Proteomic Analysis of MeJa-Induced Defense Responses in Rice against Wounding
Abstract
1. Introduction
2. Results and Discussion
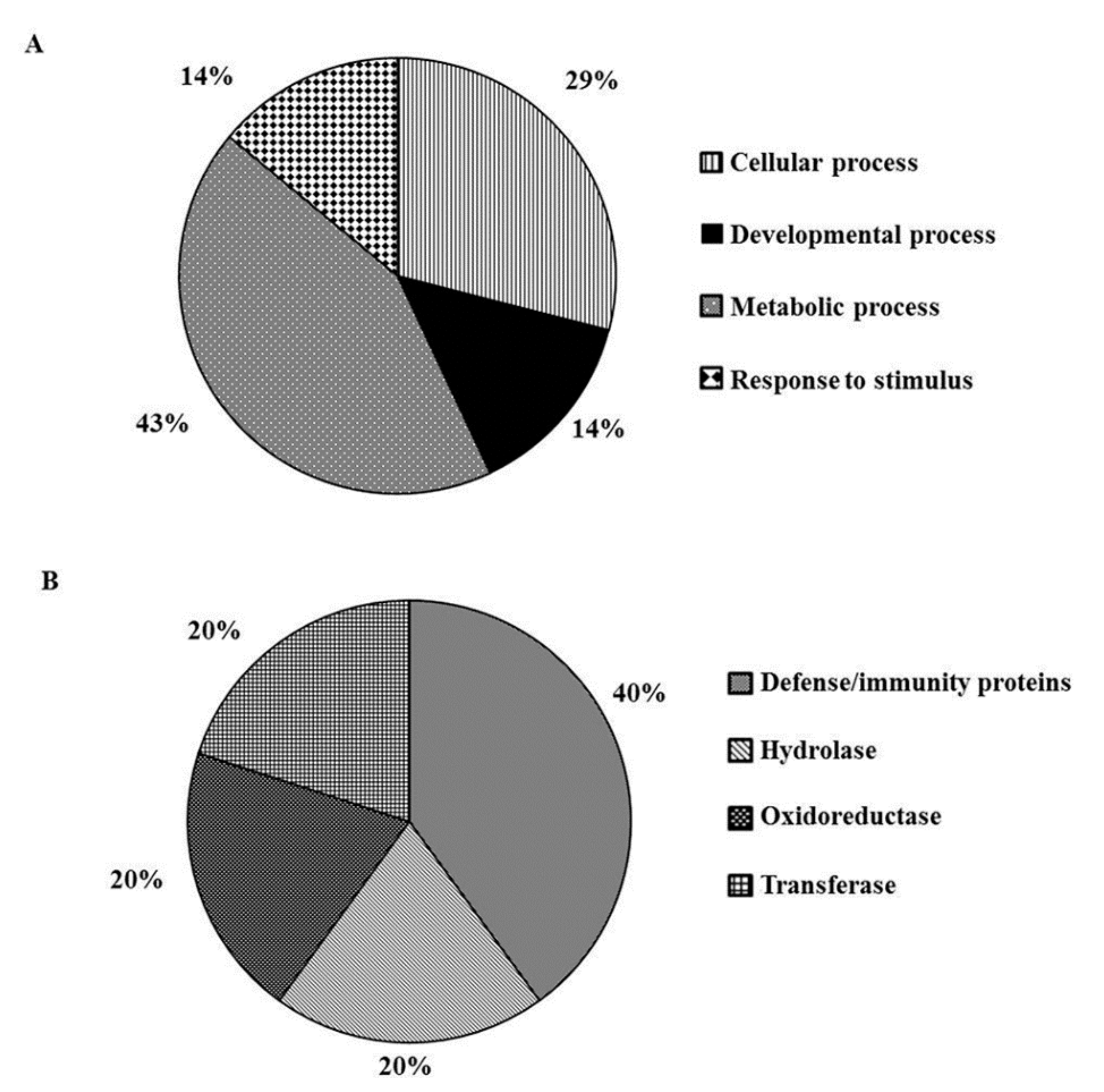
2.1. MeJA Treatment Modulates Broad Spectrum Biological Processes
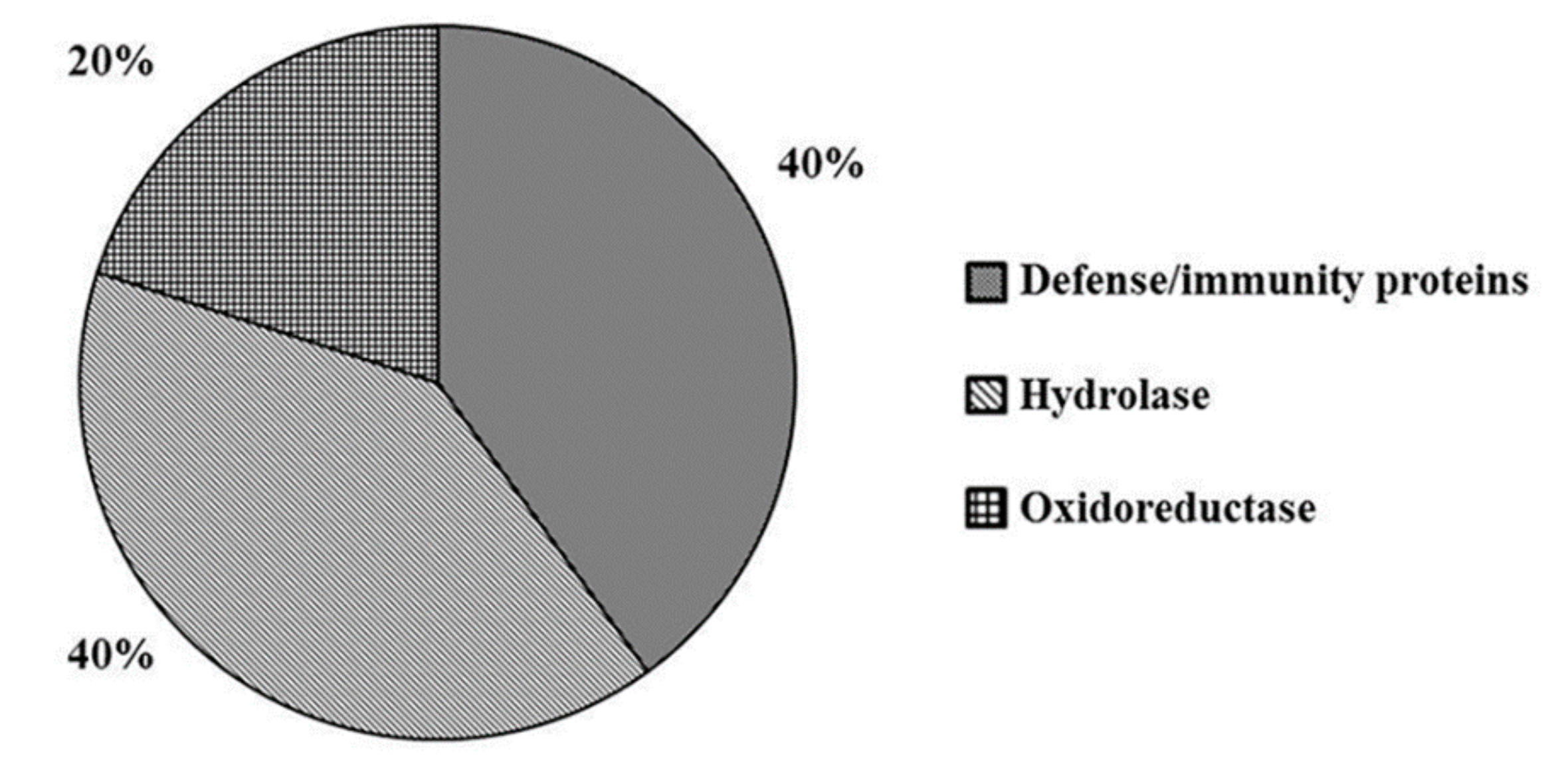
2.2. Wounding Induces Proteome Changes on Immunity-Related Proteins and Enzymes
2.3. Combined MeJA Treatment and Wounding Affect the Level of Proteins Related to Defense Processes
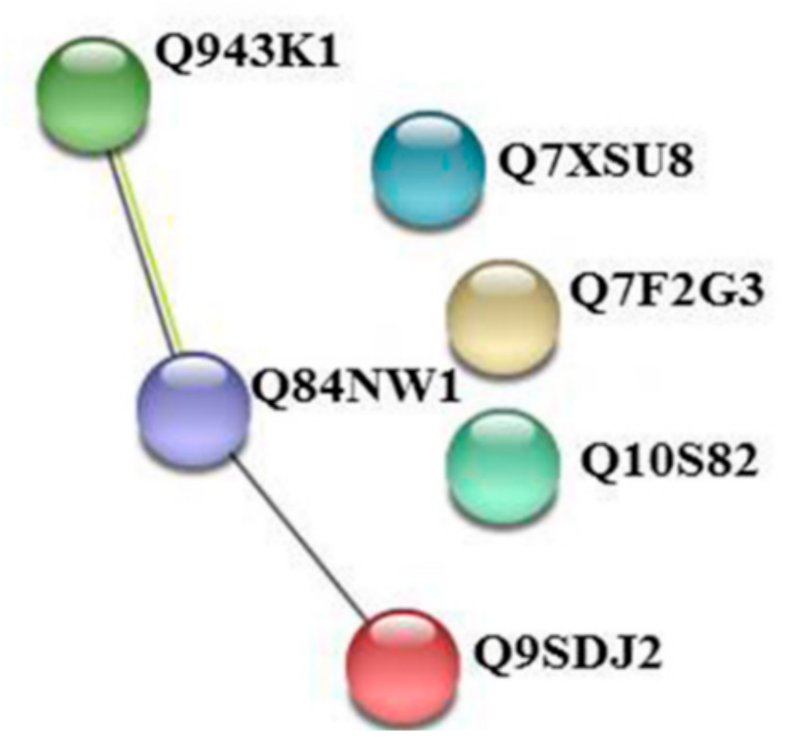
2.4. Priming-Regulated Proteins Correlate with Defense Processes
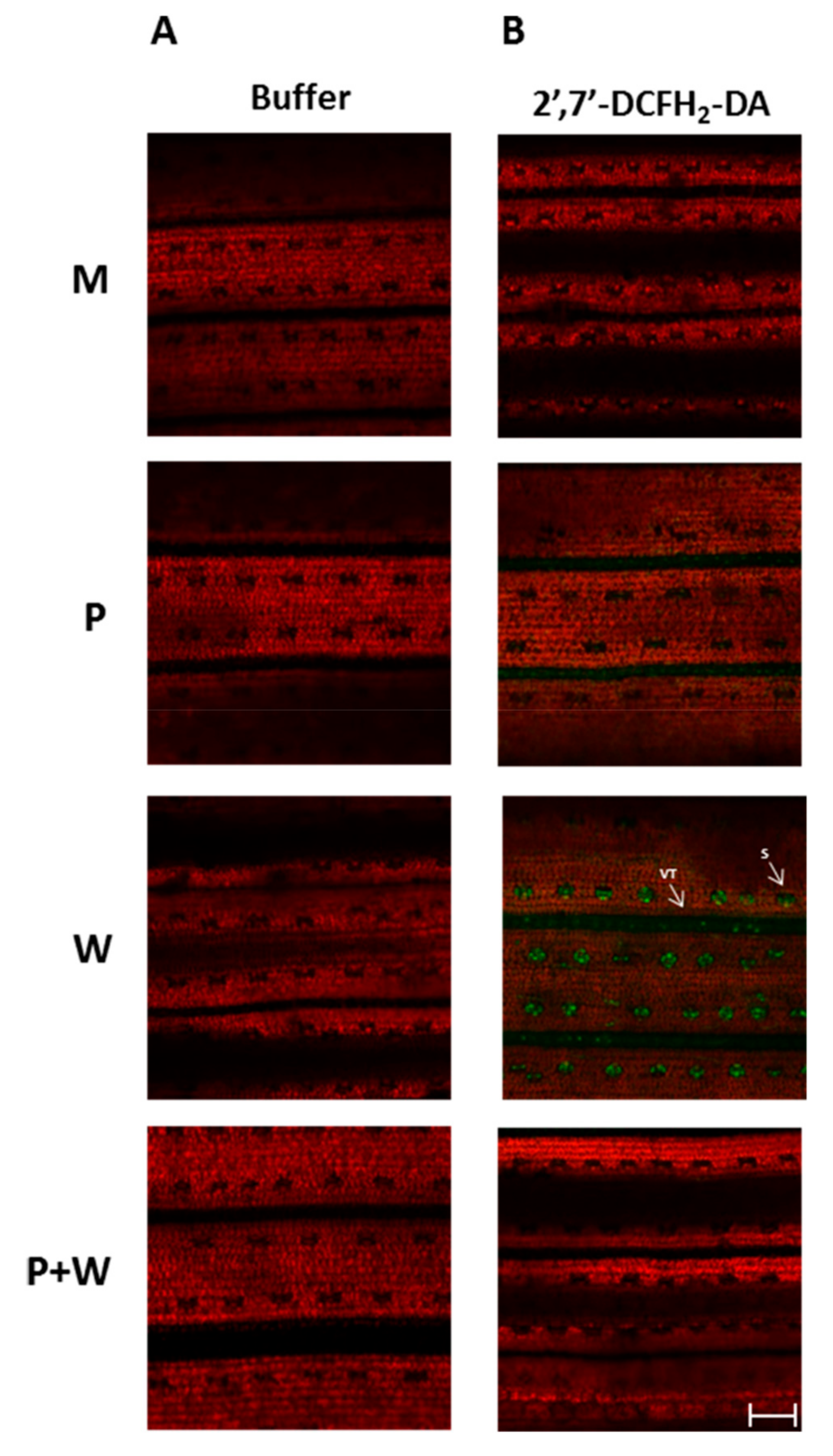
2.5. MeJA Protects Plants from Effects of Wounding-Triggered H2O2 Production
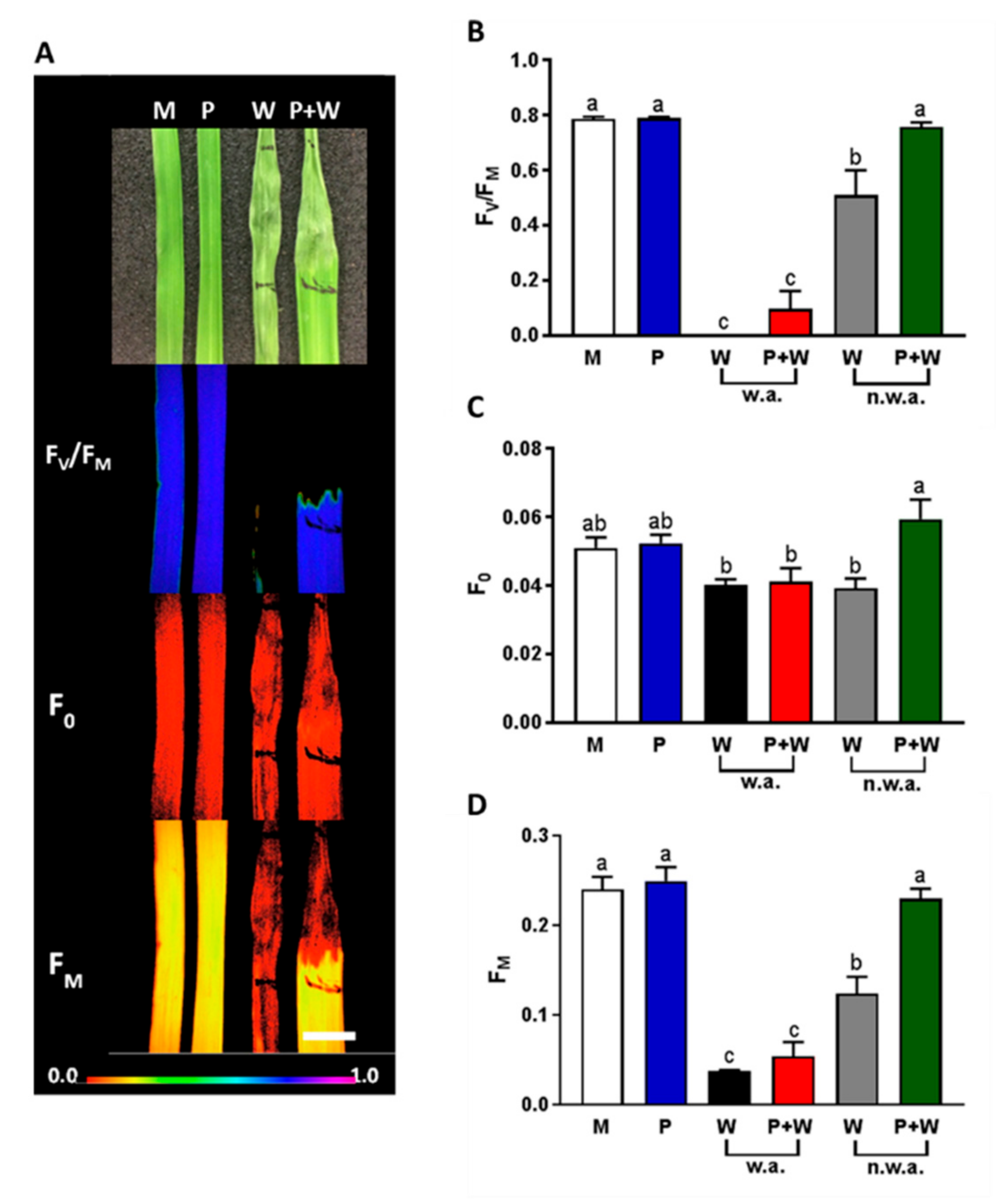
2.6. MeJA Protects Plants from Photosynthetic Damage
3. Materials and Methods
3.1. Plant Material and Treatments
3.2. Protein Sample Preparation
3.3. Proteomic Profiling and Data Analysis
3.4. ROS Detection in Rice Leaves
3.5. Chlorophyll Fluorescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MeJA | Methyl-jasmonate |
| M | Mock |
| P | MeJA- treated plants |
| W | Wounded plants |
| P + W | Wounded plants after MeJA treatment |
References
- Rasmann, S.; De Vos, M.; Jander, G. Ecological role of transgenerational resistance against biotic threats. Plant Signal. Behav. 2012, 7, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Mescher, M.C.; Carlson, J.E.; De Moraes, C.M. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol. 2008, 146, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Aranega-Bou, P.; De la, O.; Leyva, M.; Finiti, I.; García-Agustín, P.; González-Bosch, C. Priming of plant resistance by natural compounds. Hexanoic acid as a model. Front. Plant Sci. 2014, 5, 488. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone crosstalk in plant disease and defense:more than just JASMONATE-SALICYLATE antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signaling. J. Exp. Bot. 2013, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Pieterse, C.M.J.; Van Wees, S.C.M. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55. [Google Scholar]
- Howe, G.A.; Lightner, J.; Browse, J.; Ryan, C.A. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 1996, 8, 2067–2077. [Google Scholar] [CrossRef] [PubMed]
- McConn, M.; Creelman, R.A.; Bell, E.; Mullet, J.E.; Browse, J. Jasmonate is essential for insect defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 5473–5477. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, C.; Lee, G.I.; Howe, G.A. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc. Natl. Acad. Sci. USA 2002, 99, 6416–6421. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Howe, G.A. The tomato mutant spr1 is defective in systemin perception and the production of a systemic wound signal for defense gene expression. Plant J. 2003, 33, 567–576. [Google Scholar] [CrossRef]
- Conrath, U. Systemic Acquired Resistance. Plant Signal. Behav. 2006, 1, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef]
- Conrath, U. Molecular aspects of defence priming. Trends Plant Sci. 2011, 16, 524–531. [Google Scholar] [CrossRef]
- Jaskiewicz, M.; Conrath, U.; Peterhänsel, C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep. 2011, 12, 50–55. [Google Scholar] [CrossRef]
- Slaughter, A.; Daniel, X.; Flors, V.; Luna, E.; Hohn, B.; Mauch-Mani, B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 2012, 158, 835–843. [Google Scholar] [CrossRef]
- Tanou, G.; Fotopoulos, V.; Molassiotis, A. Priming against environmental challenges and proteomics in plants: Update and agricultural perspectives. Front. Plant Sci. 2012, 3, 216. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Bäurle, I. Plant Heat Adaptation: Priming in response to heat stress. F1000Research 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Švecová, E.; Proietti, S.; Caruso, C.; Colla, G.; Crinò, P. Antifungal activity of Vitex agnus-castus extract against Pythium ultimum in tomato. Crop Prot. 2013, 43, 223–230. [Google Scholar] [CrossRef]
- De Palma, M.; D’Agostino, N.; Proietti, S.; Bertini, L.; Lorito, M.; Ruocco, M.; Caruso, C.; Chiusano, M.L.; Tucci, M. Suppression Subtractive Hybridization analysis provides new insights into the tomato (Solanum lycopersicum L.) response to the plant probiotic microorganism Trichoderma longibrachiatum MK1. J. Plant Physiol. 2016, 190, 79–94. [Google Scholar] [CrossRef]
- Beckers, G.J.M.; Conrath, U. Priming for stress resistance: From the lab to the field. Curr. Opin. Plant Biol. 2007, 10, 425–431. [Google Scholar] [CrossRef]
- Beckers, G.J.; Jaskiewicz, M.; Liu, Y.; Underwood, W.R.; He, S.Y.; Zhang, S.; Conrath, U. Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell 2009, 21, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Pastor, V.; Luna, E.; Ton, J.; Cerezo García, M.; García Agustín, P.; Flors, V. Fine tuning of reactive oxygen species homeostasis regulates primed immune responses in Arabidopsis. Mol. Plant Microbe Interact. 2013, 26, 1334–1344. [Google Scholar] [CrossRef]
- Gamir, J.; Sánchez-Bel, P.; Flors, V. Molecular and physiological stages of priming: How plants prepare for environmental challenges. Plant Cell Rep. 2014, 33, 1935–1949. [Google Scholar] [CrossRef]
- Ton, J.; Mauch-Mani, B. Amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. Plant J. 2004, 38, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Tarighi, S. Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. J. Plant Physiol. 2010, 167, 201–208. [Google Scholar] [CrossRef]
- Luzzatto, T.; Golan, A.; Yishay, M.; Bilkis, I.; Ben-Ari, J.; Yedidia, I. Priming of antimicrobial phenolics during induced resistance response towards Pectobacterium carotovorum in the ornamental monocot calla lily. J. Agric. Food Chem. 2007, 55, 10315–10322. [Google Scholar] [CrossRef]
- Ding, Y.; Fromm, M.; Avramova, Z. Multiple exposures to drought ‘train’ transcriptional responses in Arabidopsis. Nat. Commun. 2012, 3, 740. [Google Scholar] [CrossRef]
- Sani, E.; Herzyk, P.; Perrella, G.; Colot, V.; Amtmann, A. Hyperosmotic priming of Arabidopsis seedlings establishes a long-term somatic memory accompanied by specific changes of the epigenome. Genome Biol. 2013, 14, R59. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Bruce, T.J.A.; Matthes, M.C.; Napier, J.A.; Pickett, J.A. Stressful ‘memories’ of plants: Evidence and possible mechanisms. Plant Sci. 2007, 173, 603–608. [Google Scholar] [CrossRef]
- Luna, E.; Bruce, T.J.A.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 317–327. [Google Scholar] [CrossRef]
- Kathiria, P.; Sidler, C.; Golubov, A.; Kalischuk, M.; Kawchuk, L.M.; Kovalchuk, I. Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial, and fungal pathogens in the progeny of infected tobacco plants. Plant Physiol. 2010, 153, 1859–1870. [Google Scholar] [CrossRef]
- Liu, Y.; Bino, R.J.; Van der Burg, W.J.; Groot, S.P.C.; Hilhorst, H.W.M. Effects of osmotic priming on dormancy and storability of tomato (Lycopersicon esculentum Mill.) seeds. Seed Sci. Res. 1996, 6, 49–55. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Paparella, S.; Arau, J.S.S.; Rossi, G.; Wijayasinghe, M.; Carbonera, D.; Balestrazzi, A. Seed priming: State of the art and new perspectives. Plant Cell Rep. 2015, 34, 1281–1293. [Google Scholar] [CrossRef]
- Hussain, S.; Yin, H.; Peng, S.; Khan, F.A.; Khan, F.; Sameeullah, M.; Hussain, H.A.; Huang, J.; Cui, K.; Nie, L. Comparative transcriptional profiling of primed and non-primed rice seedlings under submergence stress. Front. Plant Sci. 2016, 7, 1125. [Google Scholar] [CrossRef]
- Proietti, S.; Bertini, L.; Timperio, A.M.; Zolla, L.; Caporale, C.; Caruso, C. Crosstalk between salicylic acid and jasmonate in Arabidopsis investigated by an integrated proteomic and transcriptomic approach. Mol. Biosyst. 2013, 9, 1169–1187. [Google Scholar] [CrossRef]
- Kushalappa, A.C.; Gunnaiah, R. Metabolo-proteomics to discover plant biotic stress resistance genes. Trends Plant Sci. 2013, 18, 522–531. [Google Scholar] [CrossRef]
- Obata, T.; Fernie, A.R. The use of metabolomics to dissect plant responses to abiotic stresses. Cell Mol. Life Sci. 2012, 69, 3225–3243. [Google Scholar] [CrossRef]
- Gupta, B.; Saha, J.; Sengupta, A.; Gupta, K. Plant Abiotic Stress: ‘Omics’ Approach. J. Plant Biochem. Physiol. 2013, 1, e108. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Li, G.; Wu, Y.; Liu, G.; Xiao, X.; Wang, P.; Gao, T.; Xu, M.; Han, Q.; Wang, Y.; Guo, T.; Kang, G. Large-scale Proteomics Combined with Transgenic Experiments Demonstrates an Important Role of Jasmonic Acid in Potassium Deficiency Response in Wheat and Rice. Mol. Cell. Proteom. 2017, 16, 1889–1905. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Zhang, Y.L.; Chen, S.X.; Yin, G.H.; Yang, Z.Z.; Lee, S.; Liu, C.G.; Zhao, D.D.; Ma, Y.K.; Song, F.Q.; et al. Proteomics of methyl jasmonate induced defense response in maize leaves against Asian corn borer. BMC Genom. 2015, 16, 224. [Google Scholar] [CrossRef]
- Dhakarey, R.; Raorane, M.L.; Treumann, A.; Peethambaran, P.K.; Schendel, R.R.; Sahi, V.P.; Hause, B.; Bunzel, M.; Henry, A.; Kohli, A.; et al. Physiological and Proteomic Analysis of the Rice Mutant cpm2 Suggests a Negative Regulatory Role of Jasmonic Acid in Drought Tolerance. Front Plant Sci. 2017, 8, 1903. [Google Scholar] [CrossRef]
- Li, Y.; Nie, Y.; Zhang, Z.; Ye, Z.; Zou, X.; Zhang, L.; Wang, Z. Comparative proteomic analysis of methyl jasmonate-induced defense responses in different rice cultivars. Proteomics 2014, 14, 1088–1101. [Google Scholar] [CrossRef]
- Si, T.; Wang, X.; Wu, L.; Zhao, C.; Zhang, L.; Huang, M.; Cai, J.; Zhou, Q.; Dai, T.; Zhu, J.K.; et al. Nitric Oxide and Hydrogen Peroxide Mediate Wounding-Induced Freezing Tolerance through Modifications in Photosystem and Antioxidant System in Wheat. Front. Plant Sci. 2017, 8, 1284. [Google Scholar] [CrossRef]
- Savatin, D.V.; Gramegna, G.; Modesti, V.; Cervone, F. Wounding in the plant tissue: The defense of a dangerous passage. Front Plant Sci. 2014, 5, 470. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, L.; Morreel, K.; De Witte, E.; Lammertyn, F.; Van Montagu, M.; Boerjan, W.; Inzé, D.; Goossens, A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 2008, 105, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Bertini, L.; Proietti, S.; Focaracci, F.; Sabatini, B.; Caruso, C. Epigenetic control of defense genes following MeJA-induced priming in rice (O. sativa). J. Plant Physiol. 2018, 228, 166–177. [Google Scholar]
- Hickman, R.J.; Van Verk, M.C.; Van Dijken, A.J.H.; Pereira Mendes, M.; Vroegop-Vos, I.A.; Caarls, L.; Steenbergen, M.; Van der Nagel, I.; Wesselink, G.J.; Jironkin, A.; et al. Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell 2017, 29, 2086–2105. [Google Scholar] [CrossRef]
- Tobias, C.M.; Chow, E.K. Structure of the cinnamyl-alcohol dehydrogenase gene family in rice and promoter activity of a member associated with lignification. Planta 2005, 220, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H.J.; Hall, J.L.; Barber, M.S. Elicitor-induced cinnamyl alcohol dehydrogenase activity in lignifying wheat (Triticum aestivum L.) leaves. Plant Physiol. 1994, 104, 551–556. [Google Scholar] [CrossRef]
- Lynch, D.; Lidgett, A.; McInnes, R.; Huxley, H.; Jones, E.; Mahoney, N.; Spangenberg, G. Isolation and characterisation of three cinnamyl alcohol dehydrogenase homologue cDNAs from perennial ryegrass (Lolium perenne L.). J. Plant Physiol. 2002, 159, 653–660. [Google Scholar]
- Davidson, R.M.; Manosalva, P.M.; Snelling, J.; Bruce, M.; Leung, H.; Leach, J.E. Rice germin-like proteins: Allelic diversity and relationships to early stress responses. Rice 2010, 3, 43–55. [Google Scholar] [CrossRef]
- Grzelczak, Z.F.; Lane, B.G. Signal resistance of a soluble protein to enzymic proteolysis: An unorthodox approach to the isolation and purification of germin, a rare growth-related protein. Can. J. Biochem. Cell Biol. 1984, 62, 1351–1353. [Google Scholar] [CrossRef]
- Bernier, F.; Berna, A. Germins and germin-like proteins: Plant do-all proteins. But what do they do exactly? Plant Physiol. Biochem. 2001, 39, 545–554. [Google Scholar] [CrossRef]
- Lane, B.G. Oxalate, germins, and higher-plant pathogens. IUBMB Life 2002, 53, 67–75. [Google Scholar] [CrossRef]
- Dunwell, J.M.; Khuri, S.; Gane, P.J. Microbial relatives of the seed storage proteins of higher plants: Conservation of structure and diversification of function during evolution of the cupin superfamily. Microbiol. Mol. Biol. Rev. 2000, 64, 153–179. [Google Scholar] [CrossRef]
- Druka, A.; Kudrna, D.; Kannangara, C.G.; Wettstein, D.V.; Kleinhofs, A. Physical and genetic mapping of barley (Hordeum vulgare) germin-like cDNAs. Proc. Natl. Acad. Sci. USA 2002, 99, 850–855. [Google Scholar] [CrossRef]
- Lane, B.G.; Dunwell, J.M.; Ray, J.A.; Schmitt, M.R.; Cuming, A.C. Germin, a protein marker of early plant development, is an oxalate oxidase. J. Biol. Chem. 1993, 268, 12239–12242. [Google Scholar]
- Lane, B.G. Oxalate oxidases and differentiating surface structure in wheat: Germins. Biochem. J. 2000, 349, 309–321. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Baldwin, I.T. Silencing of a Germin-Like gene in Nicotiana attenuate improves performance of native herbivores. Plant Physiol. 2006, 140, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef]
- Moons, A.; Prinsen, E.; Bauw, G.; Van Montagu, M. Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. Plant Cell 1997, 9, 2243–2259. [Google Scholar] [CrossRef]
- Rakwal, R.; Komatsu, S. Role of jasmonate in the rice (Oryza sativa L.) self-defense mechanism using proteome analysis. Electrophoresis 2000, 21, 2492–2500. [Google Scholar] [CrossRef]
- Miché, L.; Battistoni, F.; Gemmer, S.; Belghazi, M.; Reinhold-Hurek, B. Upregulation of jasmonate-inducible defense proteins and differential colonization of roots of Oryza sativa cultivars with the endophyte Azoarcus sp. Mol. Plant Microb. Interact. 2006, 19, 502–511. [Google Scholar] [CrossRef]
- Xie, X.Z.; Xue, Y.J.; Zhou, J.J.; Zhang, B.; Chang, H.; Takano, M. Phytochromes regulate SA and JA signaling pathways in rice and are required for developmentally controlled resistance to Magnaporthe grisea. Mol. Plant 2011, 4, 688–696. [Google Scholar] [CrossRef]
- Cohen-Kupiec, R.; Chet, I. The molecular biology of chitin digestion. Curr. Opin. Biotechnol. 1998, 9, 270–277. [Google Scholar] [CrossRef]
- Guo, X.L.; Bai, L.R.; Su, C.Q.; Shi, L.R.; Wang, D.W. Molecular cloning and expression of drought-induced protein 3 (DIP3) encoding a class III chitinase in upland rice. Genet. Mol. Res. 2013, 12, 6860–6870. [Google Scholar] [CrossRef]
- Schlumbaum, A.; Mauch, F.; Vögeli, U.; Boller, T. Plant chitinases are potent inhibitors of fungal growth. Nature 1986, 12, 6860–6870. [Google Scholar] [CrossRef]
- Taira, T.; Toma, N.; Ishihara, M. Purification, characterization, and antifungal activity of chitinases from pineapple (Ananas comosus) leaf. Biosci. Biotechnol. Biochem. 2005, 69, 189–196. [Google Scholar] [CrossRef]
- Collinge, D.B.; Kragh, K.M.; Mikkelsen, J.D.; Nielsen, K.K.; Rasmussen, U.; Vad, K. Plant chitinases. Plant J. 1993, 3, 31–40. [Google Scholar] [CrossRef]
- Schweizer, P.; Buchala, A.; Silverman, P.; Seskar, M.; Raskin, I.; Métraux, J.-P. Jasmonate-inducible genes are activated in rice by pathogen attack without a concomitant increase in endogenous jasmonic acid levels. Plant Physiol. 1997, 114, 79–88. [Google Scholar] [CrossRef]
- Byeon, Y.; Choi, G.-H.; Lee, H.Y.; Back, K. Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice. J. Exp. Bot. 2015, 66, 6917–6925. [Google Scholar] [CrossRef]
- Tan, D.X. Preface: Melatonin and plants. J. Exp. Bot. 2015, 66, 625–626. [Google Scholar] [CrossRef]
- Lee, H.Y.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal. Res. 2014, 57, 262–268. [Google Scholar] [CrossRef]
- Kim, S.; Cho, K.S.; Kim, S.G.; Kang, S.Y.; Kang, K.Y. A rice isoflavone reductase-like gene, OsIRL, is induced by rice blast fungal elicitor. Mol. Cells 2003, 16, 224–231. [Google Scholar]
- Adie, B.A.T.; Perez-Perez, J.; Perez-Perez, M.M.; Godoy, M.; Sanchez-Serrano, J.J.; Schmelz, E.A.; Solano, R. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 2007, 19, 1665–1681. [Google Scholar] [CrossRef] [PubMed]
- Bodenhausen, N.; Reymond, P. Signaling pathways controlling induced resistance to insect herbivores in Arabidopsis. Mol. Plant Microb. Interact. 2007, 20, 1406–1420. [Google Scholar] [CrossRef]
- Mitsuhara, I.; Iwai, T.; Seo, S.; Yanagawa, Y.; Kawahigasi, H.; Hirose, S.; Ohkawa, Y.; Ohashi, Y. Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180). Mol. Genet. Genomics 2008, 279, 415–427. [Google Scholar] [CrossRef]
- Duff, S.M.G.; Gautam, S.; Plaxton, W.C. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant 1994, 90, 791–800. [Google Scholar] [CrossRef]
- Del Pozo, J.C.; Allona, I.; Rubio, V.; Leyva, A.; de la Peña, A.; Aragoncillo, C.; Paz-Ares, J. A type 5 acid phosphatase gene from Arabidopsis thaliana is induced by phosphate starvation and by some other types of phosphate mobilising/oxidative stress conditions. Plant J. 1999, 19, 579–589. [Google Scholar] [CrossRef]
- Liu, Y.; Ahn, J.-E.; Datta, S.; Salzman, R.A.; Moon, J.; Huyghues-Despointes, B.; Pittendrigh, B.; Murdock, L.L.; Koiwa, H.; Zhu-Salzman, K. Arabidopsis Vegetative Storage protein is an anti-insect acid phosphatase. Plant Physiol. 2005, 139, 1545–1556. [Google Scholar] [CrossRef]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Genevestigator V3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinform. 2008, 2008, 420747. [Google Scholar] [CrossRef]
- Tonfack, L.B.; Moummou, H.; Latché, A.; Youmbi, E.; Benichou, M.; Pech, J.C.; Van Der Rest, B. The plant SDR superfamily: Involvement in primary and secondary metabolism. Curr. Top. Plant Biol. 2011, 12, 41–53. [Google Scholar]
- Di Mario, R.J.; Clayton, H.; Mukherjee, A.; Ludwig, M.; Moroney, J.V. Plant Carbonic Anhydrases: Structures, Locations, Evolution, and Physiological Roles. Mol. Plant 2017, 10, 30–46. [Google Scholar] [CrossRef]
- Slaymaker, D.H.; Navarre, D.A.; Clark, D.; del Pozo, O.; Martin, G.B.; Klessig, D.F. The tobacco salicylic acid-binding protein 3 (SABP3) is the chloroplast carbonic anhydrase, which exhibits antioxidant activity and plays a role in the hypersensitive defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 11640–11645. [Google Scholar] [CrossRef]
- Restrepo, S.; Myers, K.L.; del Pozo, O.; Martin, G.B.; Hart, A.L.; Buell, C.R.; Fry, W.E.; Smart, C.D. Gene profiling of a compatible interaction between Phytophthora infestans and Solanum tuberosum suggests a role for carbonic anhydrase. Mol. Plant Microb. Interact. 2005, 18, 913–922. [Google Scholar] [CrossRef]
- Collins, R.M.; Afzal, M.; Ward, D.A.; Prescott, M.C.; Sait, S.M.; Rees, H.H.; Tomsett, A.B. Differential proteomic analysis of Arabidopsis thaliana genotypes exhibiting resistance or susceptibility to the insect herbivore, Plutella xylostella. PLoS ONE 2010, 5, e10103. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Sasaki, K.; Yuichi, O.; Hiraga, S.; Gotoh, Y.; Seo, S.; Mitsuhara, I.; Ito, H.; Matsui, H.; Ohashi, Y. Characterization of two rice peroxidase promoters that respond to blast fungus-infection. Mol. Genet. Genomics 2007, 278, 709–722. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef]
- Willekens, H.; Inze, D.; Van Montagu, M.; Van Camp, W. Catalases in plants. Mol. Breed. 1995, 1, 207–228. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. Hydrogen peroxide homeostasis: Activation of plant catalases by calcium/calmodulin. Proc. Natl. Acad. Sci. USA 2002, 99, 4097–4102. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, X.; Xue, M.; Zhang, X.; Li, Q. Antioxidant enzyme responses induced by whiteflies in tobacco plants in defense against aphids: Catalase may play a dominant role. PLoS ONE 2016, 11, e0165454. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.; Lee, Y.H.; Song, S.I. Rice CatA, CatB, and CatC are involved in environmental stress response, root growth, and photorespiration, respectively. J. Plant Biol. 2014, 57, 375–382. [Google Scholar] [CrossRef]
- González-Bosch, C. Priming plant resistance by activation of redox-sensitive genes. Free Radic. Biol. Med. 2018, 122, 171–180. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Carroll, M.J.; LeClere, S.; Phipps, S.M.; Meredith, J.; Chourey, P.S.; Alborn, H.T.; Teal, P.E. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. USA 2006, 103, 8894–8899. [Google Scholar] [CrossRef] [PubMed]
- Bollivar, D.W.; Beale, S.I. The Chlorophyll Biosynthetic Enzyme Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase (Characterization and Partial Purification from Chlamydomonas reinhardtii and Synechocystis sp. PCC 6803). Plant Physiol. 1996, 112, 105–114. [Google Scholar] [CrossRef]
- Bergantino, E.; Segalla, A.; Brunetta, A.; Teardo, E.; Rigoni, F.; Giacometti, G.M. Light- and pH-dependent structural changes in the PsbS subunit of photosystem II. Proc. Natl. Acad. Sci. USA 2003, 100, 15265–15270. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaji, K. Cross talk between abiotic and biotic stress responses: A current view from the point of convergence in the stress signalling network. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef]
- Yi, H.; Liu, X.; Yi, M.; Chen, G. Dual Role of Hydrogen Peroxide in Arabidopsis Guard Cells in Response to Sulfur Dioxide. Adv. Toxicol. 2014, 2014, 9. [Google Scholar] [CrossRef]
- Niu, L.; Liao, W. Hydrogen Peroxide Signaling in Plant Development and Abiotic Responses: Crosstalk with Nitric Oxide and Calcium. Front. Plant Sci. 2016, 7, 230. [Google Scholar] [CrossRef]
- Orozco-Cardenas, M.; Ryan, C.A. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 6553–6557. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Dong, F.; Gao, J.; Galbraith, D.W.; Song, C.-P. Hydrogen Peroxide Is Involved in Abscisic Acid-Induced Stomatal Closure in Vicia faba. Plant Physiol. 2001, 126, 1438–1448. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Swarbrick, P.J.; Scholes, J.D.; Rolfe, S.A. Imaging photosynthesis in wounded leaves of Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 55–69. [Google Scholar] [CrossRef]
- Tang, J.Y.; Zielinski, R.E.; Zangerl, A.R.; Crofts, A.R.; May, R.; Berenbaum, M.R.; DeLucia, E.H. The differential effects of herbivory by first and fourth instars of Trichoplusia ni (Lepidoptera: Noctuidae) on photosynthesis in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 527–536. [Google Scholar] [CrossRef]
- Scholes, J.D.; Rolfe, S.A. Chlorophyll fluorescence imaging as tool for understanding the impact of fungal diseases on plant performance; a phenomics perspective. Funct. Plant Biol. 2009, 36, 880–892. [Google Scholar] [CrossRef]
- McElrone, A.J.; Hamilton, J.G.; Krafnick, J.; Aldea, M.; Knepp, R.G.; DeLuica, E.H. Combined effects of elevated CO2 and natural climatic variation on leaf spot diseases of redbud and sweetgum trees. Environ. Pollut. 2010, 158, 108–114. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B. Chlorophyll fluorescence as a tool to monitor plant response to the environment. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 583–604. [Google Scholar]
- Melis, A. Photosystem II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Mittal, S.; Kumari, N.; Sharma, V. Differential response of salt stress on brassica juncea: Photosynthetic performance, pigment, proline, d1 and antioxidant enzymes. Plant Physiol. Biochem. 2012, 54, 17–26. [Google Scholar] [CrossRef]
- Ji, B.; Li, Z.; Gu, W.; Li, J.; Xie, T.; Wei, S. Methyl jasmonate pretreatment promotes the growth and photosynthesis of maize seedlings under saline conditions by enhancing the antioxidant defense system. Int. J. Agric. Biol. 2018, 20, 1454–1462. [Google Scholar]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Thomas, P.D.; Campbell, M.J.; Kejariwal, A.; Mi, H.; Karlak, B.; Daverman, R.; Diemer, K.; Muruganujan, A.; Narechania, A. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res. 2003, 13, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Proietti, S.; Giangrande, C.; Amoresano, A.; Pucci, P.; Molinaro, A.; Bertini, L.; Caporale, C.; Caruso, C. Xanthomonas campestris lipooligosaccharides trigger innate immunity and oxidative burst in Arabidopsis. Plant Physiol. Biochem. 2014, 85, 51–62. [Google Scholar] [CrossRef] [PubMed][Green Version]


| UniProt Code | MSU ID Code | Protein Name | Log2 Fold-Change | p-Value |
|---|---|---|---|---|
| Q10D65 | LOC_Os03g52860 | Lipoxygenase Linoleate 9S-lipoxygenase 2 | 6.66 | 0.018 |
| Q53LW0 | LOC_Os11g20160 | O-methyltransferase | 4.59 | 0.013 |
| Q01HV9 | no code | Arginine decarboxylase | 3.67 | 0.005 |
| Q0JR25 | LOC_Os01g03360 | Bowman–Birk type bran trypsin inhibitor | 3.25 | 0.014 |
| B7E4J4 | LOC_Os05g31750 | Os05g0382600 | 2.98 | 0.02 |
| Q5WMX0 | LOC_Os05g15770 | DIP3 | 2.58 | 0.002 |
| Q5Z678 | LOC_Os06g47620 | IAA-amino acid hydrolase ILR1-like 6 | 2.41 | 0.037 |
| Q8LMW8 | LOC_Os10g11500 | Os10g0191300 protein (putative PRB1-2) | 2.26 | 0.022 |
| Q5U1I3 | no code | Peroxidase | 2.24 | 0.01 |
| Q8S3P3 | LOC_Os04g56430 | DUF26-like protein | 2.23 | 0.018 |
| Q69JF3 | LOC_Os09g36700 | Os9g0538000 | 2.14 | 0.016 |
| Q7XAD8 | no code | Os07g0126400 protein (putative Prb1) | 2.07 | 0.024 |
| Q5ZCA9 | no code | Bowman–Birk type bran trypsin inhibitor (Fragment) | 2.0 | 0.008 |
| Q6YZZ7 | LOC_Os08g08970 | Germin-like protein 8-3 | 2.0 | 0.015 |
| Q75T45 | LOC_Os12g36830 | Os12g0555000 (root PR10) | 1.77 | 0.014 |
| Q8L6H4 | LOC_Os03g32314 | Allene oxide cyclase, chloroplastic | 1.72 | 0.05 |
| Q6YXT5 | LOC_Os08g02230 | Os08g0114300 protein | 1.58 | 0.02 |
| Q33E23 | LOC_Os04g45970 | Glutamate dehydrogenase 2, mitochondrial | 1.33 | 0.024 |
| Q40707 | LOC_Os12g36880 | PBZ1 | 1.26 | 0.01 |
| B9F4F6 | no code | Citrate synthase | 1.1 | 0.046 |
| Q9ATR3 | no code | Glucanase | 1.07 | 0.0012 |
| Q0JG75 | LOC_Os01g71190 | Photosystem II reaction center Psb28 protein | −1.09 | 0.002 |
| C5MRM9 | no code | PsbA (Fragment) | −1.09 | 0.019 |
| A2YVX9 | no code | Putative uncharacterized protein (Germin-like protein 8-14) | −1.09 | 0.044 |
| Q5QLS1 | LOC_Os01g47780 | Arabinogalactan protein-like | −1.1 | 0.047 |
| B7EKW3 | LOC_Os07g26690 | Aquaporin | −1.18 | 0.03 |
| Q5Z5A8 | LOC_Os06g51330 | Photosystem II stability/assembly factor HCF136 chloroplastic | −1.32 | 0.045 |
| Q6ERW9 | LOC_Os09g23540 | Probable cinnamyl alcohol dehydrogenase 8B | −1.36 | 0.041 |
| Q7F8S5 | LOC_Os02g09940 | Peroxiredoxin-2E-2, chloroplastic | −1.64 | 0.029 |
| B9FY06 | LOC_Os07g38300 | Ribosome-recycling factor, chloroplastic | −1.89 | 0.014 |
| H2KW47 | LOC_Os11g13890 | Chlorophyll A-B binding protein, chloroplastic | −1.94 | 0.014 |
| J3RG68 | no code | Photosystem I iron-sulfur center | −2.06 | 0.013 |
| UniProt Code | MSU ID Code | Protein Name | Log2 Fold-Change | p-Value |
|---|---|---|---|---|
| Q306J3 | LOC_Os12g14440 | Dirigent protein | 5.11 | 0.001 |
| Q10D65 | LOC_Os03g52860 | Linoleate 9S-lipoxygenase 2 | 4.17 | 0.019 |
| Q75T45 | LOC_Os12g36830 | Os12g0555000 (root PR10) | 3.52 | 0.001 |
| Q945E9 | LOC_Os03g18850 | JIOsPR10 | 2.38 | 0.011 |
| Q40707 | LOC_Os12g36880 | PBZ1 | 2.04 | 0.035 |
| Q5WMX0 | LOC_Os05g15770 | DIP3 | 1.63 | 0.001 |
| Q8S3P3 | LOC_Os04g56430 | DUF26-like protein | 1.63 | 0.012 |
| Q5ZCA9 | no code | Bowman–Birk type bran trypsin inhibitor (Fragment) | 1.32 | 0.007 |
| Q0JR25 | LOC_Os01g03360 | Bowman–Birk type bran trypsin inhibitor | 1.2 | 0.025 |
| Q7XAD8 | no code | Os07g0126400 protein (putative Prb1) | 1.14 | 0.036 |
| Q9FTN5 | LOC_Os01g01660 | Os01g0106400 (putative isoflavone) | −1.56 | 0.044 |
| UniProt Code. | MSU ID Code | Protein Name | Log2Fold-Change | p-Value |
|---|---|---|---|---|
| Q10D65 | LOC_Os03g52860 | Linoleate 9S-lipoxygenase 2 | 5.3 | 0.015 |
| Q5U1I3 | no code | Peroxidase | 5.1 | 0.039 |
| Q306J3 | LOC_Os12g14440 | Dirigent protein | 4.3 | 0.005 |
| Q8S3P3 | LOC_Os04g56430 | DUF26-like protein | 3.2 | 0.01 |
| Q01HV9 | no code | Arginine decarboxylase | 3.1 | 0.033 |
| Q5WMX0 | LOC_Os05g15770 | DIP3 | 3.0 | 0.01 |
| Q9ATR3 | no code | Glucanase | 2.9 | 0.043 |
| B7E4J4 | LOC_Os05g31750 | Os05g0382600 | 2.7 | 0.016 |
| Q10N98 | LOC_Os03g16950 | 33 kDa secretory protein, putative expressed | 2.7 | 0.042 |
| Q75T45 | LOC_Os12g36830 | Os12g0555000 (root PR10) | 2.7 | 0.018 |
| Q69JX7 | LOC_Os09g36680 | Drought-induced S-like ribonuclease | 2.7 | 0.017 |
| Q7XAD8 | no code | Os07g0126400 protein (putative Prb1) | 2.4 | 0.029 |
| Q8LMW8 | LOC_Os10g11500 | Os10g0191300 protein (putative PRB1-2) | 2.2 | 0.001 |
| Q0JR25 | LOC_Os01g03360 | Bowman–Birk type bran trypsin inhibitor | 2.2 | 0.037 |
| Q69JF3 | LOC_Os09g36700 | Os09g0538000 | 2.1 | 0.008 |
| Q6YXT5 | LOC_Os08g02230 | Os08g0114300 protein | 1.9 | 0.008 |
| Q5ZCA9 | no code | Bowman–Birk type bran trypsin inhibitor (Fragment) | 1.8 | 0.005 |
| Q8L6H4 | LOC_Os03g32314 | Allene oxide cyclase, chloplastic | 1.7 | 0.031 |
| Q5Z7J2 | LOC_Os06g35520 | Peroxidase | 1.7 | 0.033 |
| Q40707 | no code | PBZ1 | 1.7 | 0.037 |
| Q33E23 | LOC_Os04g45970 | Glutamate dehydrogenase 2, mitochondrial | 1.6 | 0.013 |
| Q6ZI95 | LOC_Os08g41880 | Purple acid phosphatase | 1.6 | 0.036 |
| Q0D3V1 | no code | Os07g0664300 protein | 1.6 | 0.046 |
| B9F4F6 | no code | Citrate synthase | 1.6 | 0.024 |
| S4U072 | LOC_Os04g39150 | OSJNBb0048E02.12 protein | −1.0 | 0.023 |
| Q10A54 | LOC_Os10g05069 | Alpha-mannosidase | −2.3 | 0.047 |
| UniProt Code | MSU ID Code | Protein Name | Log2 Fold-Change (P + W/W) | p-Value | Log2 Fold-Change (P + W/P) | p-Value |
|---|---|---|---|---|---|---|
| Q7F2G3 | LOC_Os01g45274 | Carbonic anhydrase, chloroplast precursor, putative, expressed | 1.48 | 0.006 | 1.33 | 0.002 |
| Q943K1 | LOC_Os01g64960 | Chlorophyll A-B binding protein, putative, expressed | 1.22 | 9.29 × 10−5 | 1.21 | 0.001 |
| Q84NW1 | LOC_Os07g32880 | ATP synthase gamma chain, putative, expressed | 1.13 | 0.015 | 1.32 | 0.027 |
| Q9SDJ2 | LOC_Os01g17170 | Magnesium-protoporphyrin IX monomethyl ester cyclase, chloroplast precursor, putative, expressed | 1.54 | 0.003 | 1.42 | 0.005 |
| Q10S82 | LOC_Os03g03910 | Catalase domain containing protein | 1.33 | 0.005 | 1.48 | 0.007 |
| Q7XSU8 | LOC_Os04g59190 | Peroxidase precursor, putative, expressed | 1.21 | 0.049 | 1.14 | 0.028 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertini, L.; Palazzi, L.; Proietti, S.; Pollastri, S.; Arrigoni, G.; Polverino de Laureto, P.; Caruso, C. Proteomic Analysis of MeJa-Induced Defense Responses in Rice against Wounding. Int. J. Mol. Sci. 2019, 20, 2525. https://doi.org/10.3390/ijms20102525
Bertini L, Palazzi L, Proietti S, Pollastri S, Arrigoni G, Polverino de Laureto P, Caruso C. Proteomic Analysis of MeJa-Induced Defense Responses in Rice against Wounding. International Journal of Molecular Sciences. 2019; 20(10):2525. https://doi.org/10.3390/ijms20102525
Chicago/Turabian StyleBertini, Laura, Luana Palazzi, Silvia Proietti, Susanna Pollastri, Giorgio Arrigoni, Patrizia Polverino de Laureto, and Carla Caruso. 2019. "Proteomic Analysis of MeJa-Induced Defense Responses in Rice against Wounding" International Journal of Molecular Sciences 20, no. 10: 2525. https://doi.org/10.3390/ijms20102525
APA StyleBertini, L., Palazzi, L., Proietti, S., Pollastri, S., Arrigoni, G., Polverino de Laureto, P., & Caruso, C. (2019). Proteomic Analysis of MeJa-Induced Defense Responses in Rice against Wounding. International Journal of Molecular Sciences, 20(10), 2525. https://doi.org/10.3390/ijms20102525





