Cashew Tree Pollen: An Unknown Source of IgE-Reactive Molecules
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Pollen Extract Preparation
4.3. Mass Spectrometry, Protein Identification, and GO Annotations
4.4. 1D and 2D Gel Electrophoresis
4.5. 1D and 2D Immunoblot
4.6. ELISA (Enzyme-Linked Immunosorbent Assay)
4.7. Phylogenetic Tree Construction
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roberts, G.; Xatzipsalti, M.; Borrego, L.M.; Custovic, A.; Halken, S.; Hellings, P.W.; Papadopoulos, N.G.; Rotiroti, G.; Scadding, G.; Timmermans, F.; et al. Paediatric rhinitis: Position paper of the European Academy of Allergy and Clinical Immunology. Allergy 2013, 68, 1102–1116. [Google Scholar] [CrossRef] [PubMed]
- Taketomi, E.A.; Sopelete, M.C.; de Sousa Moreira, P.F.; de Assis Machado Vieira, F. Pollen allergic disease: Pollens and its major allergens. Braz. J. Otorhinolaryngol. 2006, 72, 562–567. [Google Scholar] [CrossRef]
- ASBAI. Associção Brasileira de Alergia e Imunopatologia—Informações ao Público—Polinose. 2009. Available online: http://www.sbai.org.br/secao.asp?s=81&id=300 (accessed on 24 June 2016).
- D’Amato, G.; Cecchi, L.; Bonini, S.; Nunes, C.; Annesi-Maesano, I.; Behrendt, H.; Liccardi, G.; Popov, T.; van Cauwenberge, P. Allergenic pollen and pollen allergy in Europe. Allergy 2007, 62, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Carini, A. Existe a febre do feno no Brazil? Rev. Med. São Paulo 1908, 11, 23–26. [Google Scholar]
- Rosário Filho, N.A. Análise de 50 casos de polinose por gramíneas. Rev. Bras. Alergia e Imunopatol. 1987, 10, 25–32. [Google Scholar]
- Dutra, B.M.R.S.; Filho, N.A.R.; Zavadniak, A.F. Alérgenos inaláveis em Curitiba: Uma revisão de sua relevância clínica. Rev. Bras. Alerg Imunopatol. 2001, 24, 89–195. [Google Scholar]
- Jutel, M.; Agache, I.; Bonini, S.; Burks, A.W.; Calderon, M.; Canonica, W.; Cox, L.; Demoly, P.; Frew, A.J.; O’Hehir, R.; et al. International consensus on allergy immunotherapy. J. Allergy Clin. Immunol. 2015, 136, 556–568. [Google Scholar] [CrossRef]
- Valenta, R.; Linhart, B.; Swoboda, I.; Niederberger, V. Recombinant allergens for allergen-specific immunotherapy: 10 years anniversary of immunotherapy with recombinant allergens. Allergy 2011, 66, 775–783. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Dramburg, S.; Potapova, E.; Skevaki, C.; Renz, H. Molecular diagnosis for allergen immunotherapy. J. Allergy Clin. Immunol. 2019, 143, 831–843. [Google Scholar] [CrossRef]
- ACR-Institute for Tropical and Subtropical Crops SA. Cultivating Cashew Nuts. 2015. Available online: https://www.nda.agric.za/docs/Infopaks/cashew.htm (accessed on 13 April 2019).
- Embrapa. Cultivo do Cajueiro 2003. Available online: https://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Caju/CultivodoCajueiro/ (accessed on 15 May 2017).
- Reitsma, M.; Bastiaan-Net, S.; Sforza, S.; van der Valk, J.P.; van Gerth van Wijk, R.; Savelkoul, H.F.; de Jong, N.W.; Wichers, H.J. Purification and Characterization of Anacardium occidentale (Cashew) Allergens Ana o 1, Ana o 2, and Ana o 3. J. Agric. Food Chem. 2016, 64, 1191–1201. [Google Scholar] [CrossRef]
- Comstock, S.S.; Kshirsagar, H.; Robotham, J.M.; Roux, K.H.; Sathe, S.K.; Teuber, S.S. IgE-Reactive Proteins in Cashew Apple Juice Concentrate are Removed by Filtration. J. Allergy Clin. Immunol. 2006, 117, S49. [Google Scholar] [CrossRef]
- Mitsumoto, K.; Yabusaki, K.; Aoyagi, H. Classification of pollen species using autofluorescence image analysis. J. Biosci. Bioeng. 2009, 107, 90–94. [Google Scholar] [CrossRef]
- McCrone, W.C.; Delly, J.G. Ultraviolet (365 nm) and blue-violet fluorescence analysis of particles. In The Particle Atlas, 2nd ed.; Ann Arbor Science Publishers: Ann Arbor, MI, USA, 1973. [Google Scholar]
- Vieths, S.; Frank, E.; Scheurer, S.; Meyer, H.E.; Hrazdina, G.; Haustein, D. Characterization of a new IgE-binding 35-kDa protein from birch pollen with cross-reacting homologues in various plant foods. Scand. J. Immunol. 1998, 47, 263–272. [Google Scholar] [CrossRef]
- Huecas, S.; Villalba, M.; Rodriguez, R. Ole e 9, a major olive pollen allergen is a 1,3-β-glucanase—Isolation, characterization, amino acid sequence, and tissue specificity. J. Biol. Chem. 2001, 276, 27959–27966. [Google Scholar] [CrossRef]
- Gruehn, S.; Suphioglu, C.; O’Hehir, R.E.; Volkmann, D. Molecular cloning and characterization of hazel pollen protein (70 kD) as a luminal binding protein (BiP): A novel cross-reactive plant allergen. Int. Arch Allergy Immunol. 2003, 131, 91–100. [Google Scholar] [CrossRef]
- Postigo, I.; Guisantes, J.A.; Negro, J.M.; Rodriguez-Pacheco, R.; David-Garcia, D.; Martinez, J. Identification of 2 new allergens of Phoenix dactylifera using an immunoproteomics approach. J. Invest. Allergol. Clin. Immunol. 2009, 19, 504–507. [Google Scholar]
- Saha, B.; Bhattacharya, S.G. Charting novel allergens from date palm pollen (Phoenix sylvestris) using homology driven proteomics. J. Proteom. 2017, 165, 1–10. [Google Scholar] [CrossRef]
- Driessen, M.; Quanjer, P. Pollen Deposition in Intrathoracic Airways. Eur. Respir. J. 1991, 4, 359–363. [Google Scholar]
- Pinnick, R.G.; Hill, S.C.; Nachman, P.; Pendleton, J.D.; Fernandez, G.L.; Mayo, M.W.; Bruno, J.G. Fluorescence Particle Counter for Detecting Airborne Bacteria and Other Biological Particles. Aerosol. Sci. Technol. 2007, 4, 653–664. [Google Scholar] [CrossRef]
- Aronne, G.; Cavuoto, D.; Eduardo, P. Classification and counting of fluorescent pollen using an image analysis system. Biotech. Histochem. 2001, 76, 35–40. [Google Scholar] [CrossRef]
- Fonseca, A.E.; Westgate, M.E.; Doyle, R.T. Application of fluorescence microscopy and image analysis for quantifying dynamics of maize pollen shed. Crop Sci. 2002, 42, 2201–2206. [Google Scholar] [CrossRef]
- Ronneberger, O.; Schultz, E.; Burkhardt, H. Automated pollen recognition using 3D volume images from fluorescence microscopy. Aerobiologia 2002, 18, 107–115. [Google Scholar] [CrossRef]
- Fernandes, L.; Mesquita, A.M. Anacardium-Occidentale (Cashew) Pollen Allergy in Patients with Allergic Bronchial-Asthma. J. Allergy Clin. Immunol. 1995, 95, 501–504. [Google Scholar] [CrossRef]
- Menezes, E.A.; Tome, E.R.; Nunes, R.N.; Nunes, A.P.; Freire, C.C.F.; Torres, J.C.N.; Castro, F.M.; Croce, J. Extracts of Anacardium occidentale (cashew) pollen in patients with allergic bronchial asthma. J. Investig. Allergol. Clin. Immunol. 2002, 12, 25–28. [Google Scholar]
- Pablos, I.; Eichhorn, S.; Briza, P.; Asam, C.; Gartner, U.; Wolf, M.; Ebner, C.; Bohle, B.; Arora, N.; Vieths, S. Proteomic profiling of the weed feverfew, a neglected pollen allergen source. Sci. Rep. 2017, 7, 6049. [Google Scholar] [CrossRef] [PubMed]
- Karamloo, F.; Schmitz, N.; Scheurer, S.; Foetisch, K.; Hoffmann, A.; Haustein, D.; Vieths, S. Molecular cloning and characterization of a birch pollen minor allergen, Bet v 5, belonging to a family of isoflavone reductase–related proteins. J. Allergy Clin. Immunol. 1999, 104, 991–999. [Google Scholar] [CrossRef]
- Flinterman, A.E.; Akkerdaas, J.H.; Knulst, A.C.; van Ree, R.; Pasmans, S.G. Hazelnut allergy: From pollen-associated mild allergy to severe anaphylactic reactions. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 261–265. [Google Scholar] [CrossRef]
- Quiralte, J.; Palacios, L.; Rodriguez, R.; Cardaba, B.; Arias de Saavedra, J.M.; Villalba, M.; Florido, J.F.; Lahoz, C. Modelling diseases: The allergens of Olea europaea pollen. J. Investig. Allergol. Clin. Immunol. 2007, 17 (Suppl. 1), 24–30. [Google Scholar]
- EAACI. Molecular Allergology—User’s Guide. European Academy of Allergy and Clinical Immunology. 2016. Available online: https://www.eaaci.org/documents/Molecular_Allergology-web.pdf (accessed on 26 May 2016).
- Radev, Z. The Impact of Different Protein Content of Pollen on Honey Bee (Apis mellifera L.) Development. Am. J. Entomol. 2018, 2, 23–27. [Google Scholar] [CrossRef][Green Version]
- Buters, J.T.M.; Thibaudon, M.; Smith, M.; Kennedy, R.; Rantio-Lehtimäki, A.; Albertini, R.; Gerald Reese, G.; Weber, B.; Galan, C.; Brandao, R.; et al. Release of Bet v 1 from birch pollen from 5 European countries. Results from the HIALINE study. Atmos. Environ. 2012, 55, 496–505. [Google Scholar] [CrossRef]
- Galan, C.; Antunes, C.; Brandao, R.; Torres, C.; Garcia-Mozo, H.; Caeiro, E.; Ferro, R.; Prank, M.; Sofiev, M.; Albertini, R.; et al. Airborne olive pollen counts are not representative of exposure to the major olive allergen O le e 1. Allergy 2013, 68, 809–812. [Google Scholar] [CrossRef]
- CashewInfo.com. 2018. Available online: http://www.cashewinfo.com/ (accessed on 5 July 2018).
- Carpentier, S.C.; Witters, E.; Laukens, K.; Deckers, P.; Swennen, R.; Panis, B. Preparation of protein extracts from recalcitrant plant tissues: An evaluation of different methods for two-dimensional gel electrophoresis analysis. Proteomics 2005, 5, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Radauer, C.; Bublin, M.; Wagner, S.; Mari, A.; Breiteneder, H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J. Allergy Clin. Immunol. 2008, 121, 847–852. [Google Scholar] [CrossRef]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Zardi, L.; Righetti, P.G. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Vejvar, E. A novel allergen of hazel pollen belongs to the isoflavone reductase family and is a Bet v 6-homologue. 2011; Manuscript in preparation. [Google Scholar]

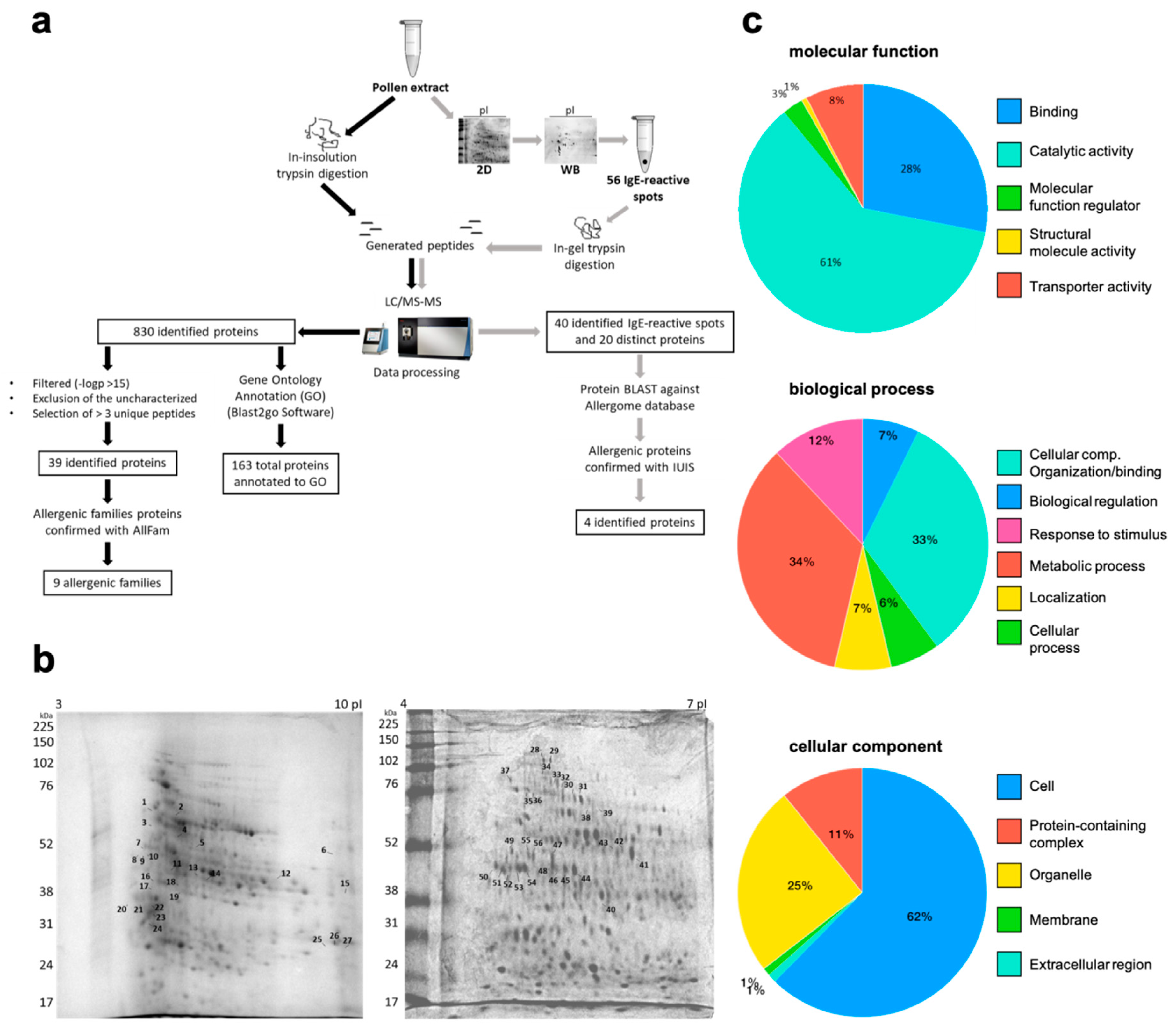
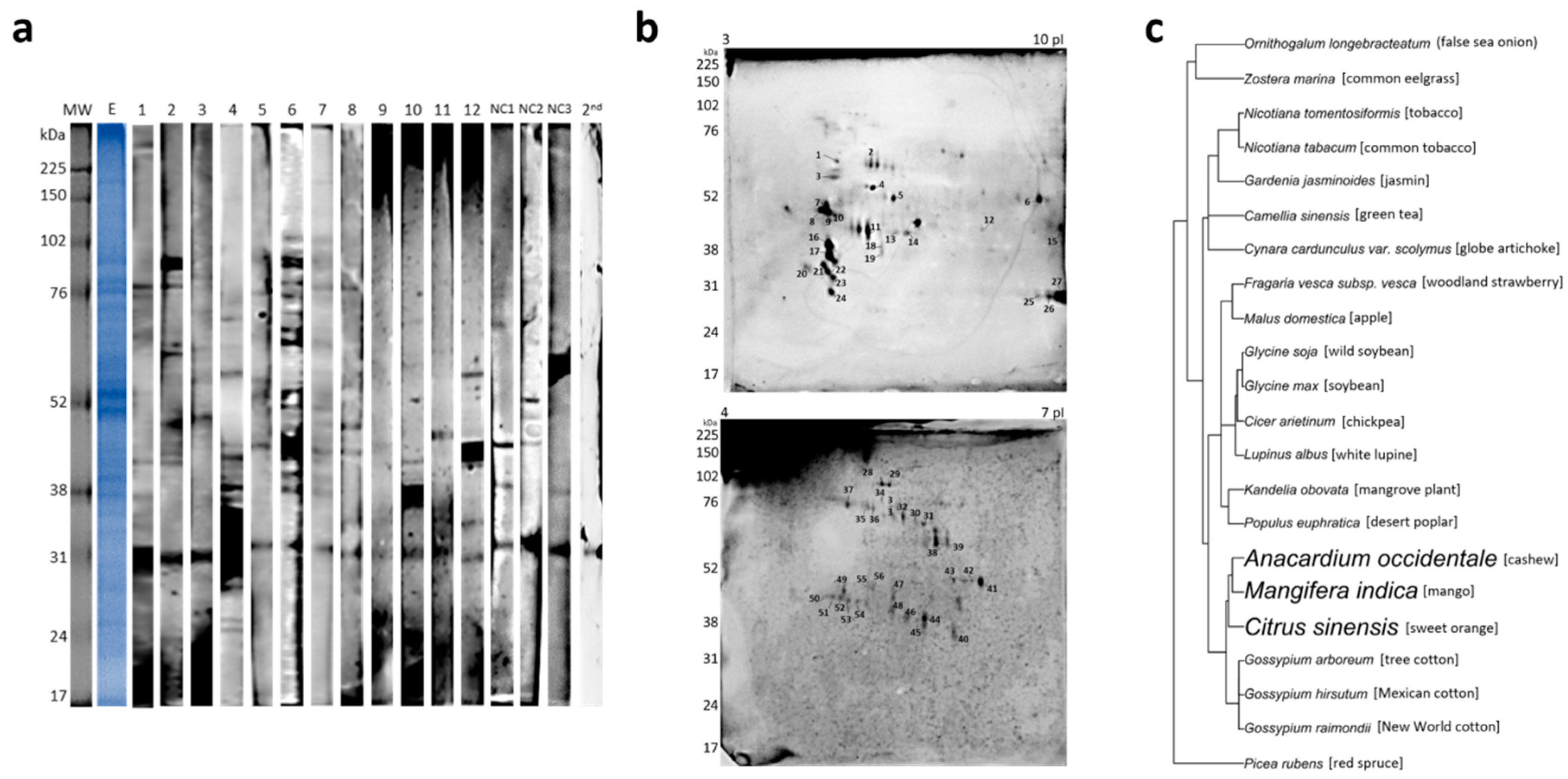

| # | Allergenic Protein Family | Protein | Organism | UP | Accession Number |
|---|---|---|---|---|---|
| 1 | β-1,3-glucanase and X8 domain | β-galactosidase | Mangifera indica | 3 | gi|68161828 |
| 2 | Isoflavone reductase family | Isoflavone reductase | Corylus avellana | 3 | gi|532961639 |
| 3 | Heat shock protein Hsp70 | Heat shock 70 kDa protein | Corchorus capsularis | 3 | gi|1137176360 |
| 4 | Glyceraldehyde 3-phosphate dehydrogenase | 6-phosphogluconate dehydrogenase, decarboxylating | Tarenaya spinosa | 3 | gi|90657561 |
| 5 | EF hand family | Calmodulin | Actinidia valvata | 5 | gi|149208297 |
| 6 | Ubiquinol-cytochrome C reductase 14kD subunit | GDP-mannose 3,5-epimerase 1 | Nicotiana tabacum | 4 | gi|1025343031 |
| 7 | Lactate/malate-dehydrogenase | Malate dehydrogenase | Punica granatum | 4 | gi|1208527593 |
| 8 | Cu/Zn superoxide dismutase | Superoxide dismutase [Cu–Zn] | Picea sitchensis | 3 | gi|116783025 |
| Zoysia japonica | 4 | gi|1150667722 | |||
| 9 | Fructose-bisphosphate aldolase Class I | Fructose-bisphosphate aldolase | Arabis alpina | 3 | gi|674241776 |
| 10 | - | Glutamine synthetase | Camellia sinensis | 3 | gi|67423358 |
| Helianthus annuus | 3 | gi|1191643447 | |||
| 11 | - | 26S protease regulatory subunit 6A | Ananas comosus | 4 | gi|1035941585 |
| 12 | - | 26S proteasome non-ATPase regulatory subunit 2 homolog A isoform X1 | Nelumbo nucifera | 5 | gi|720059466 |
| 13 | - | 3-ketoacyl-CoA thiolase 2, peroxisomal-like protein | Gossypium arboreum | 3 | gi|728824325 |
| 14 | - | 40S ribosomal protein SA | Daucus carota subsp. sativu | 3 | gi|1021032195 |
| 15 | - | Aconitate hydratase | Lupinus angustifolius | 3 | gi|1102705066 |
| 16 | - | Adenosylhomocysteinase | Daucus carota subsp. sativus | 6 | gi|1040913337 |
| 17 | - | ADK domain-containing protein/ADK_lid domain-containing protein (Fragment) | Cephalotus follicularis | 3 | tr|A0A1Q3BK62 |
| 18 | - | Aldehyde dehydrogenase 2B7 copy 2 | Bixa orellana | 4 | gi|995952955 |
| 19 | - | Alpha-1,4-glucan-protein synthase [UDP-forming] 1 | Cicer arietinum | 5 | gi|502148850 |
| 20 | - | ATP synthase subunit alpha | Nelumbo nucifera | 13 | gi|1052487924 |
| 21 | - | ATP-dependent clp protease ATP-binding subunit clpa-like cd4b, chloroplastic | Nicotiana attenuata | 3 | gi|1102148367 |
| 22 | - | Band_7 domain-containing protein | Cephalotus follicularis | 3 | tr|A0A1Q3C6R4 |
| 23 | - | Bifunctional aspartate aminotransferase and glutamate/aspartate-prephenate aminotransferase-like | Nelumbo nucifera | 3 | gi|720042258 |
| 24 | - | Caffeoyl-CoA O-methyltransferase | Morus notabilis | 4 | gi|703143062 |
| 25 | - | Citrate synthase | Spinacia oleracea | 3 | gi|902182157 |
| Helianthus annuus | 4 | gi|1191685410 | |||
| 26 | - | Clathrin heavy chain | Corchorus capsularis | 5 | gi|1137169669 |
| 27 | - | Elongation factor Tu | Phaseolus vulgaris | 4 | gi|561035855 |
| 28 | - | Eukaryotic initiation factor 4A-15 | Ananas comosus | 4 | gi|1035961582 |
| 29 | - | fumarate hydratase 1, mitochondrial | Cicer arietinum | 3 | gi|502117760 |
| 30 | - | Glucose-6-phosphate isomerase | Nelumbo nucifera | 4 | gi|719968717 |
| 31 | - | Glutamate dehydrogenase | Jatropha curcas | 3 | gi|643706362 |
| 32 | - | Ketol-acid reductoisomerase | Nelumbo nucifera | 3 | gi|720041220 |
| 33 | - | NADH dehydrogenase [ubiquinone] iron-sulfur protein 1, mitochondrial | Cicer arietinum | 4 | gi|502137547 |
| 34 | - | Peroxisomal (S)-2-hydroxy-acid oxidase GLO1-like | Nicotiana tabacum | 3 | gi|1025062071 |
| 35 | - | Phosphoenolpyruvate carboxylase | Arabidopsis lyrata subsp. lyrata | 3 | gi|297327702 |
| 36 | - | Purple acid phosphatase | Vitis vinifera | 3 | gi|147771668 |
| 37 | - | Rhamnogalacturonate lyase family protein | Theobroma cacao | 3 | gi|508723620 |
| 38 | - | Sucrose synthase | Mangifera indica | 5 | gi|425875159 |
| 39 | - | Ubiquitin-conjugating enzyme E2 variant 1D | Vigna radiata var. radiata | 5 | gi|951030072 |
| Patient | Sex | Age (y) | Symptoms | SPT | |||||
|---|---|---|---|---|---|---|---|---|---|
| Der f | Der p | Blo t | Mold | Mix Grass Pollen I | Mix Grass Pollen II | ||||
| 1 | F | 2 | AR, asthma, dermatitis | + | + | + | − | − | − |
| 2 | M | 11 | AR, asthma, conjunctivitis | + | + | + | − | − | − |
| 3 | F | 6 | AR, conjunctivitis | + | + | + | + | − | − |
| 4 | M | 17 | AR, asthma, conjunctivitis, dermatitis | + | + | + | − | − | − |
| 5 | M | 13 | AR, conjunctivitis | + | + | + | − | − | − |
| 6 | F | 65 | AR, asthma, conjunctivitis | + | + | + | − | − | − |
| 7 | M | 13 | AR, conjunctivitis | + | + | + | − | − | − |
| 8 | F | 6 | AR, asthma, conjunctivitis | + | + | + | − | − | − |
| 9 | F | 38 | AR, asthma | + | + | + | − | − | − |
| 10 | M | 7 | AR, asthma | + | + | + | − | − | − |
| 11 | F | 34 | AR, conjunctivitis | + | + | + | + | − | − |
| 12 | M | 14 | AR | + | + | + | − | − | − |
| Spot # | Allergenic Protein Family | Protein | Organism | UP | Accession Number |
|---|---|---|---|---|---|
| 2 | β-1,3-glucanase and X8 domain | PREDICTED: β-glucosidase 44-like | Citrus sinensis | 4 | gi|568833751 |
| 22 | β-1,3-glucanase | Mangifera indica | 4 | gi|87042321 | |
| 28–29–31–35–36 | β-galactosidase | Mangifera indica | 9–6–4–5–6 | gi|68161828 | |
| 7 | Prolamin superfamily | PREDICTED: α-galactosidase 3 | Fragaria vesca subsp. vesca | 2 | gi|470144145 |
| 8 | GDSL-hydrolase family | PREDICTED: GDSL esterase/lipase EXL3-like | Malus domestica | 2 | gi|658038118 |
| 19 | Isoflavone reductase family | PREDICTED: Isoflavone reductase homolog | Nicotiana tomentosiformis | 4 | gi|697122051 |
| 30 | Heat shock protein Hsp70 | Heat shock 70 kDa protein | Ornithogalum longebracteatum | 3 | gi|731910761 |
| 32 | Morus notabilis | 2 | gi|703159491 | ||
| 50 | Kandelia obovata | 4 | gi|825119573 | ||
| 52 | Gossypium arboreum | 3 | gi|728837176 | ||
| 46–48–53–54–56 | Profilin | Actin | Picea rubens | 4–3–4–3–4 | gi|6103623 |
| 55 | Gossypium hirsutum | 3 | gi|32186900 | ||
| 47 | Fructose bisphosphate aldolase class I | Fructose-bisphosphate aldolase-like protein | Gardenia jasminoides | 3 | gi|721750686 |
| 3 | - | α tubulin | Nicotiana tabacum | 4 | gi|11967906 |
| 4 | - | H(+)-transporting two-sector ATPase | Zostera marina | 4 | gi|901802009 |
| 5 | - | PREDICTED: Aminoacylase-1-like | Populus euphratica | 4 | gi|743913874 |
| 13 | - | Glutamine synthetase | Camellia sinensis | 2 | gi|42733460 |
| 15 | - | PREDICTED: Peroxisomal fatty acid β-oxidation multifunctional protein MFP2-like | Glycine max | 3 | gi|356564184 |
| 33 | - | Hypothetical protein B456_009G415000 | Gossypium raimondii | 3 | gi|763795406 |
| 39 | - | T-complex protein 1 subunit β | Glycine soja | 3 | gi|734418328 |
| 40 | - | Transketolase | Cynara cardunculus var. scolymus | 3 | gi|976910685 |
| 43 | - | Polyubiquitin | Lupinus albus | 3 | gi|89114278 |
| 44 | - | PREDICTED: Succinyl-CoA ligase [ADP-forming] subunit β, mitochondrial | Cicer arietinum | 3 | gi|502114889 |
| 45 | - | Flavanone 3-hydroxylase 2 | Mangifera indica | 2 | gi|724086306 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danella Figo, D.; De Amicis, K.; Neiva Santos de Aquino, D.; Pomiecinski, F.; Gadermaier, G.; Briza, P.; Eduardo Santos Galvão, C.; Bussador do Amaral, J.; de Oliveira Martins, C.; Fernandes Morato Castro, F.; et al. Cashew Tree Pollen: An Unknown Source of IgE-Reactive Molecules. Int. J. Mol. Sci. 2019, 20, 2397. https://doi.org/10.3390/ijms20102397
Danella Figo D, De Amicis K, Neiva Santos de Aquino D, Pomiecinski F, Gadermaier G, Briza P, Eduardo Santos Galvão C, Bussador do Amaral J, de Oliveira Martins C, Fernandes Morato Castro F, et al. Cashew Tree Pollen: An Unknown Source of IgE-Reactive Molecules. International Journal of Molecular Sciences. 2019; 20(10):2397. https://doi.org/10.3390/ijms20102397
Chicago/Turabian StyleDanella Figo, Daniele, Karine De Amicis, Denise Neiva Santos de Aquino, Fabiane Pomiecinski, Gabriele Gadermaier, Peter Briza, Clovis Eduardo Santos Galvão, Jônatas Bussador do Amaral, Carlo de Oliveira Martins, Fabio Fernandes Morato Castro, and et al. 2019. "Cashew Tree Pollen: An Unknown Source of IgE-Reactive Molecules" International Journal of Molecular Sciences 20, no. 10: 2397. https://doi.org/10.3390/ijms20102397
APA StyleDanella Figo, D., De Amicis, K., Neiva Santos de Aquino, D., Pomiecinski, F., Gadermaier, G., Briza, P., Eduardo Santos Galvão, C., Bussador do Amaral, J., de Oliveira Martins, C., Fernandes Morato Castro, F., Kalil, J., & Souza Santos, K. (2019). Cashew Tree Pollen: An Unknown Source of IgE-Reactive Molecules. International Journal of Molecular Sciences, 20(10), 2397. https://doi.org/10.3390/ijms20102397







