Gene Expression Profiles Deciphering the Pathways of Coronatine Alleviating Water Stress in Rice (Oryza sativa L.) Cultivar Nipponbare (Japonica)
Abstract
1. Introduction
2. Results
2.1. Growth of Seedlings
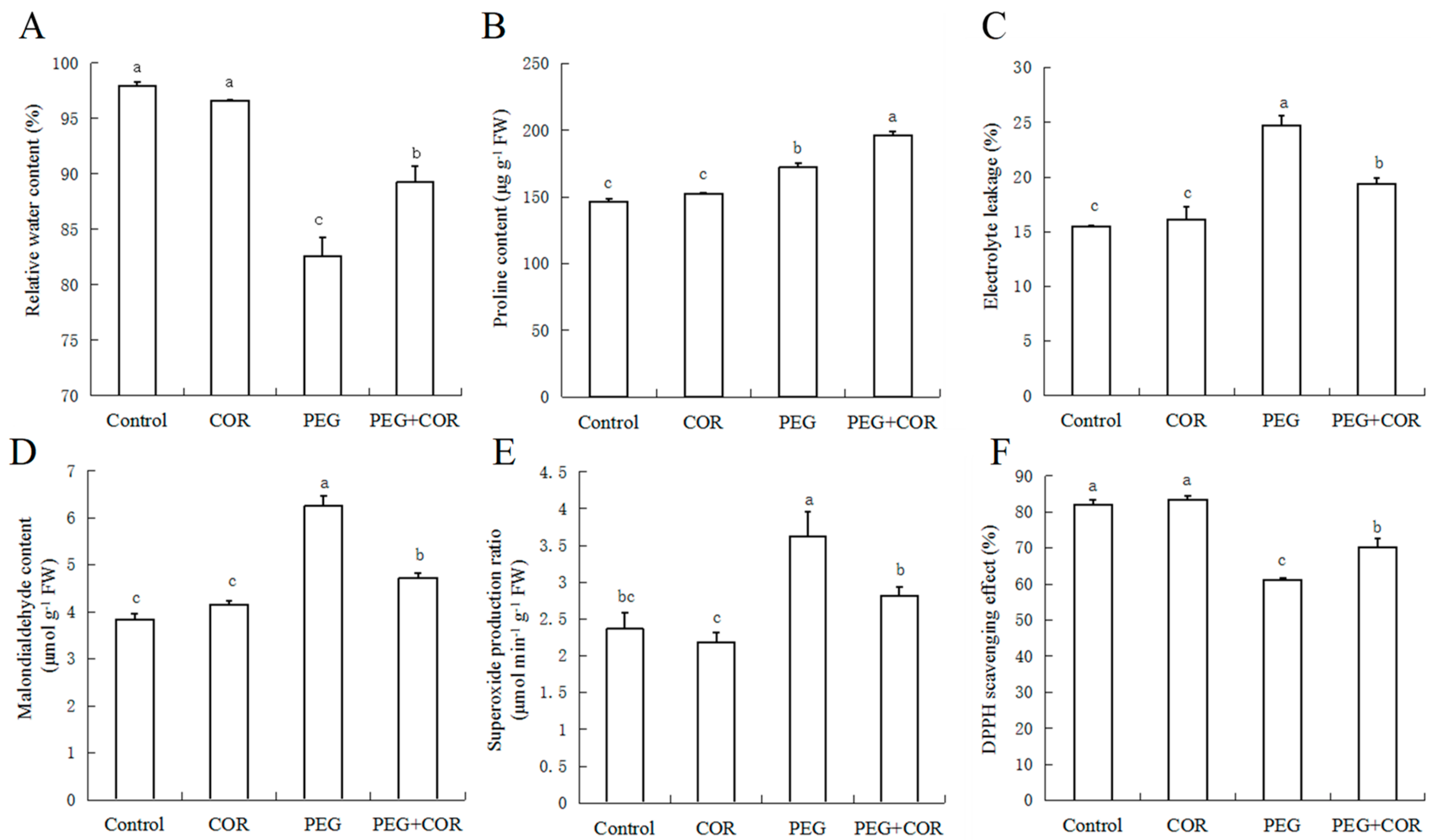
2.2. Relative Water Content, Proline Content
2.3. Relative Conductivity and MDA Content
2.4. Superoxide Radical Estimation and DPPH-Radical Scavenging Activity
2.5. Functional Analysis of the Transcriptome Map from Rice Nipponbare (Japonica) Treated by COR under Normal and Drought Stress Conditions
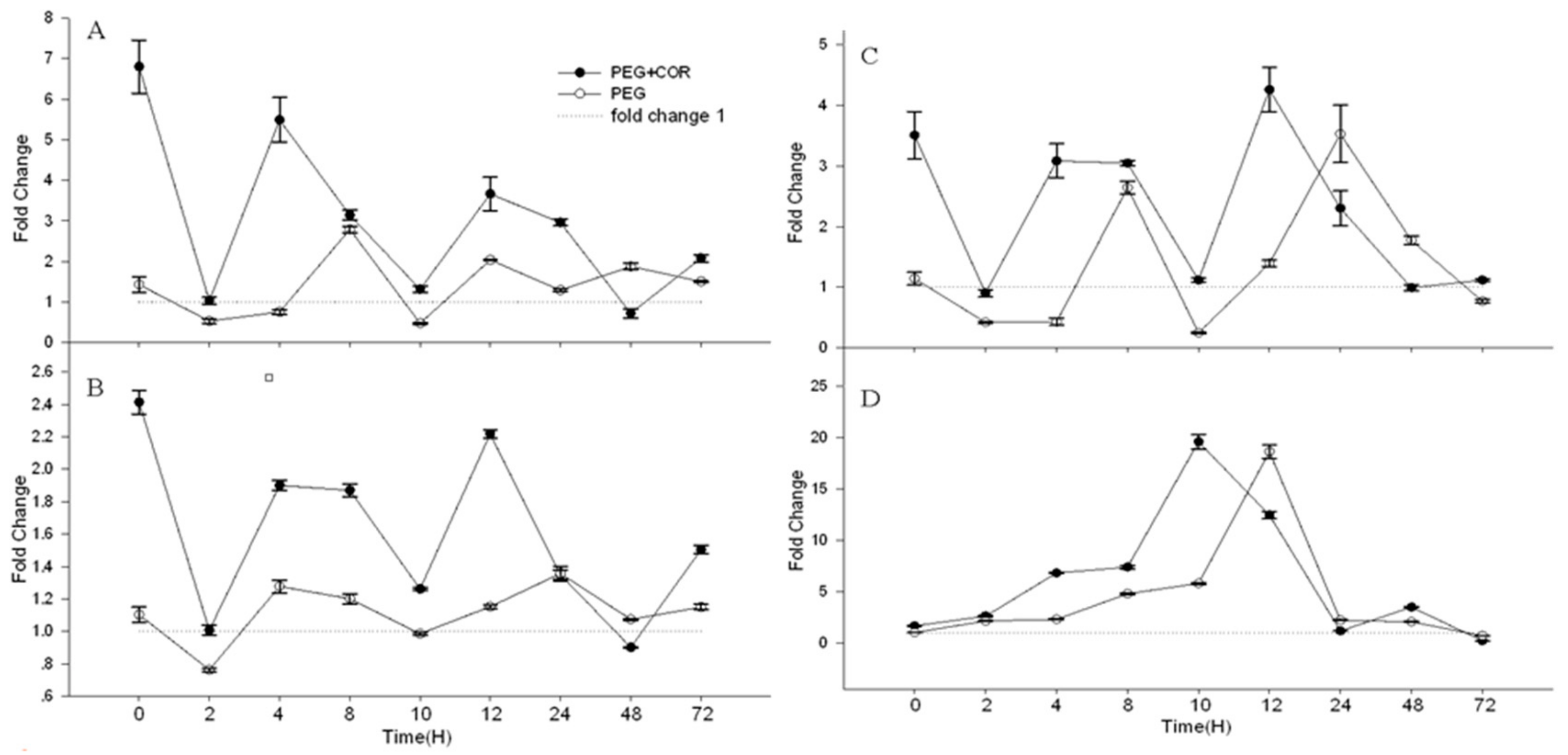
2.6. The Differentially Expressed Genes Correlated to JA, ABA and Other Hormone Biosynthetic, and Signaling Pathway under Water Stress
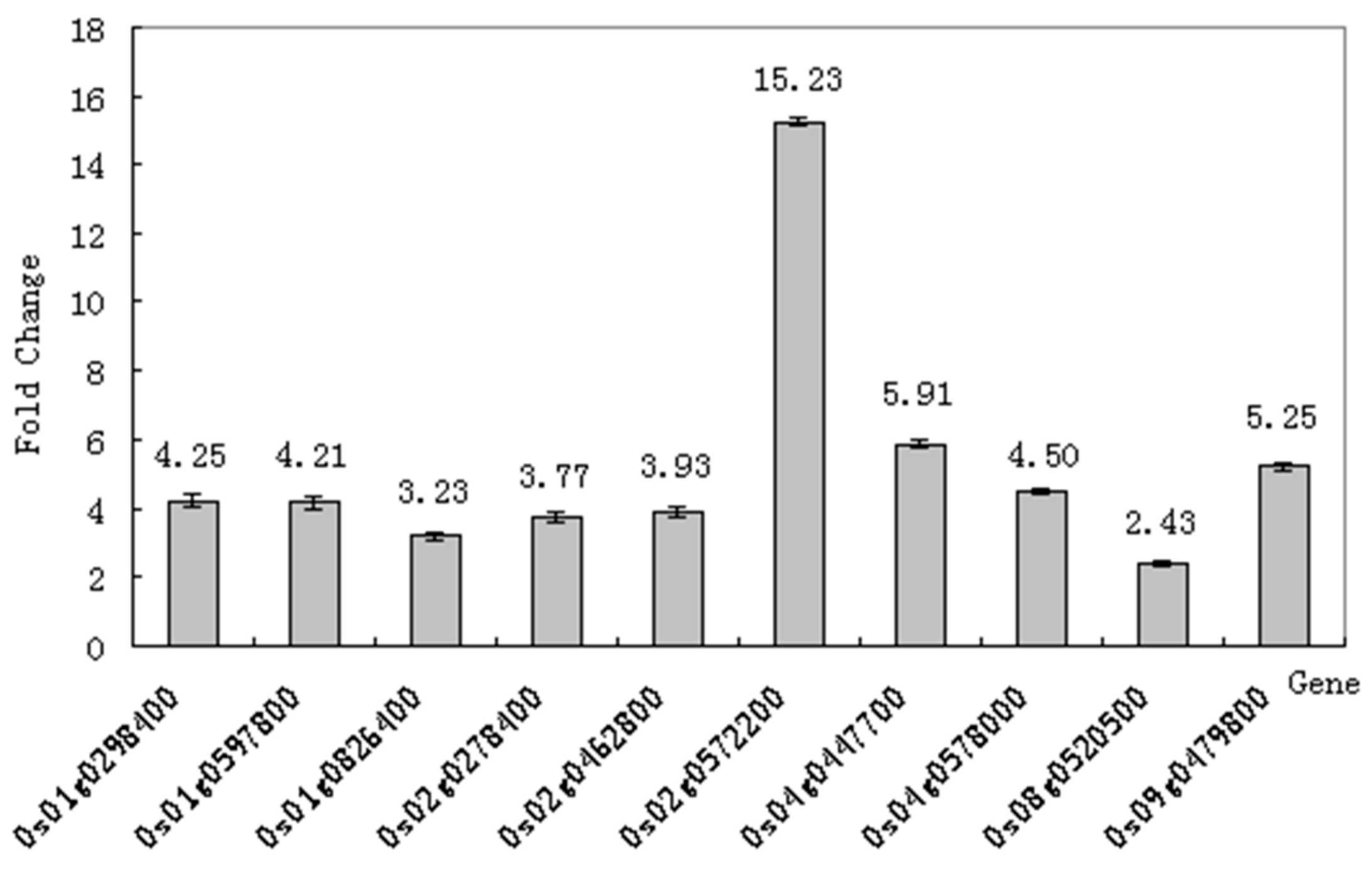
2.7. Real-Time Quantitative PCR (RT-qPCR) Verifies the Differential Expression of COR Regulated Genes
3. Discussion
3.1. Accumulation of Osmotica
3.2. The Significant Expression of Response Genes
3.3. Significantly Upregulated Transcription Factors Associated with Drought Tolerance
3.4. Changes of the Secondary Metabolic
4. Materials and Methods
4.1. Plant material, Growth Conditions and COR Treatments
4.2. Relative Water Content (RWC), Proline Concentration of Leaves
4.3. Measurements of Relative Conductivity and MDA Content
4.4. Superoxide Radical Estimation and DPPH-Radical Scavenging Activity
4.5. Affymetrix GeneChip Analysis
4.6. RNA Isolation and cDNA Preparation
4.7. Real-Time Quantitative PCR (RT-qPCR) Analysis
4.8. Statistical Analysis
5. Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| CAT | Catalase |
| CTK | Cytokinin |
| COR | Coronatine |
| DPPH | 2,2-Dipenyl-1- picrylhydrazyl |
| ETH | Ethylene |
| GR | Glutathione reductase |
| IAA | Indole acetic acid |
| LD | linear dichroism |
| MDA | Malondialdehyde |
| MeJA | Methyl jasmonic acid |
| PEG | Polyethylene glycol |
| POD | Peroxidase |
| RWC | Relative water content |
| SOD | Superoxide dismutase |
References
- Todorov, D.; Alexieva, V.; Karanov, E. Effect of putrescine, 4-PU-30, and abscisic acid on maize plants grown under normal, drought, and rewatering conditions. J. Plant Growth Regul. 1998, 17, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Lukáš, K.; Miroslava, L.; Peter, V. Effect of osmotic stress in early stages of ontogenesis on root respiration, growth, sugar content, and cell injury in maize seedlings differing in drought sensitivity. J. Integr. Plant Biol. 2006, 48, 814–822. [Google Scholar]
- Farooq, M.; Wahid, A.; Lee, D.J.; Ito, O.; Siddique, K.H.M. Advances in drought resistance of rice. Crit. Rev. Sci. 2009, 28, 199–217. [Google Scholar] [CrossRef]
- Gigon, A.; Matos, A.R.; Laffray, D.; Zuily-Fodil, Y.; Pham-Thi, A.T. Effect of drought stress on lipid metabolism in the leaves of Arabidopsis thaliana (ecotype Columbia). Ann. Bot. 2004, 94, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.; Petrova, A.; Dimitrov, I.; Tashev, T.; Olsovska, K.; Brestic, M. Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J. Agron. Crop Sci. 2018, 204, 219–227. [Google Scholar] [CrossRef]
- Ushimaru, T.; Shibasaka, M.; Tsuji, H. Resistance to oxidative injury in submerged rice seedlings after exposure to air. Plant Cell Physiol. 1994, 35, 211–218. [Google Scholar]
- Jiang, M.Y.; Zhang, J.H. Effect of Abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Shao, L.; Shu, Z.; Sun, S.L.; Peng, C.L.; Wang, X.J.; Lin, Z.F. Antioxidation of anthocyanins in photosynthesis under high temperature stress. J. Integr. Plant Biol. 2007, 49, 1341–1351. [Google Scholar] [CrossRef]
- Benard, F.J.; Runner, R.T.M. Erythrinaline alkaloids from the flowers and pods of Erythrina lysistemon and their DPPH radical scavenging properties. Phytochemistry 2004, 65, 1397–1401. [Google Scholar]
- Cao, M.; Zhang, Y.; Liu, X.; Huang, H.; Zhou, X.E.; Wang, W.; Zeng, A.; Zhao, C.; Si, T.; Du, J.; et al. Combining chemical and genetic approaches to increase drought resistance in plants. Nat. Commun. 2017, 8, 1183. [Google Scholar] [PubMed]
- Bender, C.L.; Alarcón-Chaidez, F.; Gross, D.C. Pseudomonas syringae phytotoxins: Mode of action, regulation and biosynthesis by peptide and polyketide synthetases. Microbiol. Mol. Biol. Rev. 1999, 63, 266–292. [Google Scholar] [PubMed]
- Cintas, N.A.; Koike, S.T.; Bull, C.T. A new pathovar, Pseudomonas syringae pv. Alisalensis pv nov., proposed for the causal agent of bacterial blight of broccoli and broccoli RAAB. Plant Dis. 2002, 86, 992–998. [Google Scholar] [CrossRef]
- Mitchell, R.E. Coronatine production by some phytopathogenic pseudomonads. Physiol. Plant Pathol. 1982, 20, 83–89. [Google Scholar] [CrossRef]
- Uppalapati, S.R.; Ayoubi, P.; Weng, H.; Palmer, D.A.; Mitchell, R.E.; Jones, W.; Bender, C.L. The phytotoxin coronatine and methyl jasmonate impact multiple phytohormone pathways in tomato. Plant J. 2005, 42, 201–217. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, C.; Gu, M.; Bai, Z.; Zhang, W.; Qi, T.; Cheng, Z.; Peng, W.; Luo, H.; Nan, F.; et al. The Arabidopsis CORONATINE INSENSITIVE1 Protein Is a Jasmonate Receptor. Plant Cell 2009, 21, 2220–2236. [Google Scholar] [CrossRef]
- Brooks, D.M.; Bender, C.L.; Kunkel, B.N. The pseudomonas syringaephytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Mol. Plant Pathol. 2005, 6, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Salzman, R.A. Transcriptional profiling of sorghum induced by methyl jasmonate, salicylic acid, and aminocyclopropane carboxylic acid reveals cooperative regulation and novel gene responses. Plant Physiol. 2005, 138, 352–368. [Google Scholar] [CrossRef] [PubMed]
- Feys, B.J.F.; Benedetti, C.E.; Penfold, C.N.; Turner, J.G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 1994, 6, 751–759. [Google Scholar] [CrossRef]
- Lauchli, R.; Boland, W. Indanoyl amino acid conjugates: Tunable elicitors of plant secondary metabolism. Chem. Rec. 2003, 3, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Schüler, G.; Mithöfer, A.; Baldwin, I.T.; Berger, S.; Ebel, J.; Santos, J.G.; Herrmann, G.; Höscher, D.; Kramell, R.; Kutchan, T.M.; et al. Coronalon: A powerful, tool in plant stress physiology. FEBS Lett. 2004, 563, 17–22. [Google Scholar] [CrossRef]
- Xie, Z.X.; Duan, L.S.; Tian, X.L.; Wang, B.Q.; Eneji, A.E.; Li, Z.H. Coronatine alleviates salinity stress in cotton by improving the antioxidative defense system and radical-scavenging activity. J. Plant Physiol. 2008, 165, 375–384. [Google Scholar]
- Yan, Z.F.; Wei, J.K.; Zhou, X. Effects on the resistance water stress for India millet seedling by coronatine treatment. Chin. Agric. Sci. Bull. 1999, 15, 11–14. [Google Scholar]
- Wang, B.Q.; Li, Z.H.; Eneji, A.E.; Tian, X.L.; Zhai, Z.X.; Li, J.M.; Duan, L.S. Effects of coronatine on growth, gas exchange traits, chlorophyll content, antioxidant enzymes and lipid peroxidation in maize (Zea mays L.) seedlings under simulated drought stress. Plant Prod. Sci. 2008, 11, 283–290. [Google Scholar] [CrossRef][Green Version]
- Li, X.W.; Shen, X.F.; Li, J.M.; Eneji, A.E.; Li, Z.H.; Tian, X.L.; Duan, L.S. Coronatine alleviates water deficiency stress on winter wheat seedlings. J. Integr. Plant Biol. 2010, 52, 616–625. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Liu, Y.R.; Peng, C.X.; Li, X.W.; Zhang, M.C.; Tian, X.L.; Li, J.M.; Li, Z.H.; Duan, L.S. Coronatine enhances drought tolerance in winter wheat by maintaining high photosynthetic performance. J. Plant Physiol. 2018, 228, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.F.; Chen, Y.; Li, J.M.; Duan, L.S. Coronatine effects on yield, nutrient uptake, and physio-biochemical attributes of soybean roots. J. Plant Nutr. 2018, 41, 664–671. [Google Scholar] [CrossRef]
- Wu, H.L.; Wu, X.L.; Li, Z.H.; Duan, L.S.; Zhang, M.C. Physiological evaluation of drought stress tolerance and recovery in cauliflower (Brassica oleracea L.) seedlings treated with methyl jasmonate and coronatine. J. Plant Growth Regul. 2012, 31, 113–123. [Google Scholar] [CrossRef]
- Tamogami, S.; Kodama, O. Coronatine elicits phytoalexin production in rice leaves (Oryza sativa L.) in the same manner as jasmonic acid. Phytochemistry 2000, 54, 689–694. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; Micol, J.L.; Solano, R. The JAZ family of repressors is the missing link in jasmonate signaling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Montesano, M.; Brader, G.; Palva, E.T. Pathogen derived elicitors: Searching for receptors in plants. Mol. Plant Pathol. 2003, 4, 73–79. [Google Scholar] [CrossRef]
- Lu, H.; Greenberg, J.T.; Holuigue, L. Salicylic acid signaling networks. Front. Plant Sci. 2016, 7, 238. [Google Scholar] [CrossRef]
- Glazebrook, J.; Chen, W.Q.; Estes, B.; Chang, H.S.; Nawrath, C.; Métraux, J.P. Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. Plant J. 2003, 34, 217–228. [Google Scholar] [CrossRef]
- Littleson, M.M.; Baker, C.M.; Dalençon, A.J.; Frye, E.C.; Jamieson, C.; Kennedy, A.R.; Ling, K.B.; McLachlan, M.M.; Montgomery, M.G.; Russell, C.J.; et al. Scalable total synthesis and comprehensive structure–activity relationship studies of the phytotoxin coronatine. Nat. Commun. 2018, 9, 1105. [Google Scholar] [CrossRef]
- Lin, A.I.; Zhao-Hu, L.I.; Jian-Min, L.I.; Xiao-Li, T.I.; Bao-Min, W.A.; Zhi-Xi, Z.H.; Liu-Sheng, D.U. Inducing effect of plant growth substance coronatine on drought tolerance of upland and lowland rice seedlings and its physiological mechanism. Chin. J. Rice Sci. 2008, 22, 443–446. [Google Scholar]
- Braun, Y.; Smirnova, A.; Weingart, H.; Schenk, A.; Ullrich, M. Coronatine gene expression in vitro and in planta, and protein accumulation during temperature downshift in Pseudomonas syringae. Sensors 2009, 9, 4272–4285. [Google Scholar] [CrossRef]
- Geng, X.; Jin, L.; Shimada, M.; Kim, M.G.; Mackey, D. The phytotoxin coronatine is a multifunctional component of the virulencen armament of Pseudomonas syringae. Planta 2014, 240, 1149–1165. [Google Scholar] [CrossRef]
- Ai, L.; Li, Z.H.; Xie, Z.X.; Tian, X.L.; Eneji, A.E.; Duan, L.S. Coronatine alleviates polyethylene glycol-induced water stress in two rice (Oryza sativa L.) cultivars. J. Agron. Crop Sci. 2008, 194, 360–368. [Google Scholar] [CrossRef]
- Byrne, M.E.; Barley, R.; Curtis, M.; Arroyo, J.M.; Dunham, M.; Hudson, A.; Martienssen, R.A. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 2000, 408, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Vannini, C.; Locatelli, F.; Bracale, M.; Magnani, E.; Marsoni, M.; Osnoto, M. Overexpression of the rice Osmyb4 gene increases chilling and freezing tolerance of Arabidopsis thaliana plants. Plant J. 2004, 37, 115–127. [Google Scholar] [CrossRef]
- Cominelli, E.; Gusmaroli, G.; Allegra, D.; Galbiati, M.; Wade, H.K.; Jenkins, G.I.; Tonelli, C. Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 886–894. [Google Scholar] [CrossRef]
- Wang, L.; Hua, D.; He, J.; Duan, Y.; Chen, Z.; Hong, X.; Gong, Z. Auxin Response Factor2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genet. 2011, 7, e1002172. [Google Scholar] [CrossRef]
- Selvarajan, D.; Mohan, C.; Dhandapani, V.; Nerkar, G.; Jayanarayanan, A.N.; Mohanan, M.V.; Murugan, N.; Kaur, L.; Chennappa, M.; Kumar, R.; et al. Differential gene expression profiling through transcriptome approach of Saccharum spontaneum L. under low temperature stress reveals genes potentially involved in cold acclimation. Biotech 2018, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Bürstenbinder, K.; Rzewuski, G.; Wirtz, M.; Hell, R.; Sauter, M. The role of methionine recycling for ethylene synthesis in Arabidopsis. Plant J. 2007, 49, 238–249. [Google Scholar] [CrossRef]
- Zhen, Y.; Qi, J.L.; Wang, S.S.; Su, J.; Xu, G.H.; Zhang, M.S.; Miao, L.V.; Peng, X.X.; Tian, D.; Yang, Y.H. Comparative proteome analysis of differentially expressed proteins induced by Al toxicity in soybean. Physiol. Plant. 2007, 131, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar]
- Arbona, V.; Manzi, M.; Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, S.; Yang, Y.; Huang, M.; Cheng, L.; Wei, Q.; Fei, Z.; Gao, J.; Hong, B. Transcriptome sequencing and whole genome expression profiling of chrysanthemum under dehydration stress. BMC Genom. 2013, 14, 662. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Cruz De Carvalho, M.H.; Torres-Jerez, I.V.; Kang, Y.U.; Allen, S.N.; Huhman, D.V.; Tang, Y.; Murray, J.; Sumner, L.W.; Udvardi, M.K. Global reprogramming of transcription and metabolism in M edicago truncatula during progressive drought and after rewatering. Plant Cell Environ. 2014, 37, 2553–2576. [Google Scholar] [CrossRef]
- Morales, C.G.; Pino, M.T.; Del Pozo, A. Phenological and physiological responses to drought stress and subsequent rehydration cycles in two raspberry cultivars. Sci. Hortic. 2013, 162, 234–241. [Google Scholar] [CrossRef]
- Liu, E.E.; Zong, H.; Guo, Z.F.; Li, Y.C. Effects of drought, salt and chilling stress on proline accumulation in shoot of rice seedlings. J. Trop. Subtrop. Bot. 2000, 8, 235–258. [Google Scholar]
- Kavi Kishor, P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Reduced chilling tolerance in elongating cucumber seedling radicals is related to their reduce antioxidant enzyme and DPPH-radical scavenging activity. Physiol. Plant. 2002, 115, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Todaka, D.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front. Plant Sci. 2015, 6, 84. [Google Scholar] [CrossRef]
- Joshi, R.; Wani, S.H.; Singh, B.; Bohra, A.; Dar, Z.A.; Lone, A.A.; Pareek, A.; Singla-Pareek, S.L. Transcription factors and plants response to drought stress: Current understanding and future directions. Front. Plant Sci. 2016, 7, 1029. [Google Scholar] [CrossRef] [PubMed]
- Sugano, S.; Kaminaka, H.; Rybka, Z.; Catala, R.; Salinas, J.; Matsui, K.; Ohme-Takagi, M.; Takatsuji, H. Stress-responsive zinc finger gene ZPT2-3 plays a role in drought tolerance in petunia. Plant J. 2003, 36, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Maruyama, K.; Sakuma, Y.; Meshi, T.; Iwabuchi, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis Cys/His-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 2004, 136, 2734–2746. [Google Scholar] [CrossRef]
- Dong, J.G. Cloning of a cDNA encoding 1-aminocyclop ropane-1-carboxylate synthase and expression of its mRNA in ripening apple fruit. Planta 1991, 185, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Schlagnhaufer, C.; Arteca, R.; Pell, E. Sequential expression of two 1-aminocyclop ropane-1-carboxylate synthase gene in response to biotic and abiotic stresses in potato leaves. Plant Mol. Biol. 1997, 35, 683–688. [Google Scholar] [CrossRef]
- Olson, D.C.; White, J.A.; Edelman, L.; Harkins, R.N.; Kende, H. Differential expression of two genes for 1-aminocyclop ropane-1-carboxylic acid synthase in tomato fruits. Proc. Natl. Acad. Sci. USA 1991, 88, 5340–5344. [Google Scholar] [CrossRef]
- Guiltinan, M.J.; Marcotte, J.W.R.; Quatrano, R.S. A plant leucine zipper protein that recognizes an abscisic acid response element. Science 1990, 250, 267–271. [Google Scholar] [CrossRef]
- Llorca, C.M.; Potschin, M.; Zentgraf, U. bZIPs and WRKYs: Two large transcription factor families executing two different functional strategies. Front. Plant Sci. 2014, 5, 169. [Google Scholar] [CrossRef] [PubMed]
- Fátima, C.A.; Sônia, M.B.C.; Júlio, C.M.C.; Nunes, C.C.; Martinez, C.A.; Otoni, W.C.; Fontes, E.P.B. Enhanced accumulation of BiP in transgenic plants confers tolerance to water stress. Plant Physiol. 2001, 126, 1042–1054. [Google Scholar]
- Reddy, A.S.N. Calcium: Silver bullet in signaling. Plant Sci. 2001, 160, 381–404. [Google Scholar] [CrossRef]
- Hagen, G.; Guilfoyle, T. Auxin-responsive gene expression: Genes, promoters and regulatory factors. Plant Mol. Biol. 2002, 49, 373–385. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Sharoni, A.M.; Kikuchi, S. Roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in plants. Front. Microbiol. 2013, 4, 248. [Google Scholar] [CrossRef] [PubMed]
- Franco-Zorrilla, J.M.; López-Vidriero, I.; Carrasco, J.L.; Godoy, M.; Vera, P.; Solano, R. DNA-binding specificities of plant transcription factors and their potential to define target genes. Proc. Natl. Acad. Sci. USA 2014, 111, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Shao, H.; Tang, X. Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Front. Plant Sci. 2016, 7, 67. [Google Scholar] [CrossRef]
- Shao, H.; Wang, H.; Tang, X. NAC transcription factors in plant multiple abiotic stress responses: Progress and prospects. Front. Plant Sci. 2015, 6, 902. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.L.; Seemann, J.R.; Neuman, D.; Shen, Q.J. A WRKY gene from creosote bush encodes an activator of the abscisic acid signaling pathway. J. Biol. Chem. 2004, 279, 55770–55779. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ai, C.; Jing, S.; Yu, D. Research progress on functional analysis of rice WRKY genes. Rice Sci. 2010, 17, 60–72. [Google Scholar] [CrossRef]
- Rushton, P.J.; Somssich, I.E.; Ringler, P.; Shen, Q.J. WRKY transcription factors. Plant Signal. Behav. 2014, 9, e27700. [Google Scholar] [CrossRef]
- Xie, Z.; Ruas, P.; Shen, Q.J. Regulatory networks of phytohormone abscisic acid. Vitam. Horm. 2005, 72, 235–269. [Google Scholar] [PubMed]
- Xie, Z.; Zhang, Z.L.; Zou, X.L.; Huang, J.; Ruas, P.; Thompson, D.; Shen, Q.J. Annotations and functional analyses of the rice WRKY gene superfamily reveal positive and negative regulators of abscisic acid signaling in aleurone cells. Plant Physiol. 2005, 137, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Y.; Wang, H.; Ran, X.; Li, B.; Zhang, J.; Zhang, H. Ectopic expression of a cytochrome P450 monooxygenase gene PtCYP714A3 from Populus trichocarpa reduces shoot growth and improves tolerance to salt stress in transgenic rice. Plant Biotechnol. J. 2016, 14, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Manghwar, H.; Shaban, M.; Khan, A.H.; Akbar, A.; Ali, U.; Ali, E.; Fahad, S. Phytohormones enhanced drought tolerance in plants: A coping strategy. Environ. Sci. Pollut. Res. 2018, 25, 33103–33118. [Google Scholar] [CrossRef]
- Palmer, D.A.; Bender, C.L. Effects of environmental and nutritional factors on production of the polyketide phytotoxin coronatine by Pseudomonas syringae pv. Glycinea. Appl. Environ. Microbiol. 1993, 59, 1619–1626. [Google Scholar] [PubMed]
- Sharp, R.E.; Hsiao, T.C.; Silk, W.K. Growth of the maize primary root at low water potentials. II. Role of growth and deposition of hexose and potassium in osmotic adjustment. Plant Physiol. 1990, 93, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S. Plant Physiology and Biochemistry Experiment Principle and Technology; Higher Education Press: Beijing, China, 2000. [Google Scholar]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C. Water stress tolerance of wheat (Triticum aestivum L.): Variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J. Agron. Crop Sci. 2001, 186, 63–70. [Google Scholar] [CrossRef]
- Verma, S.; Mishra, S.N. Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 2005, 162, 669–677. [Google Scholar] [CrossRef]
- Liu, F.X.; Xu, W.Y.; Wei, Q.; Zhang, Z.H.; Tan, L.B.; Di, C. Gene expression profiles deciphering rice phenotypic variation between Nipponbare (Japonica) and 93-11 (Indica) during oxidative stress. PLoS ONE 2010, 5, e8632. [Google Scholar] [CrossRef]
- Li, C.; Wong, W.H. Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc. Natl. Acad. Sci. USA 2001, 98, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wong, W.H. Model-based analysis of oligonucleotide arrays: Model validation, design issues and standard error application. Genome Biol. 2001, 2, RESEARCH0032. [Google Scholar] [PubMed]




| Treatment | Fresh Weight (g/plant) | Dry Weight (g/plant) |
|---|---|---|
| Control | 0.727a | 0.101a |
| COR | 0.700a | 0.098a |
| PEG | 0.283c | 0.063c |
| PEG+COR | 0.520b | 0.073b |
| Treatment | Total | Present | P Call (%) |
|---|---|---|---|
| Control | 57381 | 21806 | 38.00 |
| PEG | 57381 | 22827 | 39.78 |
| COR | 57381 | 21827 | 38.04 |
| PEG+COR | 57381 | 22632 | 39.44 |
| Catalogue | Gene Name | Fold Change | Annotation |
|---|---|---|---|
| Response to Stress | Os02g0572200 | 12.43 | Zn-finger, RING domain containing protein |
| Os06g0166500 | 4.98 | AUX/IAA protein family protein | |
| Os09g0474000 | 4.18 | Basic-leucine zipper (bZIP) | |
| Os07g0686800 | 3.36 | Serine/threonine protein kinase | |
| Os09g0255400 | 3.06 | Indole-3-glycerol phosphate synthase (IGPS) | |
| Os04g0578000 | 3.08 | ACC synthase | |
| Os06g0549900 | 2.71 | FAD linked oxidase, N-terminal domain containing protein | |
| Os04g0511200 | 2.26 | EFA27 for EF hand, abscisic acid | |
| Os09g0522000 | 0.49 | CBF-like protein | |
| Os08g0474000 | 0.42 | AP2 domain containing protein RAP2.6 | |
| Os01g0580500 | 0.25 | 1-aminocyclopropane-1-carboxylate oxidase | |
| Os12g0139400 | 0.09 | Two-component response regulator ARR17 | |
| Transcription Factor Gene | Os08g0520500 | 22.63 | Auxin response factor 2 |
| Os01g0298400 | 3.67 | MYB9 | |
| Os09g0417800 | 3.31 | DNA-binding WRKY domain containing protein | |
| Os03g0327800 | 3.19 | NAC-domain containing protein 29 (NAC2) | |
| Os01g0826400 | 2.82 | WRKY transcription factor 24 | |
| Os01g0904700 | 2.79 | Two-component response regulator ARR1 | |
| Os03g0437200 | 2.52 | Transcription factor WRKY3 | |
| Os04g0605100 | 2.20 | WRKY transcription factor 68 | |
| Os02g0462800 | 2.18 | WRKY transcription factor 42 | |
| Correlate to Photosynthesis and Others | Os01g0597800 | 9.34 | UDP-glucuronosyl/UDP-glucosyltransferase family protein |
| Os03g0277700 | 9.28 | Protein of unknown function DUF26 domain | |
| Os04g0447700 | 4.48 | NAD(P)H dependent 6’-deoxychalcone synthase | |
| Os06g0569500 | 3.40 | Ent-kaurene oxidase (AtKO1) ( Cytochrome P450 701A3) | |
| Os06g0549900 | 2.71 | FAD linked oxidase, N-terminal domain containing protein | |
| Os03g0709300 | 2.34 | Plastocyanin-like domain containing protein | |
| Os02g0194700 | 2.26 | Plant lipoxygenase family protein | |
| Os01g0600900 | 0.50 | Chlorophyll a-b binding protein 2, chloroplast precursor | |
| Os12g0292400 | 0.49 | Ribulose 1,5-bisphosphate carboxylase | |
| Os03g0280000 | 0.43 | ABC transporter protein | |
| Os07g0675400 | 0.16 | Aminoacyl-tRNA synthetase |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Yu, C.; Ai, L.; Zhou, Y.; Duan, L. Gene Expression Profiles Deciphering the Pathways of Coronatine Alleviating Water Stress in Rice (Oryza sativa L.) Cultivar Nipponbare (Japonica). Int. J. Mol. Sci. 2019, 20, 2543. https://doi.org/10.3390/ijms20102543
Gao W, Yu C, Ai L, Zhou Y, Duan L. Gene Expression Profiles Deciphering the Pathways of Coronatine Alleviating Water Stress in Rice (Oryza sativa L.) Cultivar Nipponbare (Japonica). International Journal of Molecular Sciences. 2019; 20(10):2543. https://doi.org/10.3390/ijms20102543
Chicago/Turabian StyleGao, Wei, Chunxin Yu, Lin Ai, Yuyi Zhou, and Liusheng Duan. 2019. "Gene Expression Profiles Deciphering the Pathways of Coronatine Alleviating Water Stress in Rice (Oryza sativa L.) Cultivar Nipponbare (Japonica)" International Journal of Molecular Sciences 20, no. 10: 2543. https://doi.org/10.3390/ijms20102543
APA StyleGao, W., Yu, C., Ai, L., Zhou, Y., & Duan, L. (2019). Gene Expression Profiles Deciphering the Pathways of Coronatine Alleviating Water Stress in Rice (Oryza sativa L.) Cultivar Nipponbare (Japonica). International Journal of Molecular Sciences, 20(10), 2543. https://doi.org/10.3390/ijms20102543





