka, K.; Nishio, K.; Nakagawa, K. Impact of cytotoxic chemotherapy on PD-L1 expression in patients with non–small cell lung cancer negative for EGFR mutation and ALK fusion. Lung Cancer 2019, 127, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.E.; Rizvi, H.; Kravets, S.; Lydon, C.; Adeni, A.; Subegdjo, S.; Hellmann, M.; Awad, M. Comparison of outcomes with PD-L1 tumor proportion score (TPS) of 50–74% vs. 75–100% in patients with non-small cell lung cancer (NSCLC) treated with first-line PD-1 inhibitors. J. Clin. Oncol. 2018, 36, 9037. [Google Scholar] [CrossRef]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef]
- Liu, J.; Blake, S.J.; Yong, M.C.; Harjunpää, H.; Ngiow, S.F.; Takeda, K.; Young, A.; O’Donnell, J.S.; Allen, S.; Smyth, M.J.; et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016, 6, 1382–1399. [Google Scholar] [CrossRef]
- Bassanelli, M.; Sioletic, S.; Martini, M.; Giacinti, S.; Viterbo, A.; Staddon, A.; Liberati, F.; Ceribelli, A. Heterogeneity of PD-L1 expression and relationship with biology of NSCLC. Anticancer Res. 2018, 38, 3789–3796. [Google Scholar] [CrossRef]
- Junttila, M.R.; de Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Taylor, C.R.; Jadhav, A.P.; Gholap, A.; Kamble, G.; Huang, J.; Gown, A.; Doshi, I.; Rimm, D.L. A Multi-institutional study to evaluate automated whole slide scoring of immunohistochemistry for expression in non-small cell lung cancer. Appl. Immunohistochem. Mol. Morphol. 2019, 27, 263–269. [Google Scholar] [CrossRef] [PubMed]
- PD-L1 IHC 22C3 pharmDx Interpretation Manual—NSCLC; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2018; pp. 1–76.
| Cases | Markers | Before Immunotherapy | After Immunotherapy | |
|---|---|---|---|---|
| Viable Tumor Area | Perinecrotic Area | |||
| Patient 1 | PD-L1 | 75.6% | 13.2% | 31.7% |
| PD-1 | 0.0% | 0.7% | 0.3% | |
| CD8 | 6.3 | 10.1 | 6.6 | |
| Patient 2 | PD-L1 | 100.0% | 58.8% | 79.7% |
| PD-1 | 0.0% | 4.5% | 1.2% | |
| CD8 | 21.5 | 28.8 | 4.4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, T.; Tateishi, A.; Bychkov, A.; Fukuoka, J. Remarkable Alteration of PD-L1 Expression after Immune Checkpoint Therapy in Patients with Non-Small-Cell Lung Cancer: Two Autopsy Case Reports. Int. J. Mol. Sci. 2019, 20, 2578. https://doi.org/10.3390/ijms20102578
Takahashi T, Tateishi A, Bychkov A, Fukuoka J. Remarkable Alteration of PD-L1 Expression after Immune Checkpoint Therapy in Patients with Non-Small-Cell Lung Cancer: Two Autopsy Case Reports. International Journal of Molecular Sciences. 2019; 20(10):2578. https://doi.org/10.3390/ijms20102578
Chicago/Turabian StyleTakahashi, Toshiaki, Akiko Tateishi, Andrey Bychkov, and Junya Fukuoka. 2019. "Remarkable Alteration of PD-L1 Expression after Immune Checkpoint Therapy in Patients with Non-Small-Cell Lung Cancer: Two Autopsy Case Reports" International Journal of Molecular Sciences 20, no. 10: 2578. https://doi.org/10.3390/ijms20102578
APA StyleTakahashi, T., Tateishi, A., Bychkov, A., & Fukuoka, J. (2019). Remarkable Alteration of PD-L1 Expression after Immune Checkpoint Therapy in Patients with Non-Small-Cell Lung Cancer: Two Autopsy Case Reports. International Journal of Molecular Sciences, 20(10), 2578. https://doi.org/10.3390/ijms20102578





