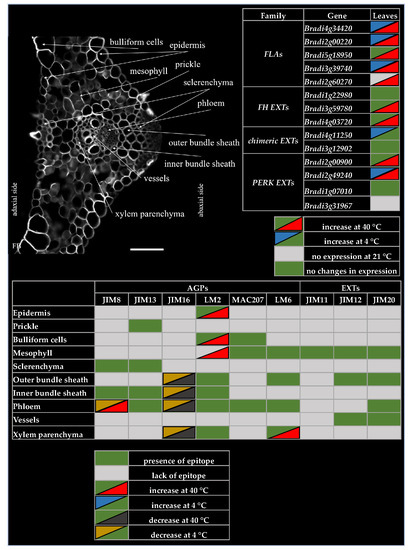
Hydroxyproline-Rich Glycoproteins as Markers of Temperature Stress in the Leaves of Brachypodium distachyon
Abstract
1. Introduction
2. Results
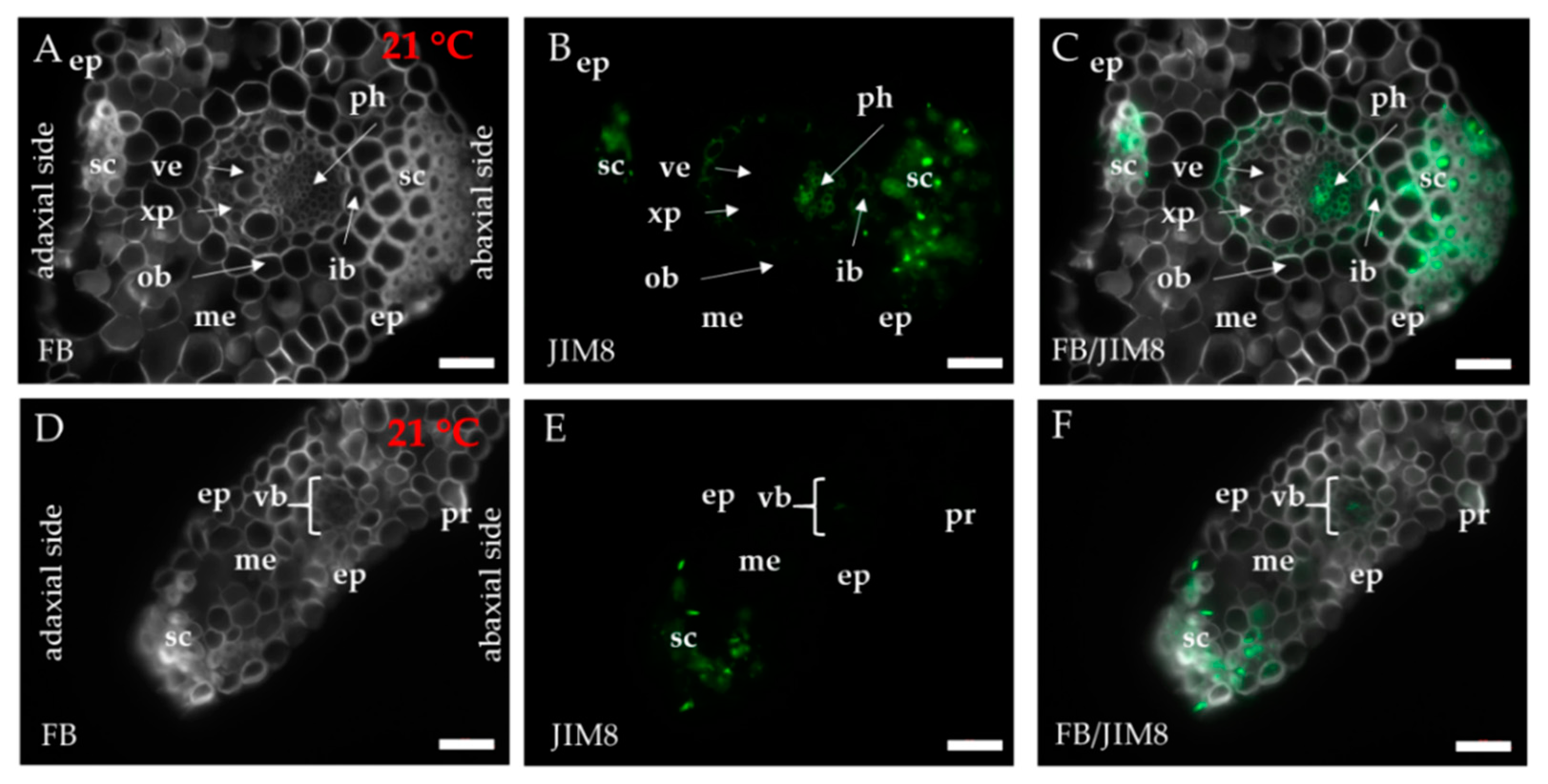
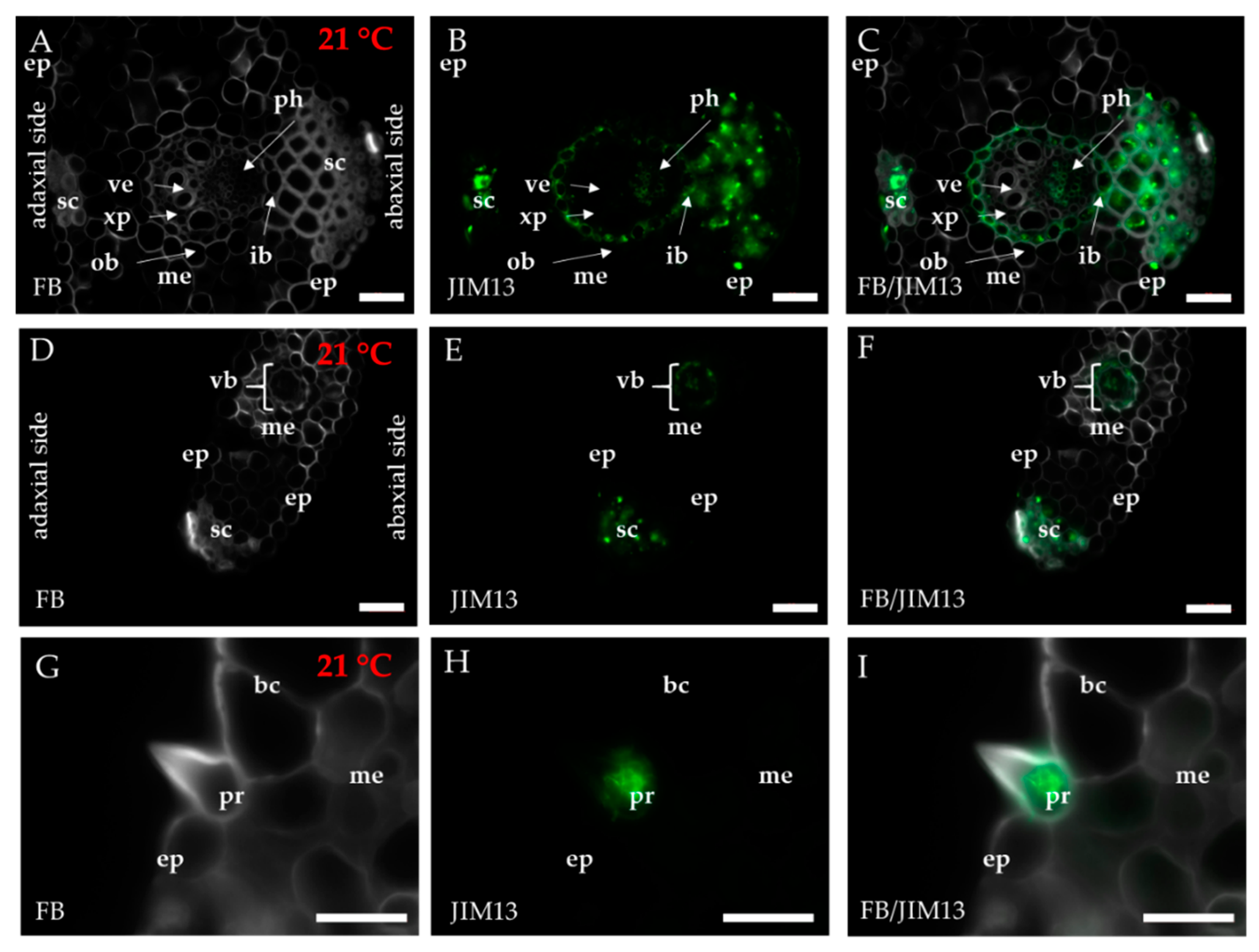
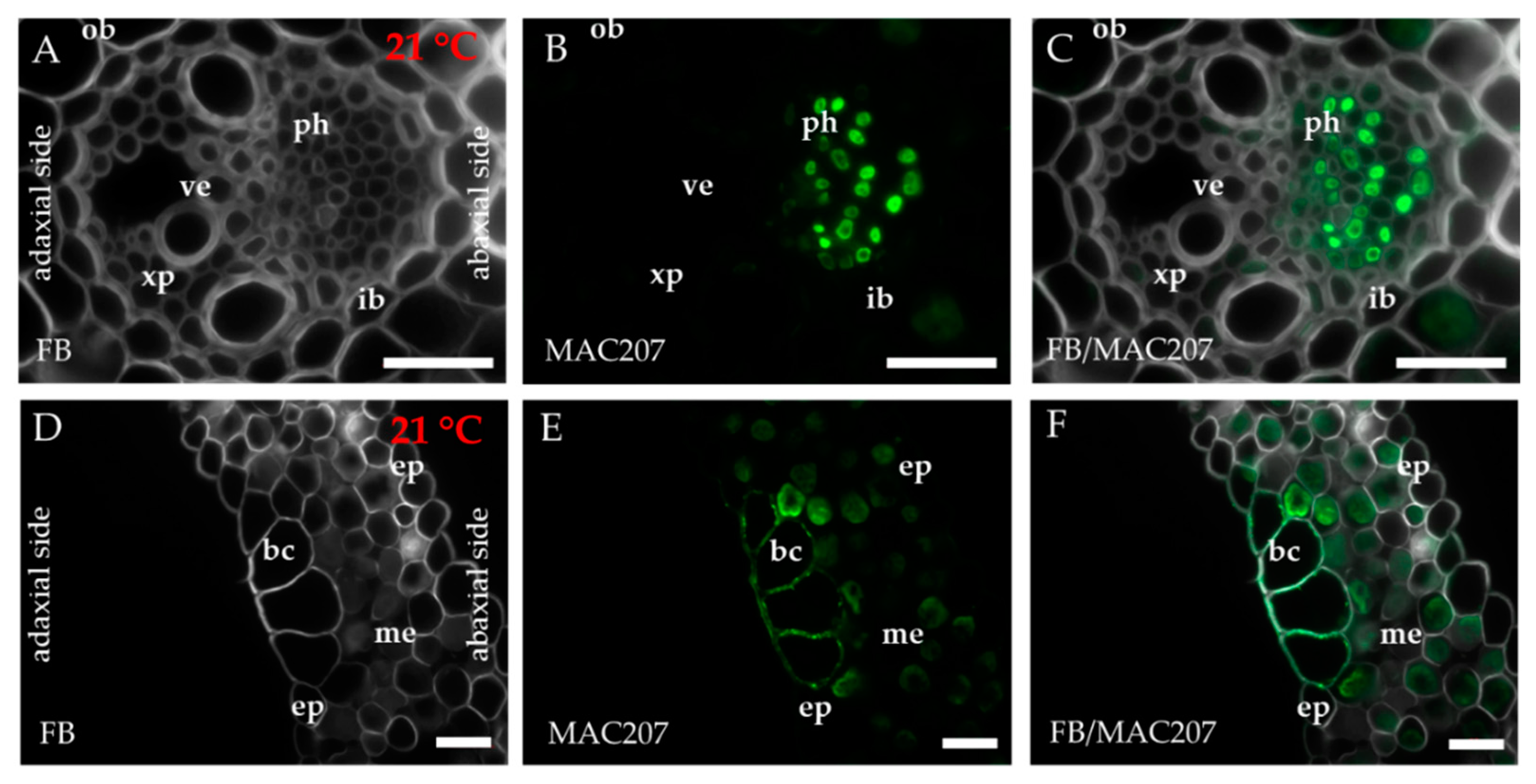
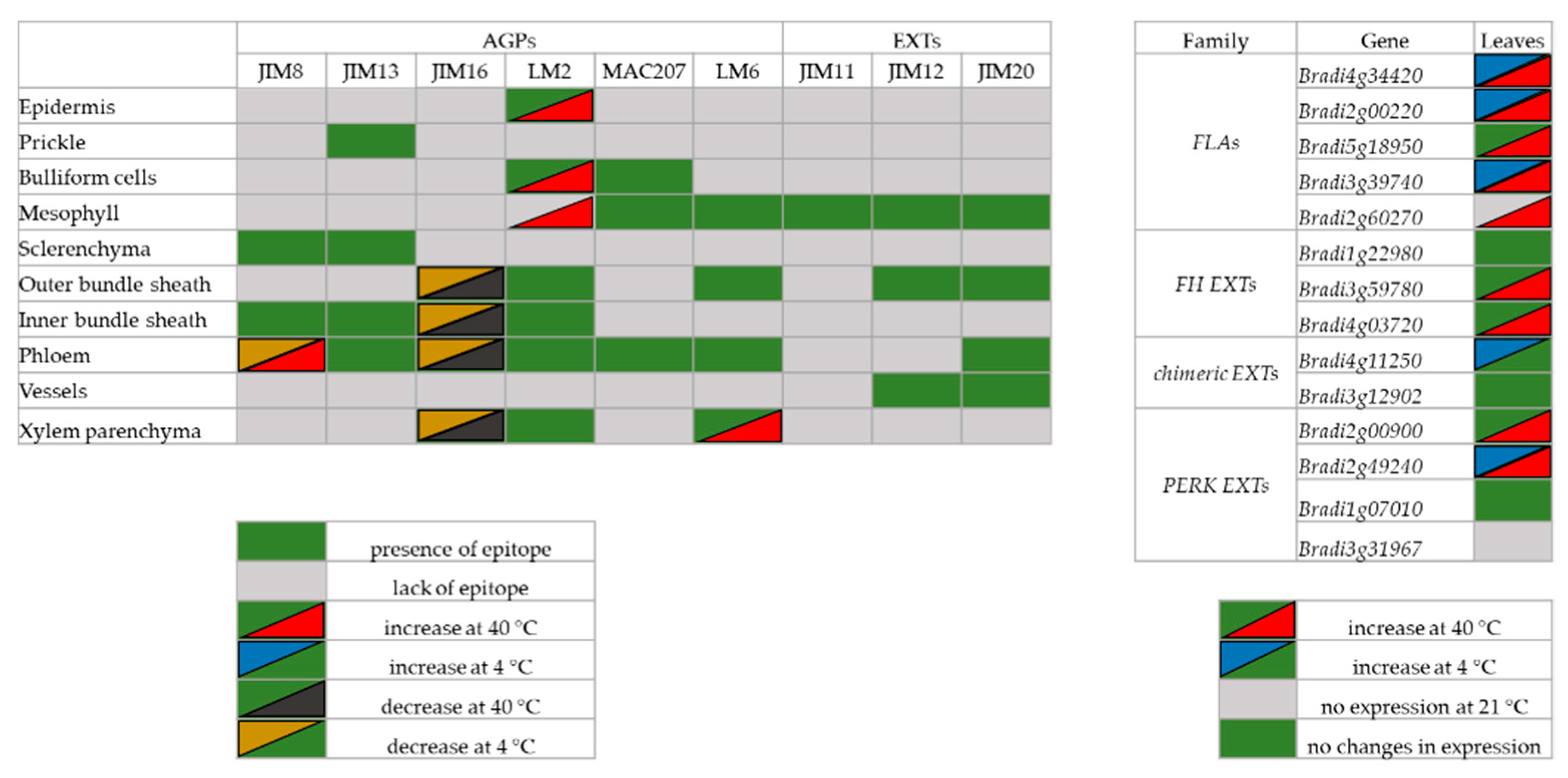
2.1. Distribution of the Epitopes of AGP and EXT in Leaves in Response to Temperature Stress
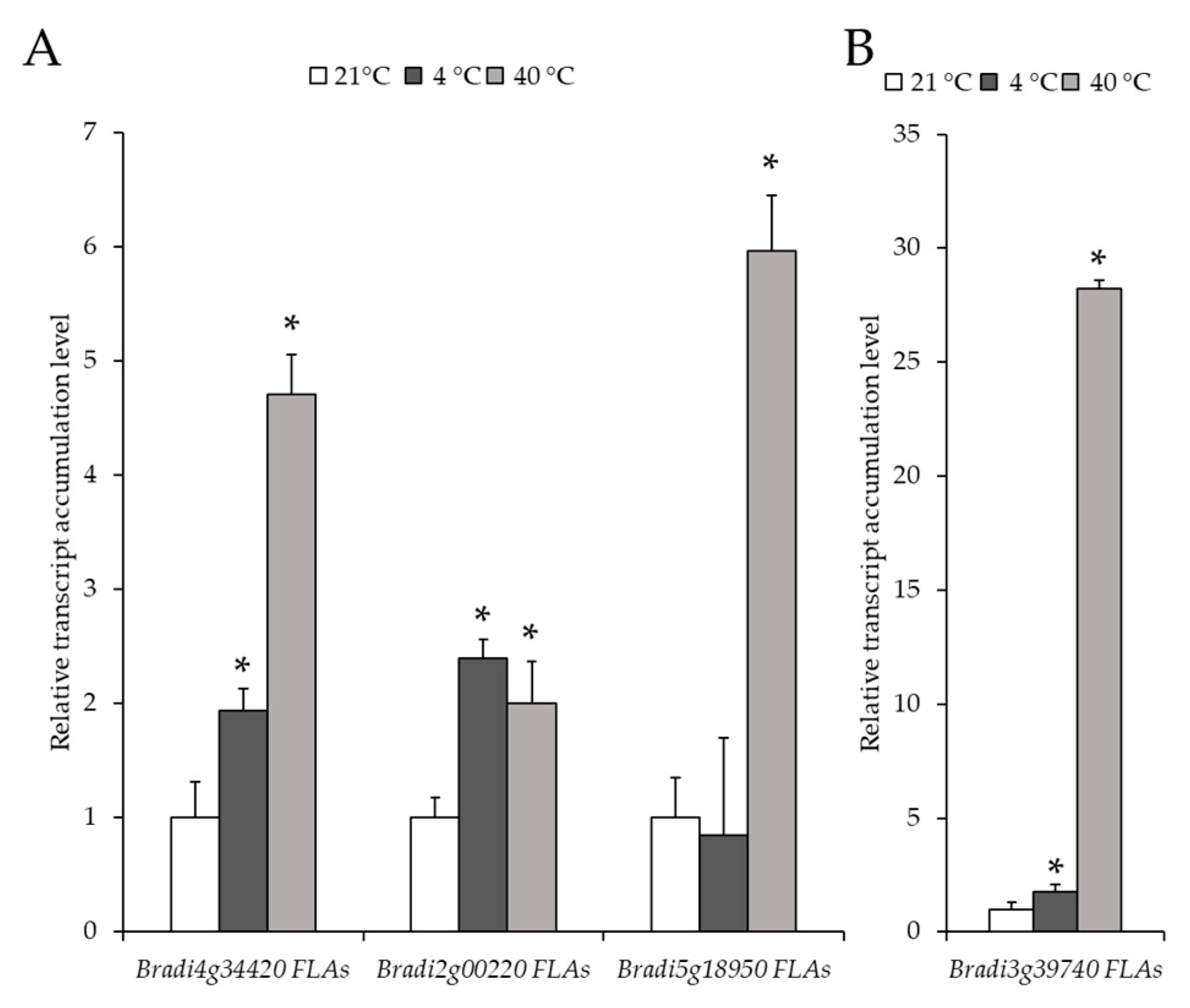
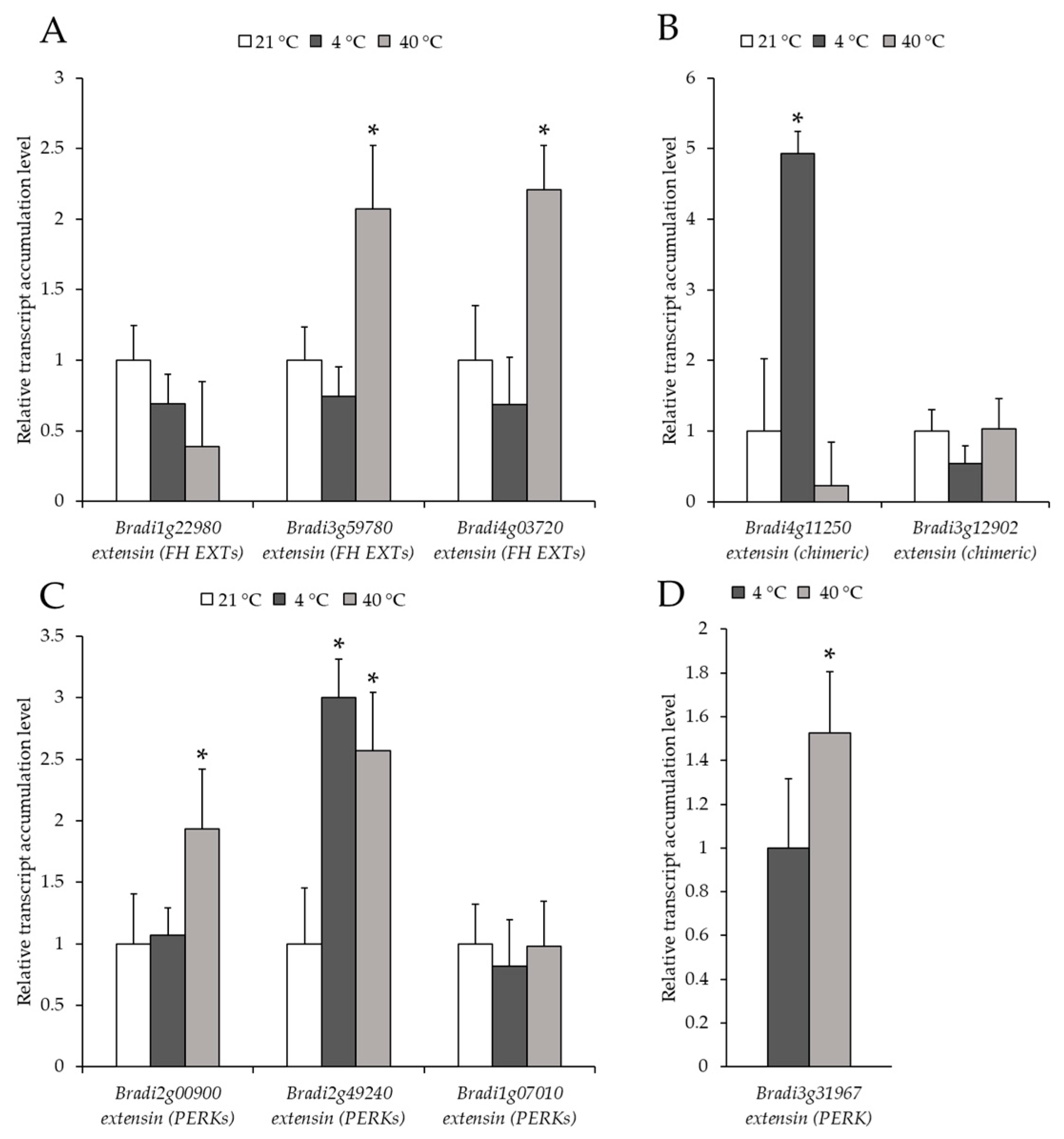
2.2. Analysis of the Level of Transcript Accumulation of the Genes Encoding the FLA, EXT and EXT-Like Receptor Kinases
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Sample Preparation
4.3. Immunohistochemistry
4.4. RT-qPCR
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AG Peptides | Arabinogalactan peptides |
| AGP | Arabinogalactan proteins |
| EXT | Extensins |
| FH EXT | Formin-homolog EXT |
| FLA | Fasciclin-like AGP |
| GPI | Glycosylphosphatidylinositol |
| HRGP | Hydroxyproline-rich glycoproteins |
| LRX | Leucine-rich repeat extensins |
| Lys-rich AGP | Lysine-rich arabinogalactan proteins |
| PBS | Phosphate-buffered saline |
| PERK | Proline-rich extensin-like receptor kinases |
| ROS | Reactive oxygen species |
| RT-qPCR | Reverse transcription-quantitative polymerase chain reaction |
Appendix A


| Genes | Description of the Genes | Primer Sequence (5′-3′) |
|---|---|---|
| Bradi1g32860 | ubiquitin | pF-GAGGGTGGACTCCTTTTGGA pR-TCCACACTCCACTTGGTGCT |
| EXTand EXT-like receptor kinase | ||
| Bradi4g11250 | extensin (chimeric EXT) | pF-GCGACTGCGACAATGATGTG |
| pR-ACCCCTTGCTAAGCCCTCTA | ||
| Bradi3g12902 | extensin (chimeric EXT) | pF-CATCTGGACCTGCCAATGGT |
| pR-TCCCAGTTTTGGAGTCTCGC | ||
| Bradi3g59780 | formin-homolog extensin (FH EXT) | pF-GATGAATGCCGGAACAGCAC |
| pR-GTGGAGAAGAGTGGTGCCTC | ||
| Bradi4g03720 | formin-homolog extensin (FH EXT) | pF-GAAGCAGATTGAGGCCGAGA |
| pR-CGCGCCTCCATCTTTTGATT | ||
| Bradi1g22980 | formin-homolog extensin (FH EXT) | pF-CAGCAGAGCCTGTTGCTTGAC |
| pR-TTCTAGGTTTCCGTGCATGAGT | ||
| Bradi2g49240 | proline-rich extensin-like receptor kinase | pF-TTCTCAGCCGTTGGGAGATG |
| (PERK) | pR-GGAAGGTCCCCAAAGTCTCG | |
| Bradi1g07010 | proline-rich extensin-like receptor kinase | pF-CCTCCACGGTAAAGGGCTG |
| (PERK) | pR-GATCCGTGGATGGCAGTCTT | |
| Bradi3g31967 | proline-rich extensin-like receptor kinase | pF-CCGTCGCCATTAAGAATCTGC |
| (PERK) | pR-GATTCTTGTGCCGAACTCGC | |
| Bradi2g00900 | proline-rich extensin-like receptor kinase (PERK) | pF-TAACTTTGAGGCACAGGTTGCT pR-AGCCATGTATCCAAAAGTCCCC |
| FLA | ||
| Bradi2g00220 | fasciclin-like arabinogalactan protein | pF-AGCTCAACAGCTCCCAGAC pR-CGAAAGCGAGTTGAGCGTG |
| Bradi5g18950 | fasciclin-like arabinogalactan protein | pF-AATAAAGGGAAGTCACCGTCGC pR-CCGTTCTTCTTGTCATGGACCT |
| Bradi4g34420 | fasciclin-like arabinogalactan protein | pF-CACATCCTCCAGATGCACGTC pR-CCGGACTCCTGGAACATGG |
| Bradi3g39740 | fasciclin-like arabinogalactan protein | pF-GTACTATTCCCTGGCGGAGTTC pR-CCATGTTGTCGGTGAGGTTGAG |
| Bradi2g60270 | fasciclin-like arabinogalactan protein | pF-AGCAGAGCAATCCTCTAGTAGC pR-TGGGTTCTTCTCGCCATTGTTA |
References
- Barlow, K.M.; Christy, B.P.; O’Leary, G.J.; Riffkin, P.A.; Nuttall, J.G. Simulating the impact of extreme heat and frost events on wheat crop production: A review. Field Crops Res. 2015, 171, 109–119. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Garvin, D.F.; Gu, Y.Q.; Hasterok, R.; Hazen, S.P.; Jenkins, G.; Mockler, T.C.; Mur, L.A.J.; Vogel, J.P. Development of genetic and genomic research resources for Brachypodium distachyon, a new model system for grass crop research. Crop Sci. 2008, 48, S-69. [Google Scholar] [CrossRef]
- International Brachypodium Initiative. Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature 2010, 463, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Janmohammadi, M.; Zolla, L.; Rinalducci, S. Low temperature tolerance in plants: Changes at the protein level. Phytochemistry 2015, 117, 76–89. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Cassin, A.M.; Lonsdale, A.; Bacic, A.; Doblin, M.S.; Schultz, C.J. Pipeline to identify hydroxyproline-rich glycoproteins. Plant Physiol. 2017, 174, 886–903. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Pereira, L.G.; Coimbra, S. Arabinogalactan proteins: Rising attention from plant biologists. Plant Reprod. 2015, 28, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Showalter, A.M.; Basu, D. Extensin and arabinogalactan-protein biosynthesis: Glycosyltransferases, research challenges, and biosensors. Front. Plant Sci. 2016, 7, 814. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Higashiyama, T. Arabinogalactan proteins and their sugar chains: Functions in plant reproduction, research methods, and biosynthesis. Plant Reprod. 2018, 31, 67–75. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Pinski, A.; Betekhtin, A.; Hupert-Kocurek, K.; Mur, L.A.J.; Hasterok, R. Defining the genetic basis of plant–endophytic bacteria interactions. Int. J. Mol. Sci. 2019, 20, 1947. [Google Scholar] [CrossRef] [PubMed]
- Betekhtin, A.; Rojek, M.; Nowak, K.; Pinski, A.; Milewska-Hendel, A.; Kurczynska, E.; Doonan, J.H.; Hasterok, R. Cell wall epitopes and endoploidy as reporters of embryogenic potential in Brachypodium distachyon callus culture. Int. J. Mol. Sci. 2018, 19, 3811. [Google Scholar] [CrossRef] [PubMed]
- Mareri, L.; Romi, M.; Cai, G. Arabinogalactan proteins: Actors or spectators during abiotic and biotic stress in plants? Plant Biosyst. 2018, 153, 173–185. [Google Scholar] [CrossRef]
- Mareri, L.; Faleri, C.; Romi, M.; Mariani, C.; Cresti, M.; Cai, G. Heat stress affects the distribution of JIM8-labelled arabinogalactan proteins in pistils of Solanum lycopersicum cv Micro-Tom. Acta Physiol. Plant 2016, 38, 184. [Google Scholar] [CrossRef]
- Yan, Y.; Takac, T.; Li, X.; Chen, H.; Wang, Y.; Xu, E.; Xie, L.; Su, Z.; Samaj, J.; Xu, C. Variable content and distribution of arabinogalactan proteins in banana (Musa spp.) under low temperature stress. Front. Plant Sci. 2015, 6, 353. [Google Scholar] [CrossRef]
- Liu, X.; Wolfe, R.; Welch, L.R.; Domozych, D.S.; Popper, Z.A.; Showalter, A.M. Bioinformatic identification and analysis of extensins in the plant kingdom. PLoS ONE 2016, 11, e0150177. [Google Scholar] [CrossRef]
- Betekhtin, A.; Rojek, M.; Milewska-Hendel, A.; Gawecki, R.; Karcz, J.; Kurczynska, E.; Hasterok, R. Spatial distribution of selected chemical cell wall components in the embryogenic callus of Brachypodium distachyon. PLoS ONE 2016, 11, e0167426. [Google Scholar] [CrossRef]
- Castilleux, R.; Plancot, B.; Ropitaux, M.; Carreras, A.; Leprince, J.; Boulogne, I.; Follet-Gueye, M.L.; Popper, Z.A.; Driouich, A.; Vicre, M. Cell wall extensins in root-microbe interactions and root secretions. J. Exp. Bot. 2018, 69, 4235–4247. [Google Scholar] [CrossRef] [PubMed]
- Baumberger, N.; Ringli, C.; Keller, B. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 2002, 12, 47–56. [Google Scholar]
- Botha, C.E. A tale of two neglected systems-structure and function of the thin- and thick-walled sieve tubes in monocotyledonous leaves. Front. Plant Sci. 2013, 4, 297. [Google Scholar] [CrossRef]
- Gawecki, R.; Sala, K.; Kurczyńska, E.U.; Świątek, P.; Płachno, B.J. Immunodetection of some pectic, arabinogalactan proteins and hemicellulose epitopes in the micropylar transmitting tissue of apomictic dandelions (Taraxacum, Asteraceae, Lactuceae). Protoplasma 2016, 254, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Milewska-Hendel, A.; Baczewska, A.H.; Sala, K.; Dmuchowski, W.; Brągoszewska, P.; Gozdowski, D.; Jozwiak, A.; Chojnacki, T.; Swiezewska, E.; Kurczynska, E. Quantitative and qualitative characteristics of cell wall components and prenyl lipids in the leaves of Tilia x euchlora trees growing under salt stress. PLoS ONE 2017, 12, e0172682. [Google Scholar] [CrossRef]
- Garaeva, L.D.; Pozdeeva, S.A.; Timofeeva, O.A.; Khokhlova, L.P. Cell-wall lectins during winter wheat cold hardening. Russ. J. Plant. Physiol. 2006, 53, 746–750. [Google Scholar] [CrossRef]
- Gong, S.Y.; Huang, G.Q.; Sun, X.; Li, P.; Zhao, L.L.; Zhang, D.J.; Li, X.B. GhAGP31, a cotton non-classical arabinogalactan protein, is involved in response to cold stress during early seedling development. Plant. Biol. 2012, 14, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Khan, S.; Ma, X. Climate change impacts on crop yield, crop water productivity and food security—A review. Prog. Nat. Sci. 2009, 19, 1665–1674. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Larsen, F.H.; Byg, I.; Damager, I.; Diaz, J.; Engelsen, S.B.; Ulvskov, P. Residue specific hydration of primary cell wall potato pectin identified by solid-state 13C single-pulse MAS and CP/MAS NMR spectroscopy. Biomacromolecules 2011, 12, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.A.; Vietor, R.J.; Jardine, G.D.; Apperley, D.C.; Jarvis, M.C. Conformation and mobility of the arabinan and galactan side-chains of pectin. Phytochemistry 2005, 66, 1817–1824. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant. Sci. 2014, 5, 771. [Google Scholar] [CrossRef]
- Moore, J.P.; Farrant, J.M.; Driouich, A. A role for pectin-associated arabinans in maintaining the flexibility of the plant cell wall during water deficit stress. Plant. Signal. Behav. 2014, 3, 102–104. [Google Scholar] [CrossRef]
- Betekhtin, A.; Pinski, A.; Milewska-Hendel, A.; Kurczynska, E.; Hasterok, R. Stability and instability processes in the calli of Fagopyrum tataricum that have different morphogenic potentials. Plant. Cell Tissue Organ. Cult. 2019, 1–15. [Google Scholar] [CrossRef]
- Xue, H.; Seifert, G.J. Fasciclin like arabinogalactan protein 4 and respiratory burst oxidase homolog d and F independently modulate abscisic acid signaling. Plant. Signal. Behav. 2015, 10, e989064. [Google Scholar] [CrossRef]
- Johnson, K.L.; Jones, B.J.; Bacic, A.; Schultz, C.J. The fasciclin-like arabinogalactan proteins of Arabidopsis. A multigene family of putative cell adhesion molecules. Plant. Physiol. 2003, 133, 1911–1925. [Google Scholar] [CrossRef]
- Seifert, G.J.; Xue, H.; Acet, T. The Arabidopsis thaliana FASCICLIN LIKE ARABINOGALACTAN PROTEIN 4 gene acts synergistically with abscisic acid signalling to control root growth. Ann. Bot. 2014, 114, 1125–1133. [Google Scholar] [CrossRef]
- Faik, A.; Abouzouhair, J.; Sarhan, F. Putative fasciclin-like arabinogalactan-proteins (FLA) in wheat (Triticum aestivum) and rice (Oryza sativa): Identification and bioinformatic analyses. Mol. Genet. Genom. 2006, 276, 478–494. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, J. Genome-wide identification, classification, and expression analysis of the arabinogalactan protein gene family in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 2647–2668. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Ahamed, K.U.; Hakeem, K.R.; Ozturk, M.; Fujita, M. Plant responses and tolerance to high temperature stress: Role of exogenous phytoprotectants. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer International Publishing: Cham, The Netherlands, 2015; pp. 385–435. [Google Scholar]
- Ellis, M.; Egelund, J.; Schultz, C.J.; Bacic, A. Arabinogalactan-proteins: Key regulators at the cell surface? Plant. Physiol. 2010, 153, 403–419. [Google Scholar] [CrossRef]
- Lima, R.B.; dos Santos, T.B.; Vieira, L.G.; Ferrarese Mde, L.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.; Petkowicz, C.L. Heat stress causes alterations in the cell-wall polymers and anatomy of coffee leaves (Coffea arabica L.). Carbohydr. Polym. 2013, 93, 135–143. [Google Scholar] [CrossRef]
- Olmos, E.; Garcia De La Garma, J.; Gomez-Jimenez, M.C.; Fernandez-Garcia, N. Arabinogalactan proteins are involved in salt-adaptation and vesicle trafficking in tobacco by-2 cell cultures. Front. Plant Sci. 2017, 8, 1092. [Google Scholar] [CrossRef]
- Zang, L.; Zheng, T.; Chu, Y.; Ding, C.; Zhang, W.; Huang, Q.; Su, X. Genome-wide analysis of the fasciclin-like arabinogalactan protein gene family reveals differential expression patterns, localization, and salt stress response in Populus. Front. Plant Sci. 2015, 6, 1140. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.; Kieliszewski, M.J.; Showalter, A.M. Salt stress upregulates periplasmic arabinogalactan proteins: Using salt stress to analyse AGP function. New Phytol. 2006, 169, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Kim, Y.; Guo, Y.; Stevenson, B.; Zhu, J.K. The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant. Cell 2002, 15, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, J.S.; Tsai, A.Y.; Xue, H.; Voiniciuc, C.; Sola, K.; Seifert, G.J.; Mansfield, S.D.; Haughn, G.W. SALT-OVERLY SENSITIVE5 mediates Arabidopsis seed coat mucilage adherence and organization through pectins. Plant Physiol. 2014, 165, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Seifert, G.J. Fascinating fasciclins: A surprisingly widespread family of proteins that mediate interactions between the cell exterior and the cell surface. Int. J. Mol. Sci. 2018, 19, 1628. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.L.; Kibble, N.A.; Bacic, A.; Schultz, C.J. A fasciclin-like arabinogalactan-protein (FLA) mutant of Arabidopsis thaliana, fla1, shows defects in shoot regeneration. PLoS ONE 2011, 6, e25154. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, W.; Li, X.; Chen, H.; Takac, T.; Samajova, O.; Fabrice, M.R.; Xie, L.; Ma, J.; Samaj, J.; et al. Expression and distribution of extensins and AGPs in susceptible and resistant banana cultivars in response to wounding and Fusarium oxysporum. Sci. Rep. 2017, 7, 42400. [Google Scholar] [CrossRef]
- Casero, P.J.; Casimiro, I.; Knox, J.P. Occurrence of cell surface arabinogalactan-protein and extensin epitopes in relation to pericycle and vascular tissue development in the root apex of four species. Planta 1998, 204, 252–259. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, Y.; Zhao, J. Roles of extensins in cotyledon primordium formation and shoot apical meristem activity in Nicotiana tabacum. J. Exp. Bot. 2008, 59, 4045–4058. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, C.; Takáč, T.; Burbach, C.; Menzel, D.; Šamaj, J. Developmental localization and the role of hydroxyproline rich glycoproteins during somatic embryogenesis of banana (Musa spp. AAA). BMC Plant. Biol. 2011, 11, 38. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, H.; Qi, H.; Zhao, J. Roles of hydroxyproline-rich glycoproteins in the pollen tube and style cell growth of tobacco (Nicotiana tabacum L.). J. Plant. Physiol. 2014, 171, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Narusaka, M.; Ishida, J.; Nanjo, T.; Fujita, M.; Oono, Y.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant. J. 2002, 31, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Kreps, J.A.; Wu, Y.; Chang, H.S.; Zhu, T.; Wang, X.; Harper, J.F. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant. Physiol. 2002, 130, 2129–2141. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, H.; Lee, Y.S.; Cho, H.T. Cell wall-associated ROOT HAIR SPECIFIC 10, a proline-rich receptor-like kinase, is a negative modulator of Arabidopsis root hair growth. J. Exp. Bot. 2016, 67, 2007–2022. [Google Scholar] [CrossRef]
- Dar, N.A.; Amin, I.; Wani, W.; Shafiq, A.; Shikari, A.B.; Wani, S.H.; Masoodi, K.Z. Abscisic acid: A key regulator of abiotic stress tolerance in plants. Plant. Gene 2017, 11, 106–111. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, G.; Zhou, Y.; Zhang, Z.; Wang, W.; Du, Y.; Wu, Z.; Song, C.P. Plasma membrane-associated proline-rich extensin-like receptor kinase 4, a novel regulator of Ca signalling, is required for abscisic acid responses in Arabidopsis thaliana. Plant. J. 2009, 60, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.F.; Goring, D.R. The proline-rich, extensin-like receptor kinase-1 (PERK1) gene is rapidly induced by wounding. Plant. Mol. Biol. 2002, 50, 667–685. [Google Scholar] [CrossRef]
- Zhao, C.; Zayed, O.; Yu, Z.; Jiang, W.; Zhu, P.; Hsu, C.C.; Zhang, L.; Tao, W.A.; Lozano-Duran, R.; Zhu, J.K. Leucine-rich repeat extensin proteins regulate plant salt tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2018, 115, 13123–13128. [Google Scholar] [CrossRef]
- Irizarry, I.; White, J.F. Bacillus amyloliquefaciens alters gene expression, ROS production and lignin synthesis in cotton seedling roots. J. Appl. Microbiol. 2018, 124, 1589–1603. [Google Scholar] [CrossRef]
- Sujkowska-Rybkowska, M.; Borucki, W. Accumulation and localization of extensin protein in apoplast of pea root nodule under aluminum stress. Micron 2014, 67, 10–19. [Google Scholar] [CrossRef]
- Francin-Allami, M.; Merah, K.; Albenne, C.; Rogniaux, H.; Pavlovic, M.; Lollier, V.; Sibout, R.; Guillon, F.; Jamet, E.; Larre, C. Cell wall proteomic of Brachypodium distachyon grains: A focus on cell wall remodeling proteins. Proteomics 2015, 15, 2296–2306. [Google Scholar] [CrossRef]
- Calderan-Rodrigues, M.J.; Guimarães Fonseca, J.; de Moraes, F.E.; Vaz Setem, L.; Carmanhanis Begossi, A.; Labate, C.A. Plant cell wall proteomics: A focus on monocot species, Brachypodium distachyon, Saccharum spp. and Oryza sativa. Int. J. Mol. Sci. 2019, 20, 1975. [Google Scholar] [CrossRef] [PubMed]
- Douche, T.; San Clemente, H.; Burlat, V.; Roujol, D.; Valot, B.; Zivy, M.; Pont-Lezica, R.; Jamet, E. Brachypodium distachyon as a model plant toward improved biofuel crops: Search for secreted proteins involved in biogenesis and disassembly of cell wall polymers. Proteomics 2013, 13, 2438–2454. [Google Scholar] [CrossRef] [PubMed]
- Francin-Allami, M.; Lollier, V.; Pavlovic, M.; San Clemente, H.; Rogniaux, H.; Jamet, E.; Guillon, F.; Larre, C. Understanding the remodelling of cell walls during Brachypodium distachyon grain development through a sub-cellular quantitative proteomic approach. Proteomes 2016, 4, 21. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, G.X.; Lv, D.W.; Li, X.H.; Yan, Y.M. N-linked glycoproteome profiling of seedling leaf in Brachypodium distachyon L. J. Proteome Res. 2015, 14, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Priest, H.D.; Fox, S.E.; Rowley, E.R.; Murray, J.R.; Michael, T.P.; Mockler, T.C. Analysis of global gene expression in Brachypodium distachyon reveals extensive network plasticity in response to abiotic stress. PLoS ONE 2014, 9, e87499. [Google Scholar] [CrossRef]
- Hong, S.Y.; Park, J.H.; Cho, S.H.; Yang, M.S.; Park, C.M. Phenological growth stages of Brachypodium distachyon: Codification and description. Weed Res. 2011, 51, 612–620. [Google Scholar] [CrossRef]
- Sala, K.; Malarz, K.; Barlow, P.W.; Kurczyńska, E.U. Distribution of some pectic and arabinogalactan protein epitopes during Solanum lycopersicum (L.) adventitious root development. BMC Plant. Biol. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Betekhtin, A.; Milewska-Hendel, A.; Chajec, L.; Rojek, M.; Nowak, K.; Kwasniewska, J.; Wolny, E.; Kurczynska, E.; Hasterok, R. 5-azacitidine induces cell death in a tissue culture of Brachypodium distachyon. Int. J. Mol. Sci. 2018, 19, 1806. [Google Scholar] [CrossRef] [PubMed]
- Pennell, R.I.; Janniche, L.; Kjellbom, P.; Scofield, G.N.; Peart, J.M.; Roberts, K. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant. Cell 1991, 3, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Yates, E.A.; Knox, J.P. Investigations into the occurrence of plant cell surface epitopes in exudate gums. Carbohydr. Polym. 1994, 24, 281–286. [Google Scholar] [CrossRef]
- Yates, E.A.; Valdor, J.F.; Haslam, S.M.; Morris, H.R.; Dell, A.; Mackie, W.; Knox, J.P. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 1996, 6, 131–139. [Google Scholar] [CrossRef]
- Knox, J.P.; Linstead, P.J.; Peart, J.; Cooper, C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant. J. 1991, 1, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.; Yates, E.A.; Willats, W.G.T.; Martin, H.; Knox, J.P. Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 1996, 198, 452–459. [Google Scholar] [CrossRef]
- Bradley, D.J.; Wood, E.A.; Larkins, A.P.; Galfre, G.; Butcher, G.W.; Brewin, N.J. Isolation of monoclonal antibodies reacting with peribacteroid membranes and other components of pea root nodules containing Rhizobium leguminosarum. Planta 1988, 173, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Pennell, R.I.; Knox, J.P.; Scofield, G.N.; Selvendran, R.R.; Roberts, K. A family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J. Cell. Biol. 1989, 108, 1967–1977. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Marcus, S.E.; Knox, J.P. Generation of a monoclonal antibody specific to (1→5)-α-l-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef]
- El-Tantawy, A.A.; Solis, M.T.; Da Costa, M.L.; Coimbra, S.; Risueno, M.C.; Testillano, P.S. Arabinogalactan protein profiles and distribution patterns during microspore embryogenesis and pollen development in Brassica napus. Plant. Reprod. 2013, 26, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.; Beven, A.; Donovan, N.; Neill, S.J.; Peart, J.; Roberts, K.; Knox, J.P. Localization of cell wall proteins in relation to the developmental anatomy of the carrot root apex. Plant. J. 1994, 5, 237–246. [Google Scholar] [CrossRef]
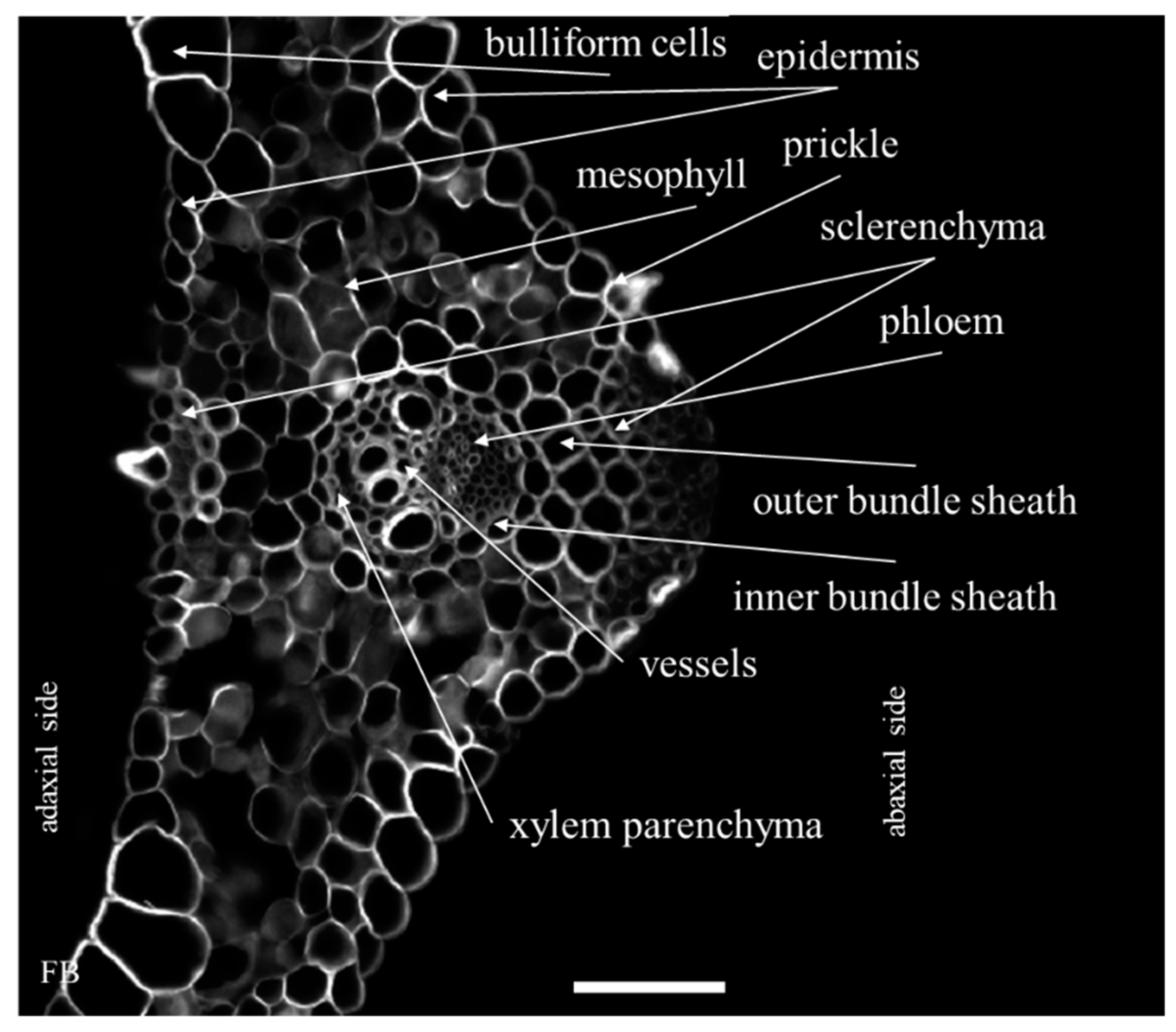
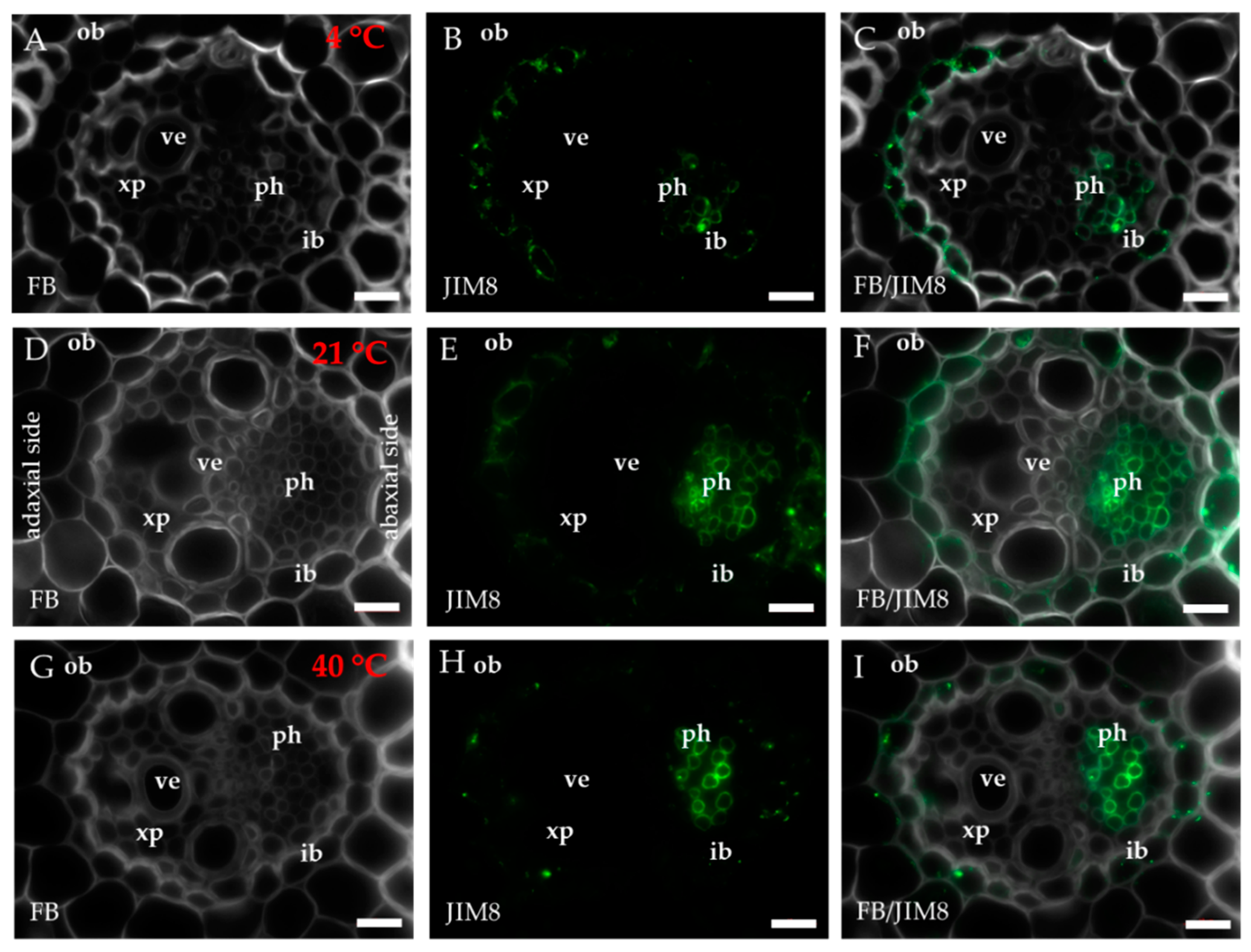
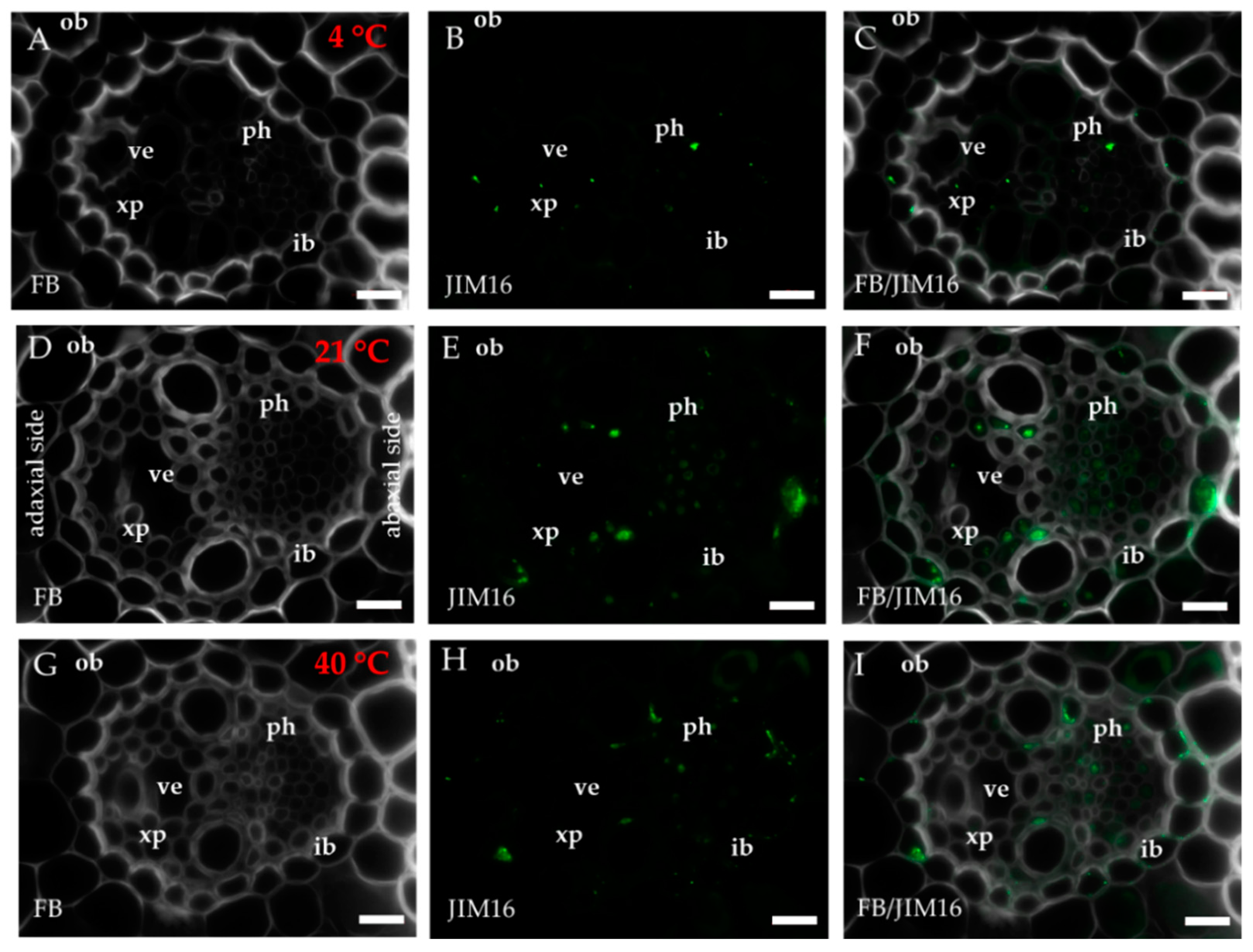
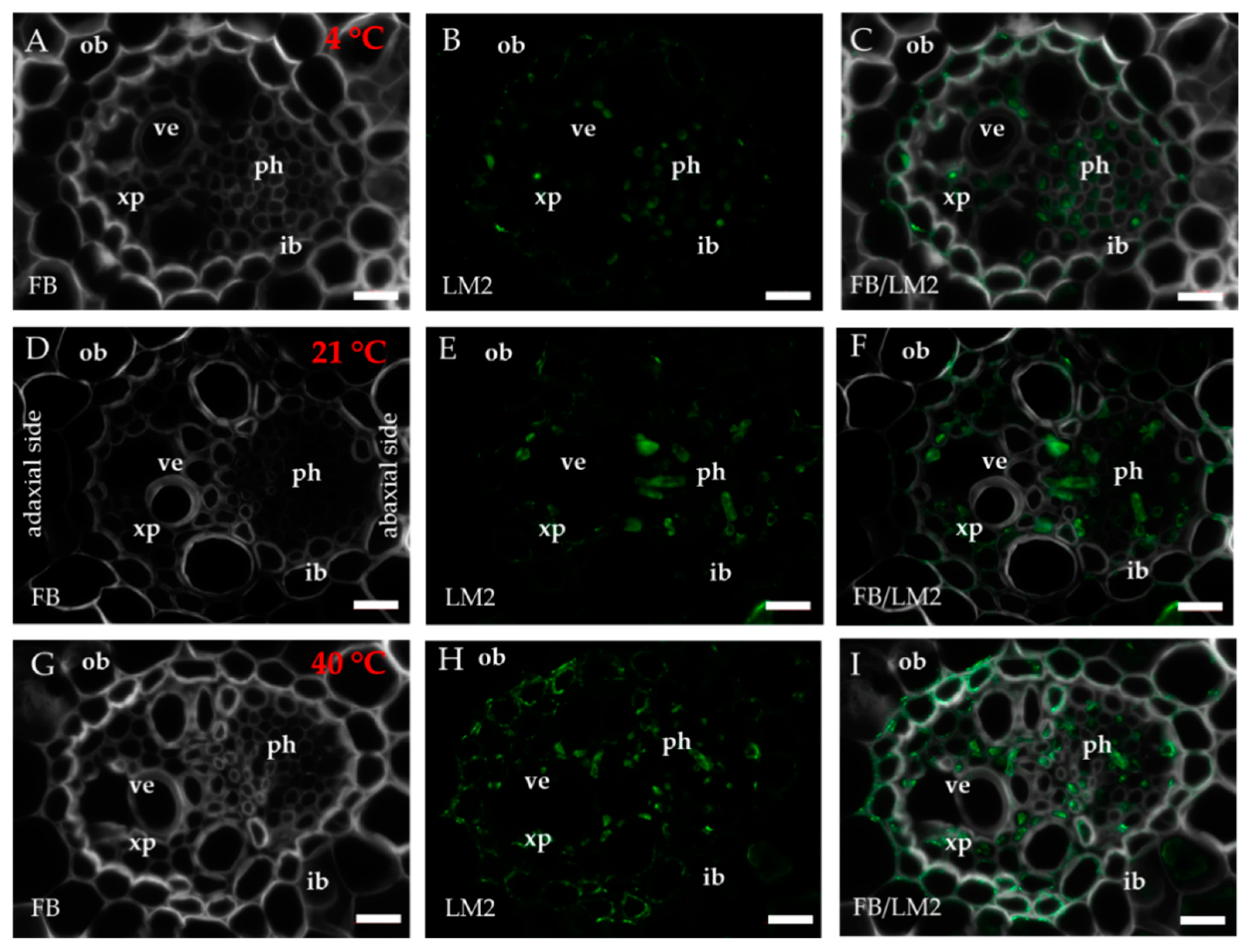
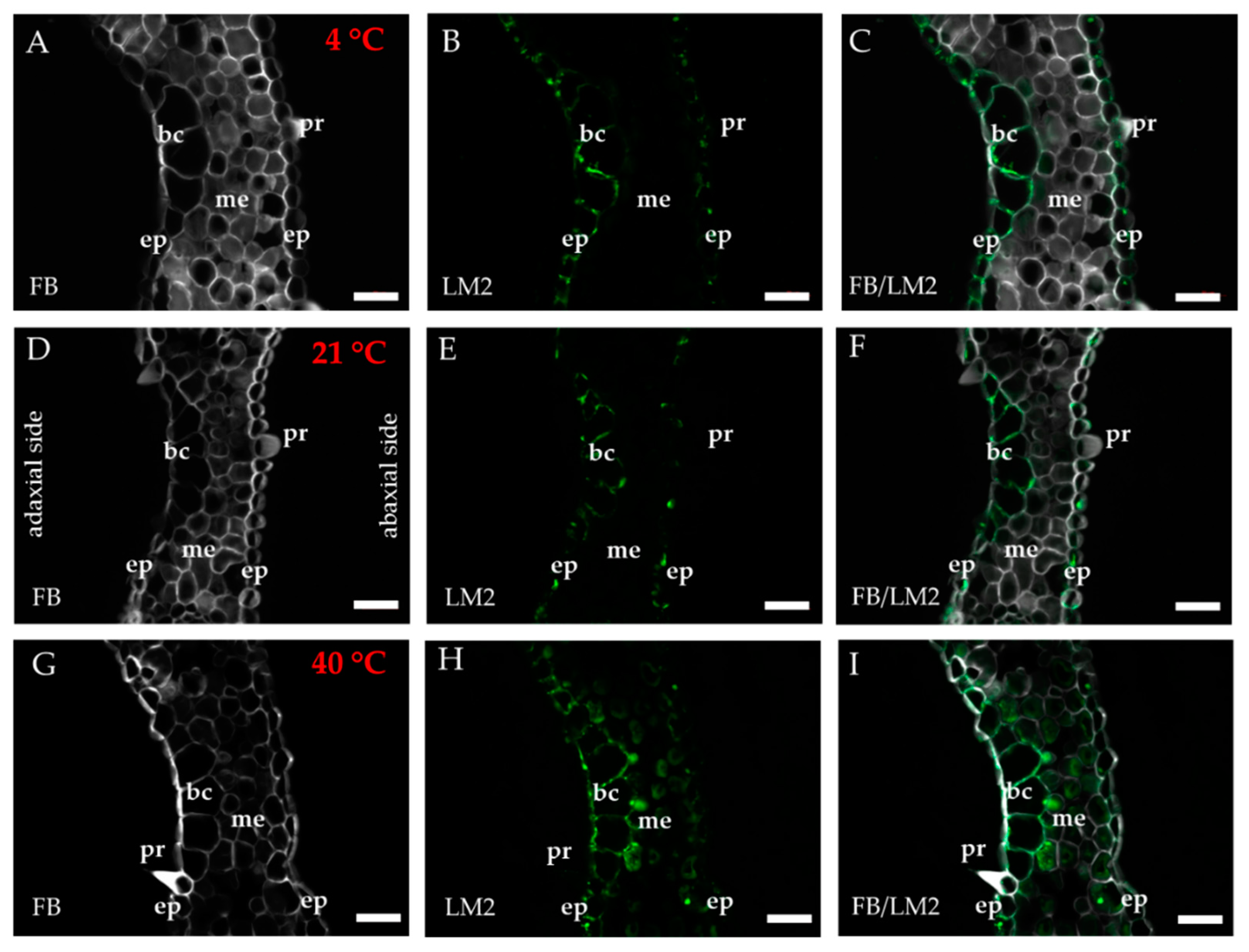
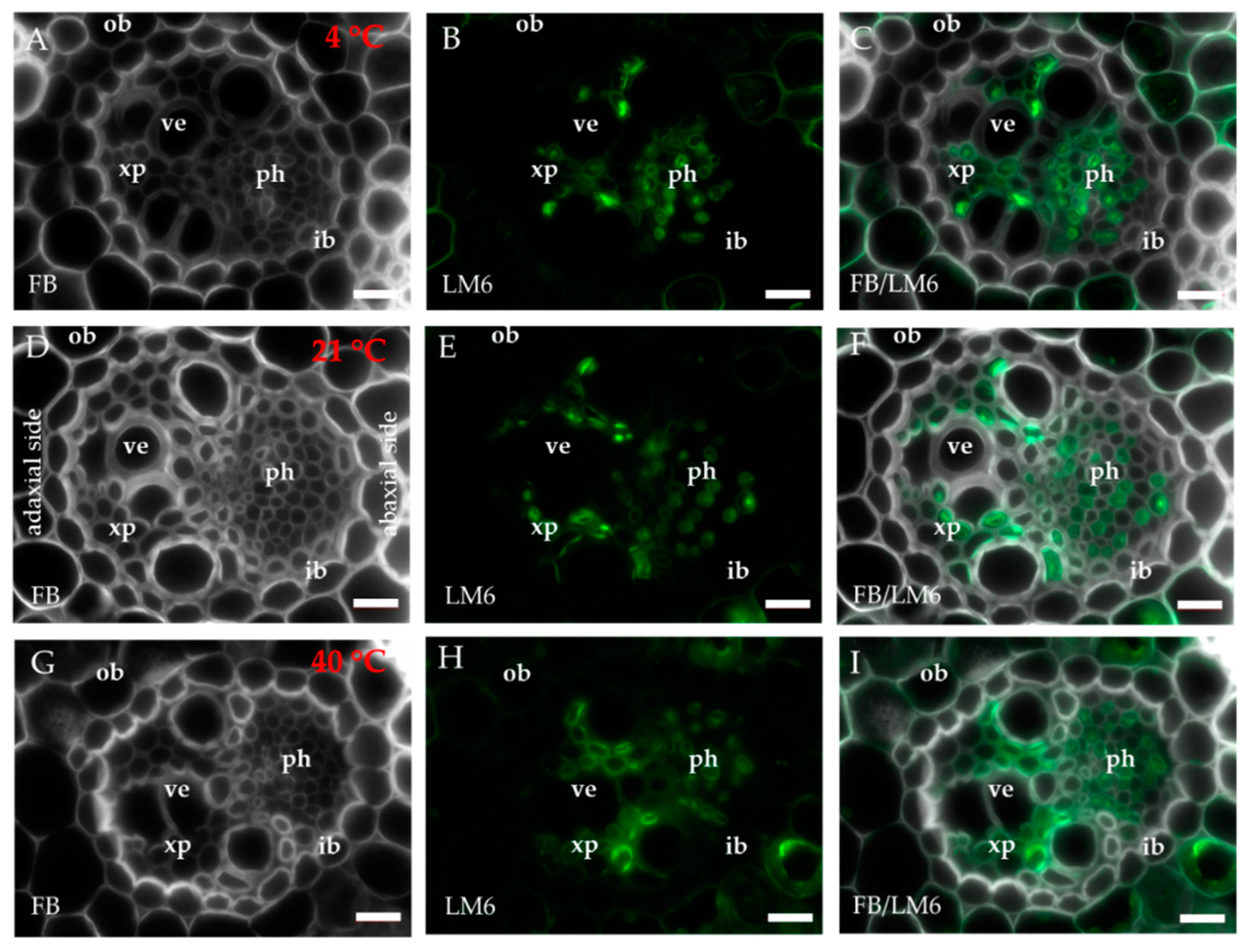
| Antibody | Epitope | References |
|---|---|---|
| AGP | ||
| JIM8 | Arabinogalactan | [73] |
| JIM13 | (β)GlcA1->3(α)GalA1->2Rha | [74,75,76] |
| JIM16 | AGP glycan | [74,75,76] |
| LM2 | β-linked GlcA | [75,77] |
| MAC207 | (β)GlcA1->3(α)GalA1->2Rha | [74,75,78,79] |
| Pectin/AGP | ||
| LM6 | (1-5)-α-l-arabinosyl residues, can also bind to some AGP | [80,81] |
| EXT | ||
| JIM11 | Extensin | [74,82] |
| JIM12 | Extensin | [82] |
| JIM20 | Extensin | [82] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinski, A.; Betekhtin, A.; Sala, K.; Godel-Jedrychowska, K.; Kurczynska, E.; Hasterok, R. Hydroxyproline-Rich Glycoproteins as Markers of Temperature Stress in the Leaves of Brachypodium distachyon. Int. J. Mol. Sci. 2019, 20, 2571. https://doi.org/10.3390/ijms20102571
Pinski A, Betekhtin A, Sala K, Godel-Jedrychowska K, Kurczynska E, Hasterok R. Hydroxyproline-Rich Glycoproteins as Markers of Temperature Stress in the Leaves of Brachypodium distachyon. International Journal of Molecular Sciences. 2019; 20(10):2571. https://doi.org/10.3390/ijms20102571
Chicago/Turabian StylePinski, Artur, Alexander Betekhtin, Katarzyna Sala, Kamila Godel-Jedrychowska, Ewa Kurczynska, and Robert Hasterok. 2019. "Hydroxyproline-Rich Glycoproteins as Markers of Temperature Stress in the Leaves of Brachypodium distachyon" International Journal of Molecular Sciences 20, no. 10: 2571. https://doi.org/10.3390/ijms20102571
APA StylePinski, A., Betekhtin, A., Sala, K., Godel-Jedrychowska, K., Kurczynska, E., & Hasterok, R. (2019). Hydroxyproline-Rich Glycoproteins as Markers of Temperature Stress in the Leaves of Brachypodium distachyon. International Journal of Molecular Sciences, 20(10), 2571. https://doi.org/10.3390/ijms20102571