Lipocalin 2: A New Antimicrobial in Mast Cells
Abstract
1. Introduction
2. Results
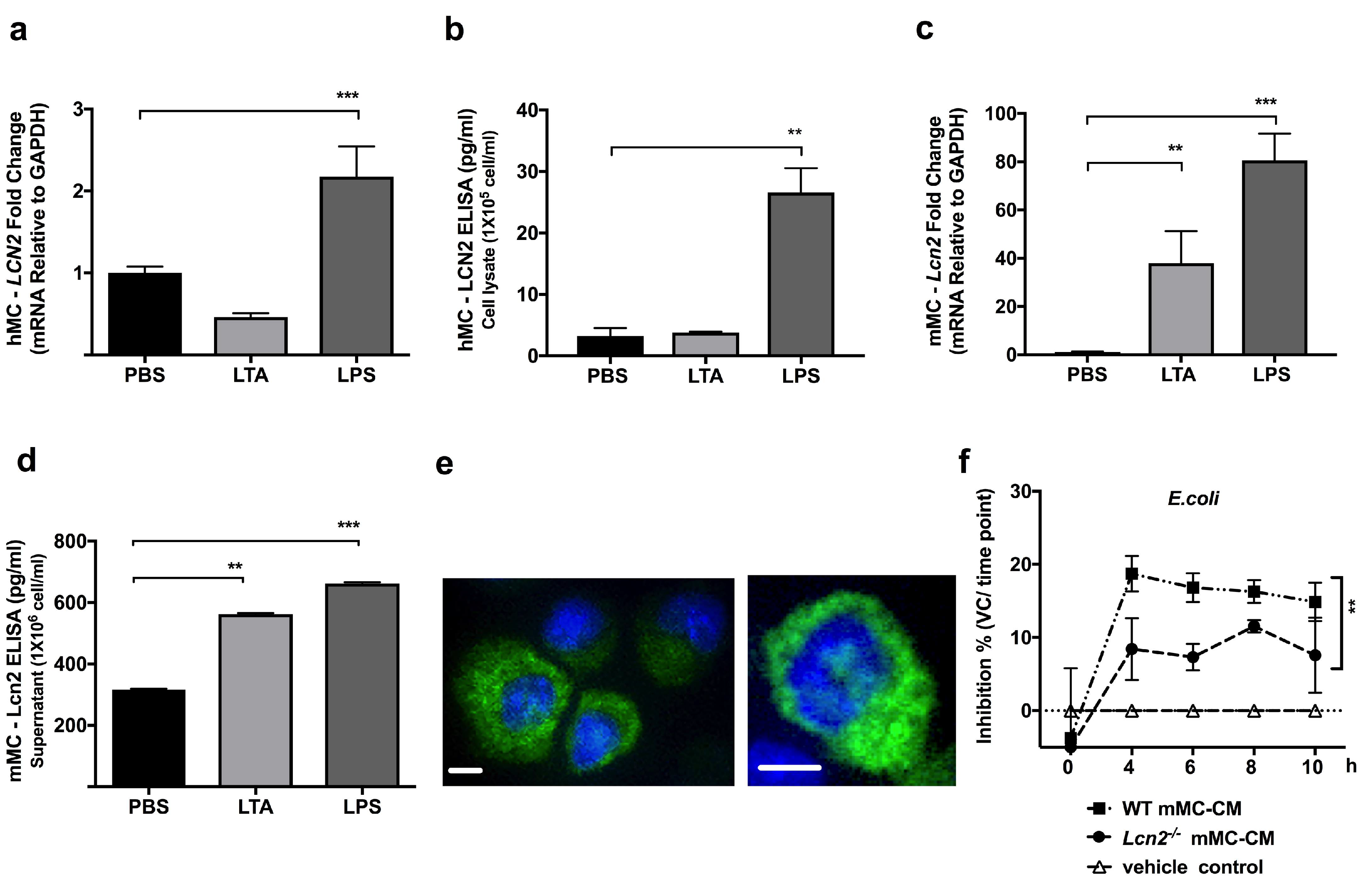
2.1. TLR4 Ligand LPS, a Gram-Negative Bacteria Byproduct, Induces LCN2 Expression and Production in both Human and Mouse MCs
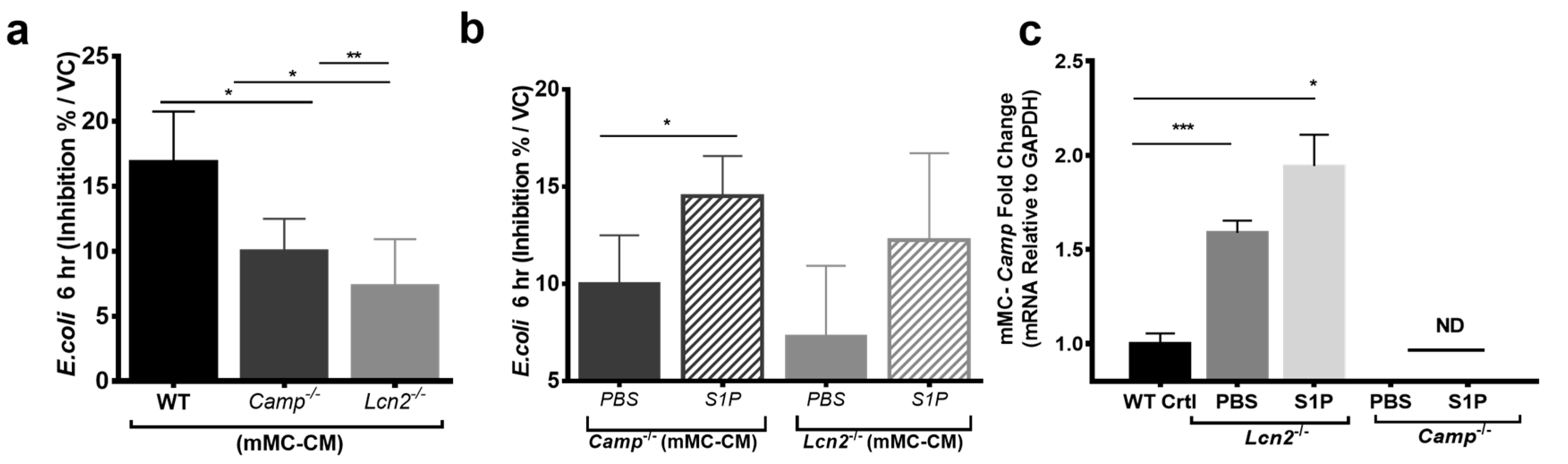
2.2. Lcn2-/- MCs Kill E. coli Less Efficiently
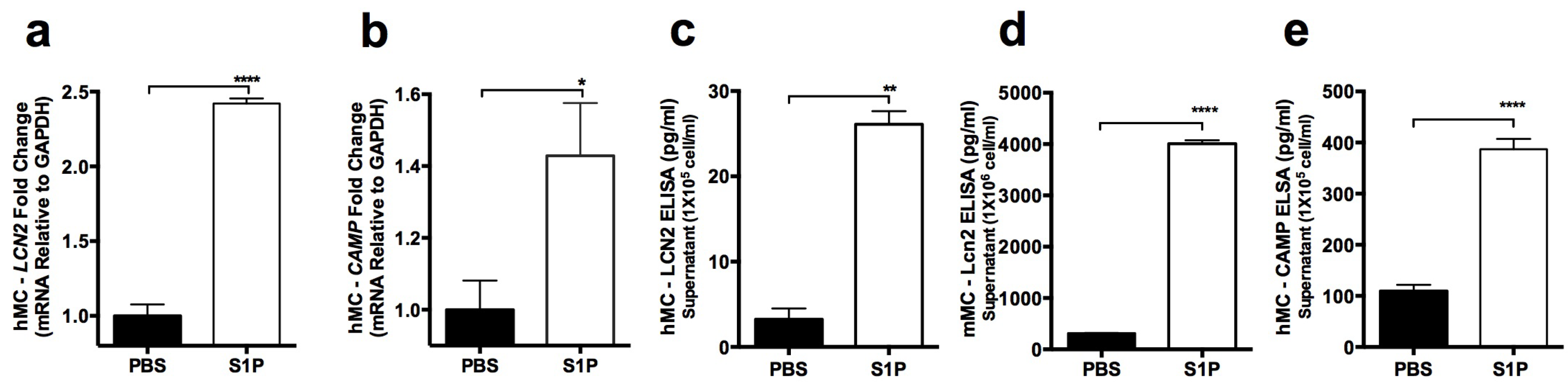
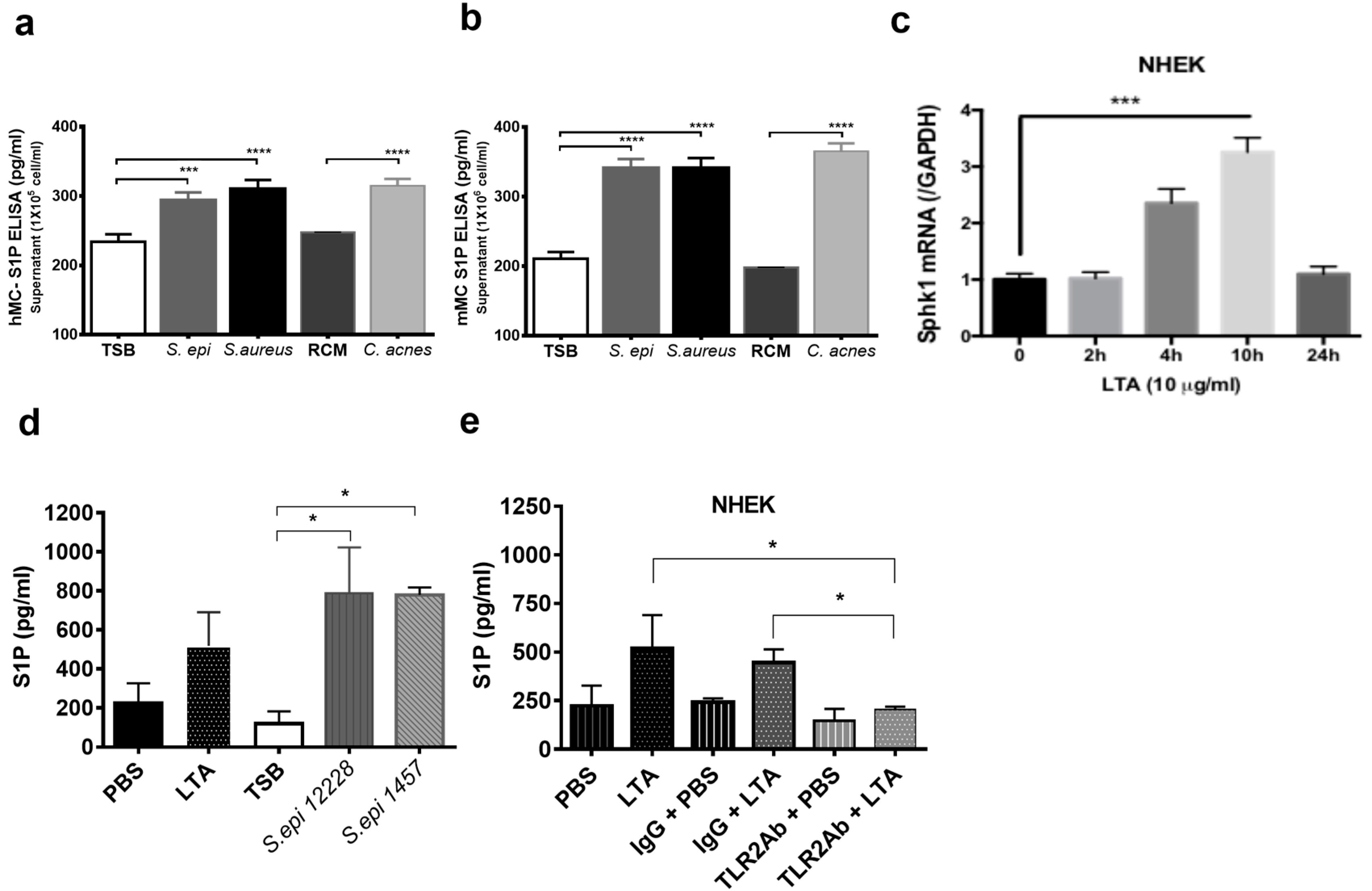
2.3. S1P Increases the Release of LCN2 and CAMP from Mast Cells
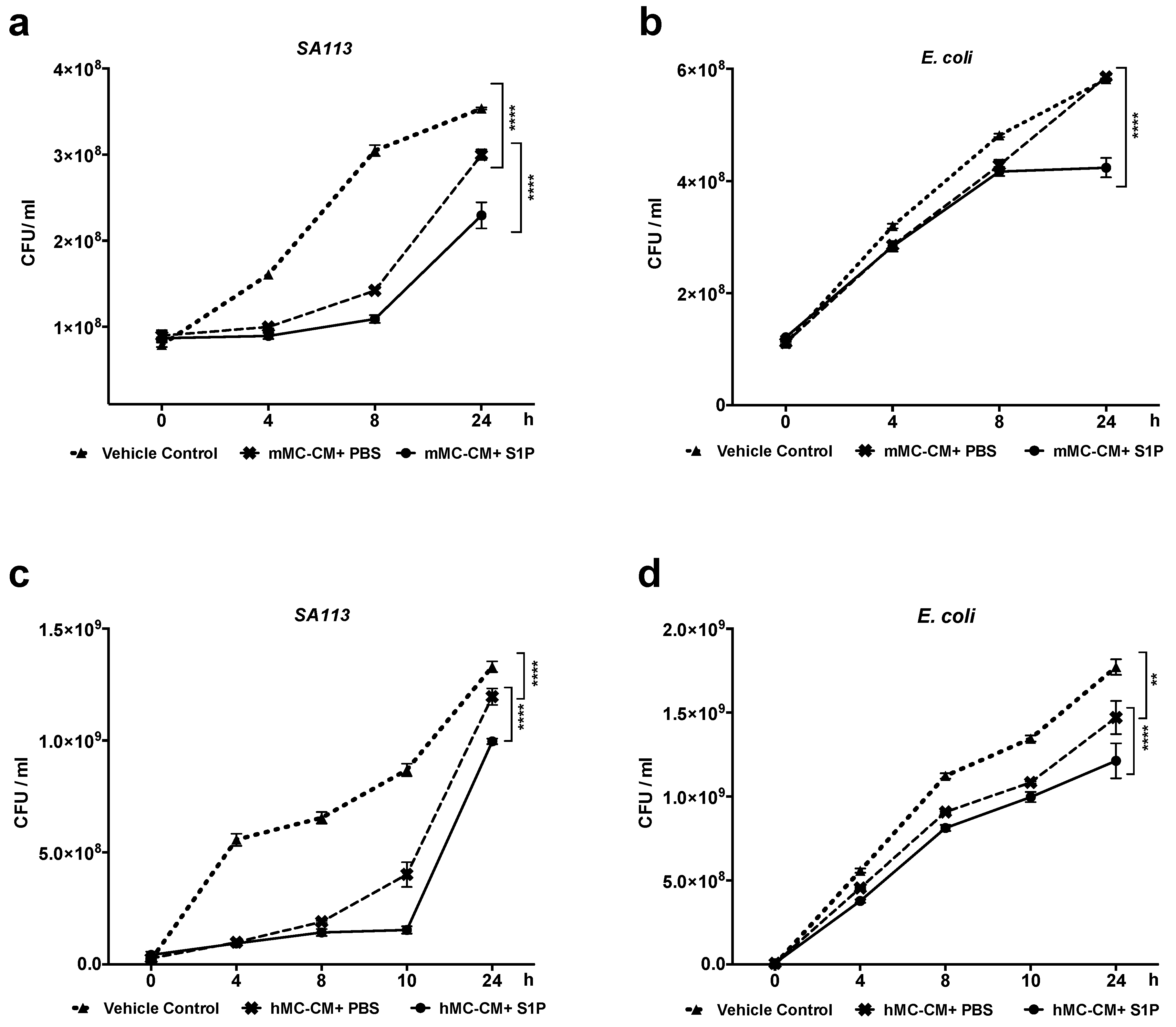
2.4. S1P Increase Antimicrobial Activity of hMCs and mMCs
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cells
4.3. Evaluation of S1P Concentration in Cell Medium
4.4. Real-Time Quantitative RT-PCR
4.5. ELISA
4.6. Antimicrobial Liquid Assays
4.7. Immunofluorescence Staining
4.8. Re-analysis of FANTOM 5 Data
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bischoff, S.C.; Kramer, S. Human mast cells, bacteria, and intestinal immunity. Immunol. Rev. 2007, 217, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Jawdat, D.M. Mast cells in innate immunity. J. Allergy Clin. Immunol. 2004, 114, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Oki, T.; Eto, K.; Izawa, K.; Yamanishi, Y.; Inagaki, N.; Frampton, J.; Kitamura, T.; Kitaura, J. Evidence that integrin alpha iib beta 3-dependent interaction of mast cells with fibrinogen exacerbates chronic inflammation. J. Biol. Chem. 2009, 284, 31463–31472. [Google Scholar] [CrossRef]
- Urb, M.; Sheppard, D.C. The role of mast cells in the defence against pathogens. PLoS Pathog. 2012, 8, e1002619. [Google Scholar] [CrossRef]
- Di Nardo, A.; Yamasaki, K.; Dorschner, R.A.; Lai, Y.; Gallo, R.L. Mast cell cathelicidin antimicrobial peptide prevents invasive group a streptococcus infection of the skin. J. Immunol. 2008, 180, 7565–7573. [Google Scholar] [CrossRef]
- Wang, Z.; Lai, Y.; Bernard, J.J.; Macleod, D.T.; Cogen, A.L.; Moss, B.; Di Nardo, A. Skin mast cells protect mice against vaccinia virus by triggering mast cell receptor s1pr2 and releasing antimicrobial peptides. J. Immunol. 2012, 188, 345–357. [Google Scholar] [CrossRef]
- Di Nardo, A.; Vitiello, A.; Gallo, R.L. Cutting edge: Mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 2003, 170, 2274–2278. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of tlr2 and tlr4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef]
- Motakis, E.; Guhl, S.; Ishizu, Y.; Itoh, M.; Kawaji, H.; de Hoon, M.; Lassmann, T.; Carninci, P.; Hayashizaki, Y.; Zuberbier, T.; et al. Redefinition of the human mast cell transcriptome by deep-cage sequencing. Blood 2014, 123, e58–e67. [Google Scholar] [CrossRef]
- Flo, T.H.; Smith, K.D.; Sato, S.; Rodriguez, D.J.; Holmes, M.A.; Strong, R.K.; Akira, S.; Aderem, A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 2004, 432, 917–921. [Google Scholar] [CrossRef]
- Berger, T.; Togawa, A.; Duncan, G.S.; Elia, A.J.; You-Ten, A.; Wakeham, A.; Fong, H.E.; Cheung, C.C.; Mak, T.W. Lipocalin 2-deficient mice exhibit increased sensitivity to escherichia coli infection but not to ischemia-reperfusion injury. Proc. Nat. Acad. Sci. USA 2006, 103, 1834–1839. [Google Scholar] [CrossRef]
- ulfone-Paus, S. Tlr signaling in mast cells: Common and unique features. Front. Immunol. 2012, 3, 185. [Google Scholar]
- Kurashima, Y.; Amiya, T.; Fujisawa, K.; Shibata, N.; Suzuki, Y.; Kogure, Y.; Hashimoto, E.; Otsuka, A.; Kabashima, K.; Sato, S.; et al. The enzyme cyp26b1 mediates inhibition of mast cell activation by fibroblasts to maintain skin-barrier homeostasis. Immunity 2014, 40, 530–541. [Google Scholar] [CrossRef]
- Wang, Z.; Mascarenhas, N.; Eckmann, L.; Miyamoto, Y.; Sun, X.; Kawakami, T.; Di Nardo, A. Skin microbiome promotes mast cell maturation by triggering stem cell factor production in keratinocytes. J. Allergy Clin. Immunol. 2017, 139, 1205–1216. [Google Scholar] [CrossRef]
- Olivera, A.; Allende, M.L.; Proia, R.L. Shaping the landscape: Metabolic regulation of s1p gradients. Biochim. Biophys. Acta 2013, 1831, 193–202. [Google Scholar] [CrossRef]
- Park, K.; Ikushiro, H.; Seo, H.S.; Shin, K.O.; Kim, Y.I.; Kim, J.Y.; Lee, Y.M.; Yano, T.; Holleran, W.M.; Elias, P.; et al. Er stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular s1p signaling complex. Proc. Nat. Acad. Sci. USA 2016, 113, E1334–E1342. [Google Scholar] [CrossRef]
- Rosen, H.; Sanna, M.G.; Cahalan, S.M.; Gonzalez-Cabrera, P.J. Tipping the gatekeeper: S1p regulation of endothelial barrier function. Trends Immunol. 2007, 28, 102–107. [Google Scholar] [CrossRef]
- Borkham-Kamphorst, E.; van de Leur, E.; Zimmermann, H.W.; Karlmark, K.R.; Tihaa, L.; Haas, U.; Tacke, F.; Berger, T.; Mak, T.W.; Weiskirchen, R. Protective effects of lipocalin-2 (lcn2) in acute liver injury suggest a novel function in liver homeostasis. Biochim. Biophys. Acta 2013, 1832, 660–673. [Google Scholar] [CrossRef]
- Borregaard, N.; Cowland, J.B. Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein. Biometals 2006, 19, 211–215. [Google Scholar] [CrossRef]
- Moschen, A.R.; Adolph, T.E.; Gerner, R.R.; Wieser, V.; Tilg, H. Lipocalin-2: A master mediator of intestinal and metabolic inflammation. Trends Endocrinol. Metab. 2017, 28, 388–397. [Google Scholar] [CrossRef]
- Shao, S.; Cao, T.; Jin, L.; Li, B.; Fang, H.; Zhang, J.; Zhang, Y.; Hu, J.; Wang, G. Increased lipocalin-2 contributes to the pathogenesis of psoriasis by modulating neutrophil chemotaxis and cytokine secretion. J. Invest. Dermatol. 2016, 136, 1418–1428. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Van Brocklyn, J.R.; Williams, J.B. The control of the balance between ceramide and sphingosine-1-phosphate by sphingosine kinase: Oxidative stress and the seesaw of cell survival and death. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 163, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Proia, R.L.; Olivera, A. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 2008, 8, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Kulinski, J.M.; Munoz-Cano, R.; Olivera, A. Sphingosine-1-phosphate and other lipid mediators generated by mast cells as critical players in allergy and mast cell function. Eur. J. Pharmacol. 2015, 778, 56–67. [Google Scholar] [CrossRef]
- Olivera, A.; Rivera, J. An emerging role for the lipid mediator sphingosine-1-phosphate in mast cell effector function and allergic disease. Adv. Exp. Med. Biol. 2011, 716, 123–142. [Google Scholar]
- Olivera, A.; Rivera, J. Sphingolipids and the balancing of immune cell function: Lessons from the mast cell. J. Immunol. 2005, 174, 1153–1158. [Google Scholar] [CrossRef]
- Park, K.; Elias, P.M.; Shin, K.O.; Lee, Y.M.; Hupe, M.; Borkowski, A.W.; Gallo, R.L.; Saba, J.; Holleran, W.M.; Uchida, Y. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol. Cell Biol. 2013, 33, 752–762. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. A new shield for a cytokine storm. Cell 2011, 146, 861–862. [Google Scholar] [CrossRef][Green Version]
- Melendez, A.J. Sphingosine kinase signalling in immune cells: Potential as novel therapeutic targets. Biochim. Biophys. Acta 2008, 1784, 66–75. [Google Scholar] [CrossRef]
- Oskeritzian, C.A.; Hait, N.C.; Wedman, P.; Chumanevich, A.; Kolawole, E.M.; Price, M.M.; Falanga, Y.T.; Harikumar, K.B.; Ryan, J.J.; Milstien, S.; et al. The sphingosine-1-phosphate/sphingosine-1-phosphate receptor 2 axis regulates early airway t-cell infiltration in murine mast cell-dependent acute allergic responses. J. Allergy Clin. Immunol. 2015, 135, 1008–1018. [Google Scholar] [CrossRef]
- Oskeritzian, C.A.; Price, M.M.; Hait, N.C.; Kapitonov, D.; Falanga, Y.T.; Morales, J.K.; Ryan, J.J.; Milstien, S.; Spiegel, S. Essential roles of sphingosine-1-phosphate receptor 2 in human mast cell activation, anaphylaxis, and pulmonary edema. J. Exp. Med. 2010, 207, 465–474. [Google Scholar] [CrossRef]
- Chumanevich, A.; Wedman, P.; Oskeritzian, C.A. Sphingosine-1-phosphate/sphingosine-1-phosphate receptor 2 axis can promote mouse and human primary mast cell angiogenic potential through upregulation of vascular endothelial growth factor-a and matrix metalloproteinase-2. Mediat. Inflamm. 2016, 2016, 1503206. [Google Scholar] [CrossRef]
- Raffatellu, M.; George, M.D.; Akiyama, Y.; Hornsby, M.J.; Nuccio, S.P.; Paixao, T.A.; Butler, B.P.; Chu, H.; Santos, R.L.; Berger, T.; et al. Lipocalin-2 resistance confers an advantage to salmonella enterica serotype typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe 2009, 5, 476–486. [Google Scholar] [CrossRef]
- Miao, Q.; Ku, A.T.; Nishino, Y.; Howard, J.M.; Rao, A.S.; Shaver, T.M.; Garcia, G.E.; Le, D.N.; Karlin, K.L.; Westbrook, T.F.; et al. Tcf3 promotes cell migration and wound repair through regulation of lipocalin 2. Nat. Commun. 2014, 5, 4088. [Google Scholar] [CrossRef]
- Kirshenbaum, A.S.; Metcalfe, D.D. Growth of human mast cells from bone marrow and peripheral blood-derived cd34+ pluripotent progenitor cells. Methods Mol. Biol. 2006, 315, 105–112. [Google Scholar]
- Kao, S.J.; Lei, H.C.; Kuo, C.T.; Chang, M.S.; Chen, B.C.; Chang, Y.C.; Chiu, W.T.; Lin, C.H. Lipoteichoic acid induces nuclear factor-kappab activation and nitric oxide synthase expression via phosphatidylinositol 3-kinase, akt, and p38 mapk in raw 264.7 macrophages. Immunology 2005, 115, 366–374. [Google Scholar] [CrossRef]
- Lehner, M.D.; Morath, S.; Michelsen, K.S.; Schumann, R.R.; Hartung, T. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different toll-like receptors independent of paracrine mediators. J. Immunol. 2001, 166, 5161–5167. [Google Scholar] [CrossRef]
- Tancowny, B.P.; Karpov, V.; Schleimer, R.P.; Kulka, M. Substance p primes lipoteichoic acid-and pam3cysserlys4-mediated activation of human mast cells by up-regulating toll-like receptor 2. Immunology 2010, 131, 220–230. [Google Scholar] [CrossRef]
- Luu-The, V.; Paquet, N.; Calvo, E.; Cumps, J. Improved real-time rt-pcr method for high-throughput measurements using second derivative calculation and double correction. Biotechniques 2005, 38, 287–293. [Google Scholar] [CrossRef]
- Murakami, M.; Ohtake, T.; Dorschner, R.A.; Schittek, B.; Garbe, C.; Gallo, R.L. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J. Investig. Dermatol. 2002, 119, 1090–1095. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-L.; Wang, Z.; Igawa, S.; Choi, J.E.; Werbel, T.; Di Nardo, A. Lipocalin 2: A New Antimicrobial in Mast Cells. Int. J. Mol. Sci. 2019, 20, 2380. https://doi.org/10.3390/ijms20102380
Chang Y-L, Wang Z, Igawa S, Choi JE, Werbel T, Di Nardo A. Lipocalin 2: A New Antimicrobial in Mast Cells. International Journal of Molecular Sciences. 2019; 20(10):2380. https://doi.org/10.3390/ijms20102380
Chicago/Turabian StyleChang, Yu-Ling, Zhenping Wang, Satomi Igawa, Jae Eun Choi, Tyler Werbel, and Anna Di Nardo. 2019. "Lipocalin 2: A New Antimicrobial in Mast Cells" International Journal of Molecular Sciences 20, no. 10: 2380. https://doi.org/10.3390/ijms20102380
APA StyleChang, Y.-L., Wang, Z., Igawa, S., Choi, J. E., Werbel, T., & Di Nardo, A. (2019). Lipocalin 2: A New Antimicrobial in Mast Cells. International Journal of Molecular Sciences, 20(10), 2380. https://doi.org/10.3390/ijms20102380




