Ribosome Structure, Function, and Early Evolution
Abstract
1. Introduction
2. Initiation of Translation
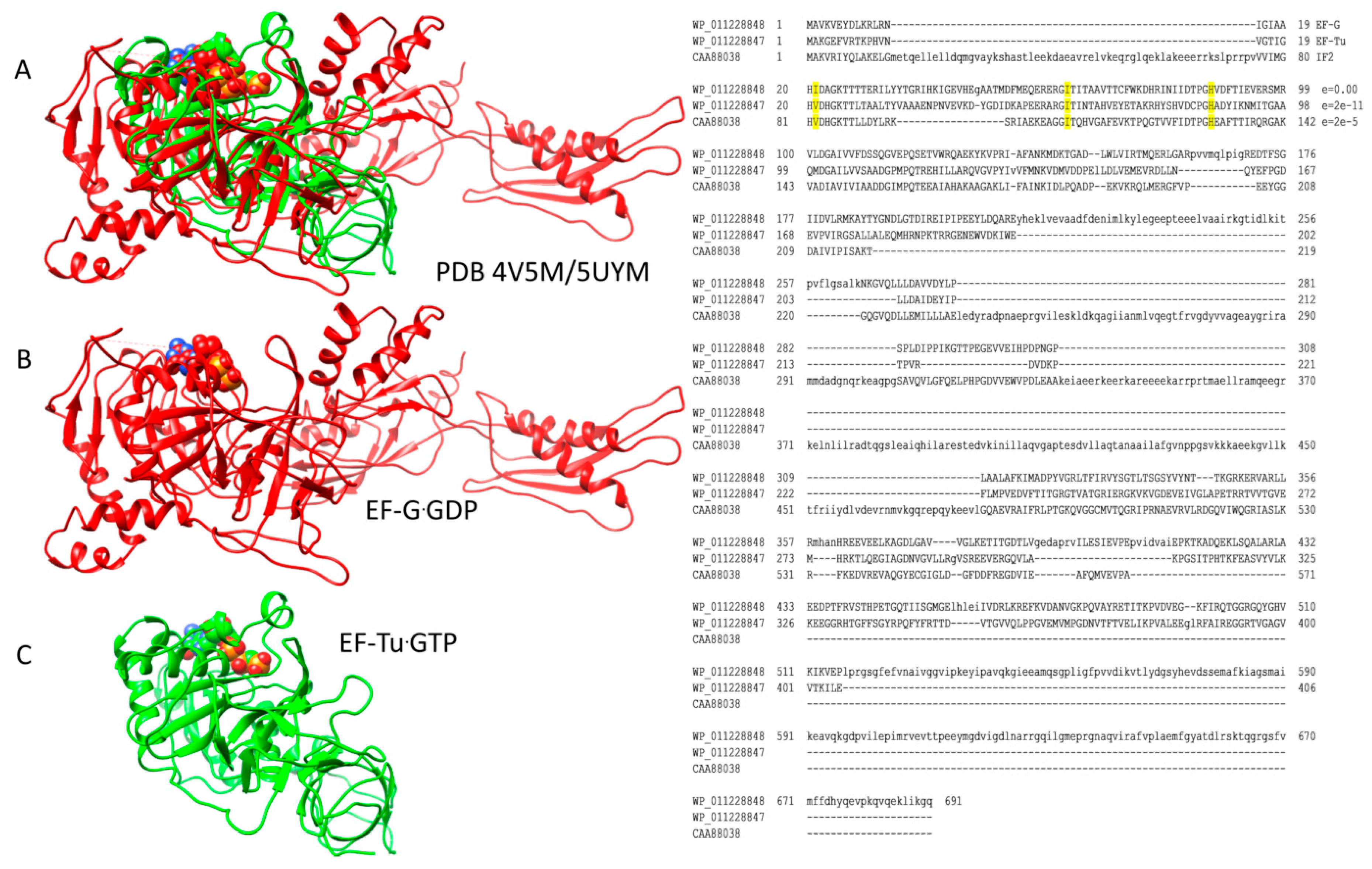
2.1. Homologous GTPases in Initiation and Elongation of Translation
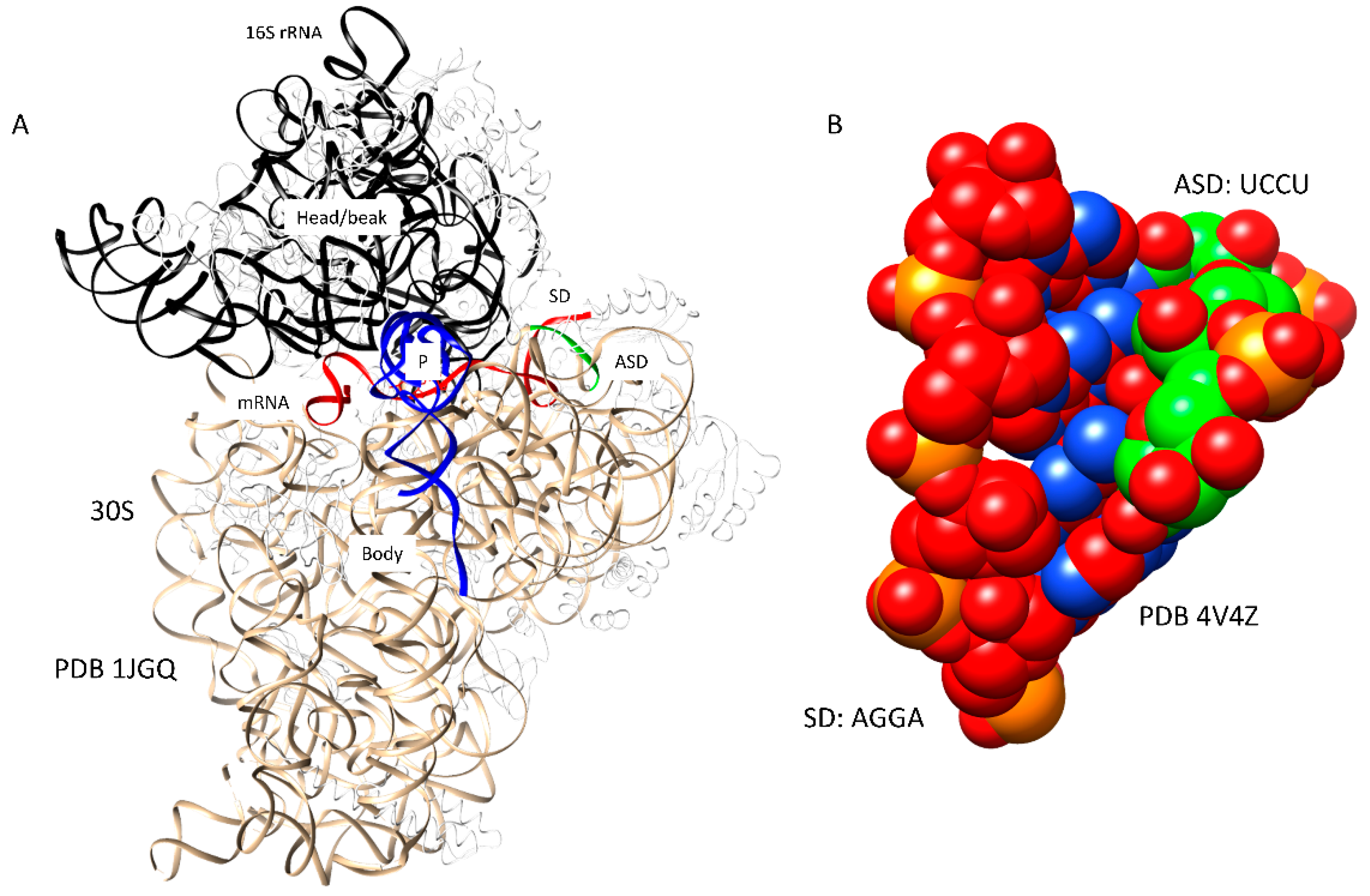
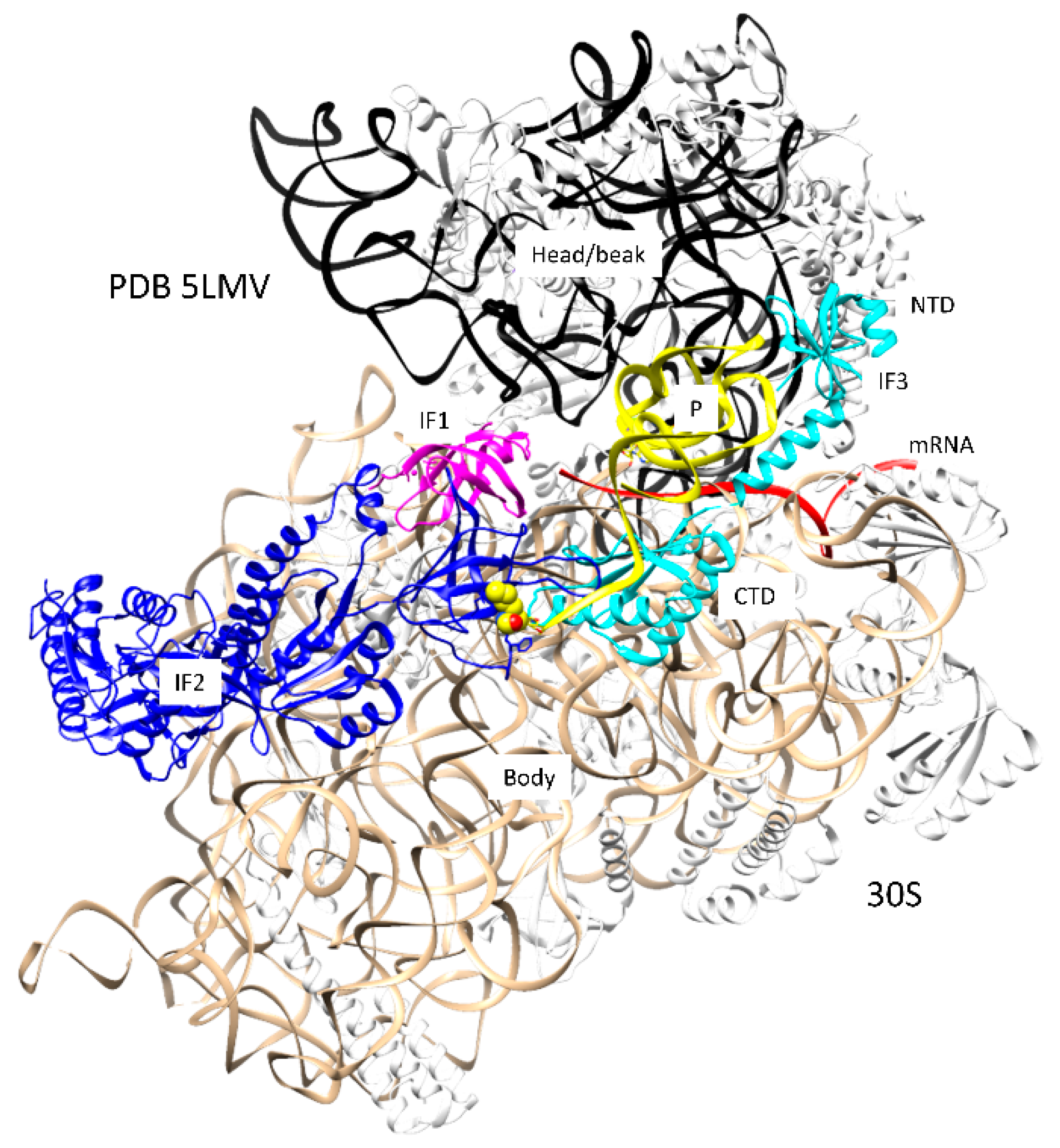
2.2. Mechanism of Initiation
3. Elongation of Translation
3.1. Molecular Motor
3.2. tRNA as a Relatively Stiff Adapter
3.3. tRNA Entry
3.4. Forming the Accurate Codon-Anticodon Latch and Closing the 30S Subunit Conformation
3.5. Accommodation
3.6. Peptide Bond Formation
3.7. Translocation
3.8. EF-G·GTP/GDP in Translocation
3.9. 30S-50S Intersubunit Bridges in Translocation
3.10. Ratchet Pawls
3.11. Kink-Turns and Micro-Motions
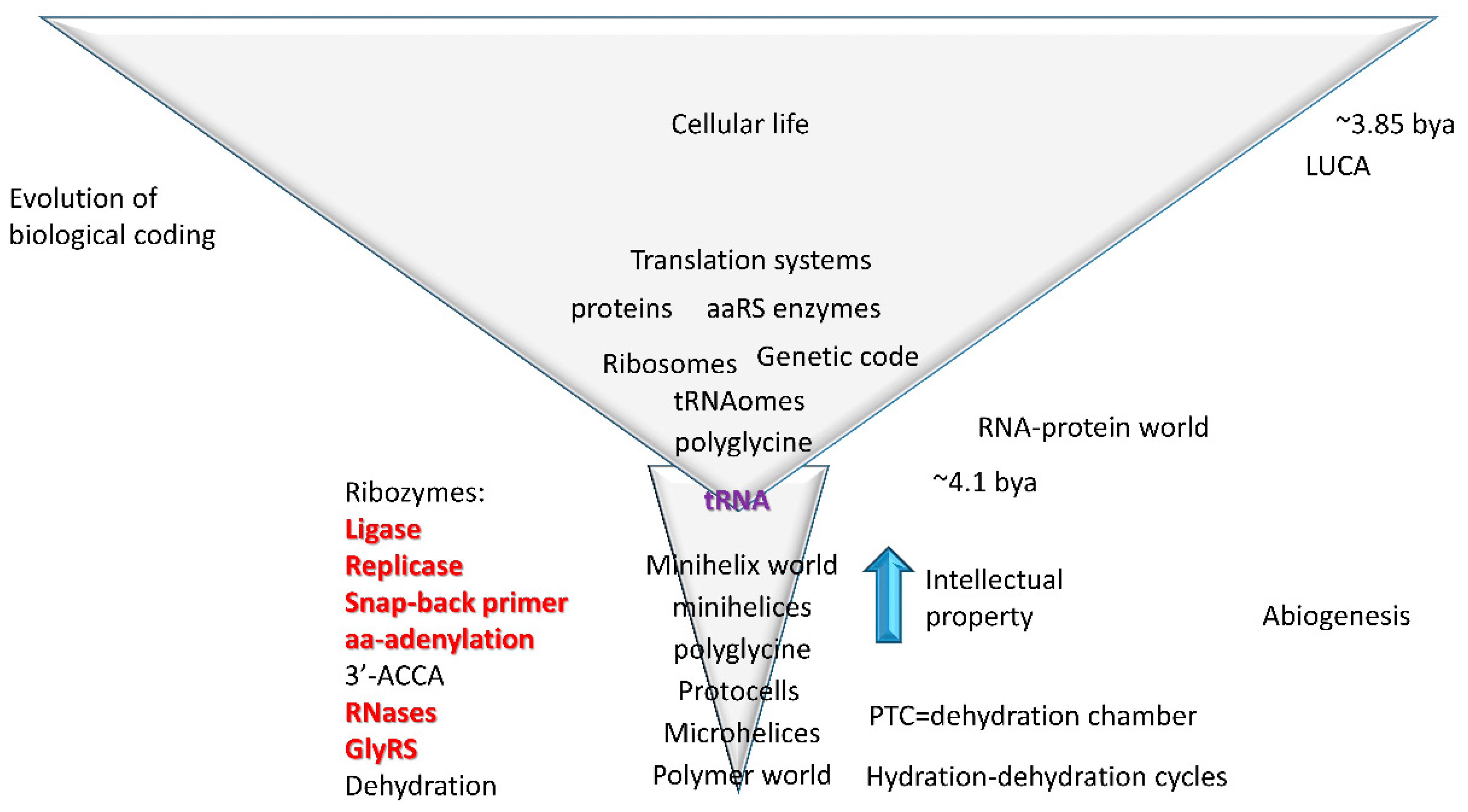
4. Evolution of Translation
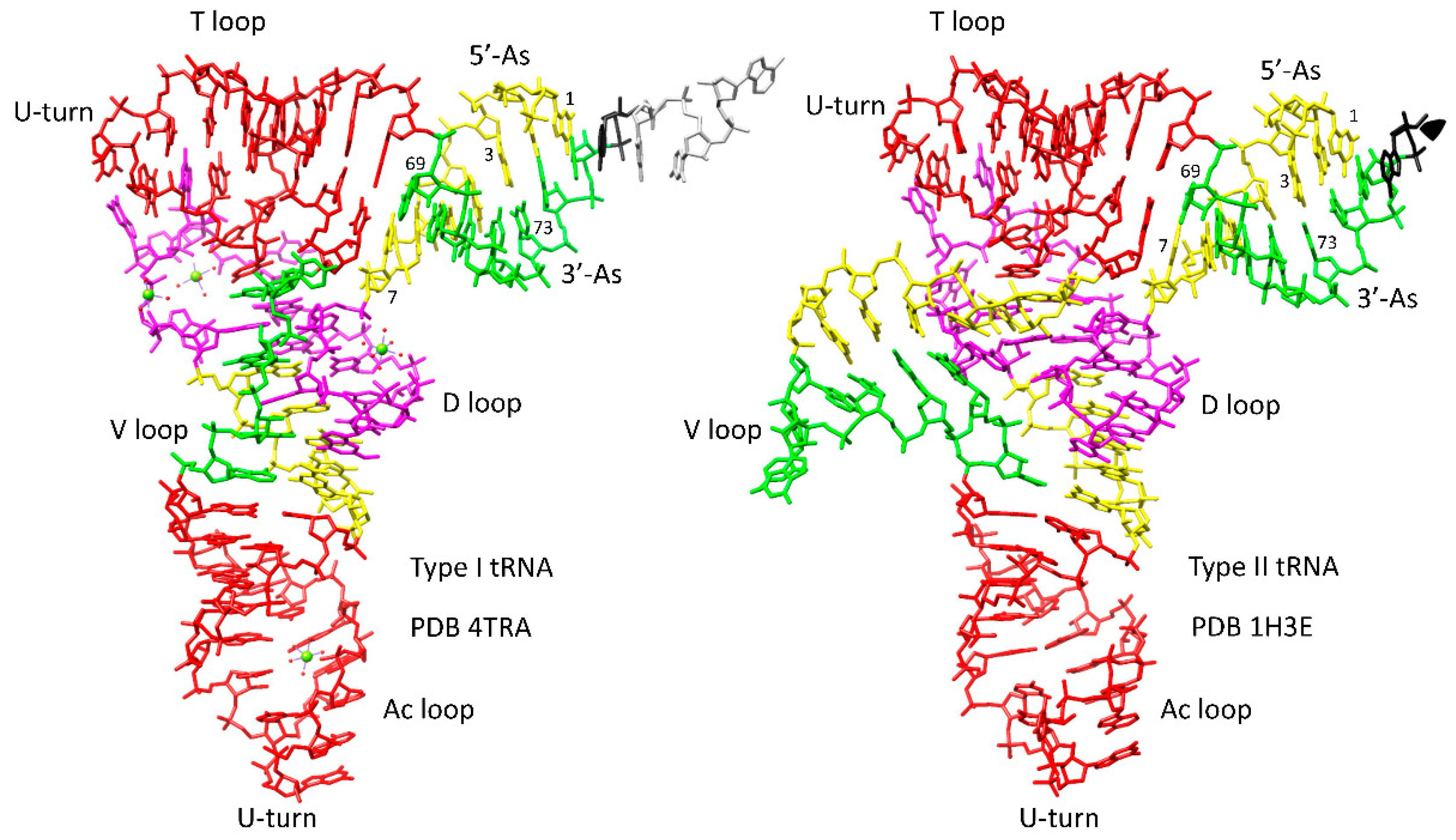
4.1. tRNA Evolution
4.2. tRNA as Core Evolutionary Intellectual Property
4.3. Aminoacyl-tRNA Synthetase Evolution
4.4. rRNA Evolution
4.5. Evolution of the Genetic Code
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| aaRS | Aminoacyl-tRNA synthetase (i.e., GlyRS) |
| A-site | Aminoacyl site |
| As | Acceptor stems |
| ASL | Anticodon stem loop |
| Ac loop | Anticodon loop |
| EF | Elongation Factor |
| E-site | Exit site |
| IF | Initiation Factor |
| LUCA | Last universal common (cellular) ancestor |
| P-site | Peptidyl site |
| PTC | Peptidyl Transferase Center |
| SRL | Sarcin-ricin loop |
| T loop | T loop or TΨC loop |
| V loop | Variable loop |
References
- Laursen, B.S.; Sorensen, H.P.; Mortensen, K.K.; Sperling-Petersen, H.U. Initiation of protein synthesis in bacteria. Microbiol. Mol. Biol. Rev. 2005, 69, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Rodnina, M.V.; Fischer, N.; Maracci, C.; Stark, H. Ribosome dynamics during decoding. Philos. Trans. R Soc. Lond. B Biol. Sci. 2017, 372. [Google Scholar] [CrossRef] [PubMed]
- Maracci, C.; Rodnina, M.V. Review: Translational GTPases. Biopolymers 2016, 105, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, R.M.; Ramakrishnan, V. Structural basis of the translational elongation cycle. Annu. Rev. Biochem. 2013, 82, 203–236. [Google Scholar] [CrossRef]
- Agirrezabala, X.; Frank, J. Elongation in translation as a dynamic interaction among the ribosome, tRNA, and elongation factors EF-G and EF-Tu. Q. Rev. Biophys. 2009, 42, 159–200. [Google Scholar] [CrossRef]
- Sergiev, P.V.; Bogdanov, A.A.; Dontsova, O.A. How can elongation factors EF-G and EF-Tu discriminate the functional state of the ribosome using the same binding site? FEBS Lett. 2005, 579, 5439–5442. [Google Scholar] [CrossRef]
- Rodnina, M.V.; Gromadski, K.B.; Kothe, U.; Wieden, H.J. Recognition and selection of tRNA in translation. FEBS Lett. 2005, 579, 938–942. [Google Scholar] [CrossRef]
- Holtkamp, W.; Wintermeyer, W.; Rodnina, M.V. Synchronous tRNA movements during translocation on the ribosome are orchestrated by elongation factor G and GTP hydrolysis. Bioessays 2014, 36, 908–918. [Google Scholar] [CrossRef]
- Rodnina, M.V.; Wintermeyer, W. The ribosome as a molecular machine: The mechanism of tRNA-mRNA movement in translocation. Biochem. Soc. Trans. 2011, 39, 658–662. [Google Scholar] [CrossRef]
- Ling, C.; Ermolenko, D.N. Structural insights into ribosome translocation. Wiley Int. Rev. RNA 2016, 7, 620–636. [Google Scholar] [CrossRef]
- Frank, J. Intermediate states during mRNA-tRNA translocation. Curr. Opin. Struct. Biol. 2012, 22, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Kavaliauskas, D.; Chen, C.; Liu, W.; Cooperman, B.S.; Goldman, Y.E.; Knudsen, C.R. Structural dynamics of translation elongation factor Tu during aa-tRNA delivery to the ribosome. Nucleic Acids Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Warshel, A. EF-Tu and EF-G are activated by allosteric effects. Proc. Natl. Acad. Sci. USA 2018, 115, 3386–3391. [Google Scholar] [CrossRef] [PubMed]
- Salsi, E.; Farah, E.; Netter, Z.; Dann, J.; Ermolenko, D.N. Movement of elongation factor G between compact and extended conformations. J. Mol. Biol. 2015, 427, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.G.; Lin, J.; Steitz, T.A. Elongation factor 4 remodels the A-site tRNA on the ribosome. Proc. Natl. Acad. Sci. USA 2016, 113, 4994–4999. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Alonso, J.P.; Fabbretti, A.; Kaminishi, T.; Iturrioz, I.; Brandi, L.; Gil-Carton, D.; Gualerzi, C.O.; Fucini, P.; Connell, S.R. Structure of a 30S pre-initiation complex stalled by GE81112 reveals structural parallels in bacterial and eukaryotic protein synthesis initiation pathways. Nucleic Acids Res. 2017, 45, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Llacer, J.L.; Wimberly, B.T.; Kieft, J.S.; Ramakrishnan, V. Large-Scale Movements of IF3 and tRNA during Bacterial Translation Initiation. Cell 2016, 167, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Yusupova, G.Z.; Yusupov, M.M.; Cate, J.H.; Noller, H.F. The path of messenger RNA through the ribosome. Cell 2001, 106, 233–241. [Google Scholar] [CrossRef]
- Yusupova, G.; Jenner, L.; Rees, B.; Moras, D.; Yusupov, M. Structural basis for messenger RNA movement on the ribosome. Nature 2006, 444, 391–394. [Google Scholar] [CrossRef]
- Ling, C.; Ermolenko, D.N. Initiation factor 2 stabilizes the ribosome in a semirotated conformation. Proc. Natl. Acad. Sci. USA 2015, 112, 15874–15879. [Google Scholar] [CrossRef]
- Liu, T.; Kaplan, A.; Alexander, L.; Yan, S.; Wen, J.D.; Lancaster, L.; Wickersham, C.E.; Fredrick, K.; Noller, H.; Tinoco, I.; et al. Direct measurement of the mechanical work during translocation by the ribosome. Elife 2014, 3, e03406. [Google Scholar] [CrossRef] [PubMed]
- Ratje, A.H.; Loerke, J.; Mikolajka, A.; Brunner, M.; Hildebrand, P.W.; Starosta, A.L.; Donhofer, A.; Connell, S.R.; Fucini, P.; Mielke, T.; et al. Head swivel on the ribosome facilitates translocation by means of intra-subunit tRNA hybrid sites. Nature 2010, 468, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Gao, H.; Sengupta, J.; Gao, N.; Taylor, D.J. The process of mRNA-tRNA translocation. Proc. Natl. Acad. Sci. USA 2007, 104, 19671–19678. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, I.; Pohl, C.; Rodnina, M.V. Optimization of speed and accuracy of decoding in translation. EMBO J. 2010, 29, 3701–3709. [Google Scholar] [CrossRef]
- Gromadski, K.B.; Rodnina, M.V. Kinetic determinants of high-fidelity tRNA discrimination on the ribosome. Mol. Cell. 2004, 13, 191–200. [Google Scholar] [CrossRef]
- Rodnina, M.V.; Wintermeyer, W. Fidelity of aminoacyl-tRNA selection on the ribosome: Kinetic and structural mechanisms. Annu. Rev. Biochem. 2001, 70, 415–435. [Google Scholar] [CrossRef]
- Pak, D.; Root-Bernstein, R.; Burton, Z.F. tRNA structure and evolution and standardization to the three nucleotide genetic code. Transcription 2017, 8, 205–219. [Google Scholar] [CrossRef]
- Li, W.; Agirrezabala, X.; Lei, J.; Bouakaz, L.; Brunelle, J.L.; Ortiz-Meoz, R.F.; Green, R.; Sanyal, S.; Ehrenberg, M.; Frank, J. Recognition of aminoacyl-tRNA: A common molecular mechanism revealed by cryo-EM. EMBO J. 2008, 27, 3322–3331. [Google Scholar] [CrossRef]
- Zhou, J.; Lancaster, L.; Donohue, J.P.; Noller, H.F. Crystal structures of EF-G-ribosome complexes trapped in intermediate states of translocation. Science 2013, 340, 1236086. [Google Scholar] [CrossRef]
- Noel, J.K.; Whitford, P.C. How EF-Tu can contribute to efficient proofreading of aa-tRNA by the ribosome. Nat. Commun. 2016, 7, 13314. [Google Scholar] [CrossRef]
- Nguyen, K.; Yang, H.; Whitford, P.C. How the Ribosomal A-Site Finger Can Lead to tRNA Species-Dependent Dynamics. J. Phys. Chem. B 2017, 121, 2767–2775. [Google Scholar] [CrossRef]
- Whitford, P.C.; Sanbonmatsu, K.Y. Simulating movement of tRNA through the ribosome during hybrid-state formation. J. Chem. Phys. 2013, 139, 121919. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Perrier, J.; Whitford, P.C. Disorder guides domain rearrangement in elongation factor Tu. Proteins 2018, 86, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Nissen, P.; Kjeldgaard, M.; Thirup, S.; Polekhina, G.; Reshetnikova, L.; Clark, B.F.; Nyborg, J. Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu, and a GTP analog. Science 1995, 270, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Sanbonmatsu, K.Y.; Joseph, S.; Tung, C.S. Simulating movement of tRNA into the ribosome during decoding. Proc. Natl. Acad. Sci. USA 2005, 102, 15854–15859. [Google Scholar] [CrossRef]
- Whitford, P.C.; Onuchic, J.N.; Sanbonmatsu, K.Y. Connecting energy landscapes with experimental rates for aminoacyl-tRNA accommodation in the ribosome. J. Am. Chem. Soc. 2010, 132, 13170–13171. [Google Scholar] [CrossRef]
- Whitford, P.C.; Geggier, P.; Altman, R.B.; Blanchard, S.C.; Onuchic, J.N.; Sanbonmatsu, K.Y. Accommodation of aminoacyl-tRNA into the ribosome involves reversible excursions along multiple pathways. RNA 2010, 16, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Ieong, K.W.; Uzun, U.; Selmer, M.; Ehrenberg, M. Two proofreading steps amplify the accuracy of genetic code translation. Proc. Natl. Acad. Sci. USA 2016, 113, 13744–13749. [Google Scholar] [CrossRef]
- Aqvist, J.; Kamerlin, S.C. The conformation of a catalytic loop is central to GTPase activity on the ribosome. Biochemistry 2015, 54, 546–556. [Google Scholar] [CrossRef]
- Shi, X.; Khade, P.K.; Sanbonmatsu, K.Y.; Joseph, S. Functional role of the sarcin-ricin loop of the 23S rRNA in the elongation cycle of protein synthesis. J. Mol. Biol. 2012, 419, 125–138. [Google Scholar] [CrossRef]
- Sanbonmatsu, K.Y. Alignment/misalignment hypothesis for tRNA selection by the ribosome. Biochimie 2006, 88, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Valle, M.; Zavialov, A.; Li, W.; Stagg, S.M.; Sengupta, J.; Nielsen, R.C.; Nissen, P.; Harvey, S.C.; Ehrenberg, M.; Frank, J. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryo-electron microscopy. Nat. Struct. Biol. 2003, 10, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Loveland, A.B.; Demo, G.; Grigorieff, N.; Korostelev, A.A. Ensemble cryo-EM elucidates the mechanism of translation fidelity. Nature 2017, 546, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Rozov, A.; Wolff, P.; Grosjean, H.; Yusupov, M.; Yusupova, G.; Westhof, E. Tautomeric G*U pairs within the molecular ribosomal grip and fidelity of decoding in bacteria. Nucleic Acids Res. 2018, 46, 7425–7435. [Google Scholar] [CrossRef] [PubMed]
- Pak, D.; Du, N.; Kim, Y.; Sun, Y.; Burton, Z.F. Rooted tRNAomes and evolution of the genetic code. Transcription 2018, 9, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Pak, D.; Kim, Y.; Burton, Z.F. Aminoacyl-tRNA synthetase evolution and sectoring of the genetic code. Transcription 2018, 9, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Whitford, P.C. Capturing Transition States for tRNA Hybrid-State Formation in the Ribosome. J. Phys. Chem. B 2016, 120, 8768–8775. [Google Scholar] [CrossRef]
- Zhou, J.; Lancaster, L.; Donohue, J.P.; Noller, H.F. How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. Science 2014, 345, 1188–1191. [Google Scholar] [CrossRef]
- Bowman, J.C.; Hud, N.V.; Williams, L.D. The ribosome challenge to the RNA world. J. Mol. Evol. 2015, 80, 143–161. [Google Scholar] [CrossRef]
- Wallin, G.; Aqvist, J. The transition state for peptide bond formation reveals the ribosome as a water trap. Proc. Natl. Acad. Sci. USA 2010, 107, 1888–1893. [Google Scholar] [CrossRef]
- Sanbonmatsu, K.Y. Flipping through the genetic code: New developments in discrimination between cognate and near-cognate tRNAs and the effect of antibiotics. J. Mol. Biol. 2014, 426, 3197–3200. [Google Scholar] [CrossRef]
- Wohlgemuth, I.; Brenner, S.; Beringer, M.; Rodnina, M.V. Modulation of the rate of peptidyl transfer on the ribosome by the nature of substrates. J. Biol. Chem. 2008, 283, 32229–32235. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Socan, J.; Himo, F.; Aqvist, J. Mechanistic alternatives for peptide bond formation on the ribosome. Nucleic Acids Res. 2018, 46, 5345–5354. [Google Scholar] [CrossRef] [PubMed]
- Rodnina, M.V. The ribosome as a versatile catalyst: Reactions at the peptidyl transferase center. Curr. Opin. Struct. Biol. 2013, 23, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.J.; Trobro, S.; He, S.L.; Aqvist, J.; Green, R. A Role for the 2′ OH of peptidyl-tRNA substrate in peptide release on the ribosome revealed through RF-mediated rescue. Chem. Biol. 2012, 19, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Sund, J.; Ander, M.; Aqvist, J. Principles of stop-codon reading on the ribosome. Nature 2010, 465, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.; Whitford, P.C. Steric interactions lead to collective tilting motion in the ribosome during mRNA-tRNA translocation. Nat. Commun. 2016, 7, 10586. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cui, X.; Beausang, J.F.; Zhang, H.; Farrell, I.; Cooperman, B.S.; Goldman, Y.E. Elongation factor G initiates translocation through a power stroke. Proc. Natl. Acad. Sci. USA 2016, 113, 7515–7520. [Google Scholar] [CrossRef]
- Mace, K.; Giudice, E.; Chat, S.; Gillet, R. The structure of an elongation factor G-ribosome complex captured in the absence of inhibitors. Nucleic Acids Res. 2018, 46, 3211–3217. [Google Scholar] [CrossRef]
- Lin, J.; Gagnon, M.G.; Bulkley, D.; Steitz, T.A. Conformational changes of elongation factor G on the ribosome during tRNA translocation. Cell 2015, 160, 219–227. [Google Scholar] [CrossRef]
- Liu, Q.; Fredrick, K. Intersubunit Bridges of the Bacterial Ribosome. J. Mol. Biol. 2016, 428, 2146–2164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fredrick, K. Contribution of intersubunit bridges to the energy barrier of ribosomal translocation. Nucleic Acids Res. 2013, 41, 565–574. [Google Scholar] [CrossRef]
- Sergiev, P.V.; Lesnyak, D.V.; Kiparisov, S.V.; Burakovsky, D.E.; Leonov, A.A.; Bogdanov, A.A.; Brimacombe, R.; Dontsova, O.A. Function of the ribosomal E-site: A mutagenesis study. Nucleic Acids Res. 2005, 33, 6048–6056. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kowiatek, B.; Opron, K.; Burton, Z.F. Type-II tRNAs and Evolution of Translation Systems and the Genetic Code. Int. J. Mol. Sci 2018, 19, 3275. [Google Scholar] [CrossRef] [PubMed]
- Root-Bernstein, R.; Kim, Y.; Sanjay, A.; Burton, Z.F. tRNA evolution from the proto-tRNA minihelix world. Transcription 2016, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Nagaswamy, U.; Fox, G.E. RNA ligation and the origin of tRNA. Orig. Life Evol. Biosph. 2003, 33, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. The origin of the tRNA molecule: Independent data favor a specific model of its evolution. Biochimie 2012, 94, 1464–1466. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M. A comparison among the models proposed to explain the origin of the tRNA molecule: A synthesis. J. Mol. Evol. 2009, 69, 1–9. [Google Scholar] [CrossRef]
- Widmann, J.; Di Giulio, M.; Yarus, M.; Knight, R. tRNA creation by hairpin duplication. J. Mol. Evol. 2005, 61, 524–530. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.; Surman, A.J.; Cooper, G.J.; Suarez-Marina, I.; Hosni, Z.; Lee, M.P.; Cronin, L. Formation of oligopeptides in high yield under simple programmable conditions. Nat. Commun. 2015, 6, 8385. [Google Scholar] [CrossRef]
- Becker, S.; Schneider, C.; Okamura, H.; Crisp, A.; Amatov, T.; Dejmek, M.; Carell, T. Wet-dry cycles enable the parallel origin of canonical and non-canonical nucleosides by continuous synthesis. Nat. Commun. 2018, 9, 163. [Google Scholar] [CrossRef]
- Perona, J.J.; Gruic-Sovulj, I. Synthetic and editing mechanisms of aminoacyl-tRNA synthetases. Top Curr. Chem. 2014, 344, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Rodin, S.N.; Ohno, S. Two types of aminoacyl-tRNA synthetases could be originally encoded by complementary strands of the same nucleic acid. Orig. Life Evol. Biosph. 1995, 25, 565–589. [Google Scholar] [CrossRef]
- Carter, C.W., Jr. Coding of Class I and II Aminoacyl-tRNA Synthetases. Adv. Exp. Med. Biol. 2017, 966, 103–148. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rodriguez, L.; Erdogan, O.; Jimenez-Rodriguez, M.; Gonzalez-Rivera, K.; Williams, T.; Li, L.; Weinreb, V.; Collier, M.; Chandrasekaran, S.N.; Ambroggio, X.; et al. Functional Class I and II Amino Acid-activating Enzymes Can Be Coded by Opposite Strands of the Same Gene. J. Biol. Chem. 2015, 290, 19710–19725. [Google Scholar] [CrossRef]
- Carter, C.W., Jr.; Li, L.; Weinreb, V.; Collier, M.; Gonzalez-Rivera, K.; Jimenez-Rodriguez, M.; Erdogan, O.; Kuhlman, B.; Ambroggio, X.; Williams, T.; et al. The Rodin-Ohno hypothesis that two enzyme superfamilies descended from one ancestral gene: An unlikely scenario for the origins of translation that will not be dismissed. Biol. Direct. 2014, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.N.; Yardimci, G.G.; Erdogan, O.; Roach, J.; Carter, C.W., Jr. Statistical evaluation of the Rodin-Ohno hypothesis: Sense/antisense coding of ancestral class I and II aminoacyl-tRNA synthetases. Mol. Biol. Evol. 2013, 30, 1588–1604. [Google Scholar] [CrossRef]
- Rodin, A.S.; Rodin, S.N.; Carter, C.W., Jr. On primordial sense-antisense coding. J. Mol. Evol. 2009, 69, 555–567. [Google Scholar] [CrossRef]
- Root-Bernstein, R.; Root-Bernstein, M. The ribosome as a missing link in prebiotic evolution II: Ribosomes encode ribosomal proteins that bind to common regions of their own mRNAs and rRNAs. J. Theor. Biol. 2016, 397, 115–127. [Google Scholar] [CrossRef]
- Root-Bernstein, M.; Root-Bernstein, R. The ribosome as a missing link in the evolution of life. J. Theor. Biol. 2015, 367, 130–158. [Google Scholar] [CrossRef]
- Bernhardt, H.S.; Patrick, W.M. Genetic code evolution started with the incorporation of glycine, followed by other small hydrophilic amino acids. J. Mol. Evol. 2014, 78, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, H.S.; Tate, W.P. Evidence from glycine transfer RNA of a frozen accident at the dawn of the genetic code. Biol. Direct. 2008, 3, 53. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Novozhilov, A.S. Origin and Evolution of the Universal Genetic Code. Annu. Rev. Genet. 2017, 51, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Novozhilov, A.S. Origin and evolution of the genetic code: The universal enigma. IUBMB Life 2009, 61, 99–111. [Google Scholar] [CrossRef] [PubMed]
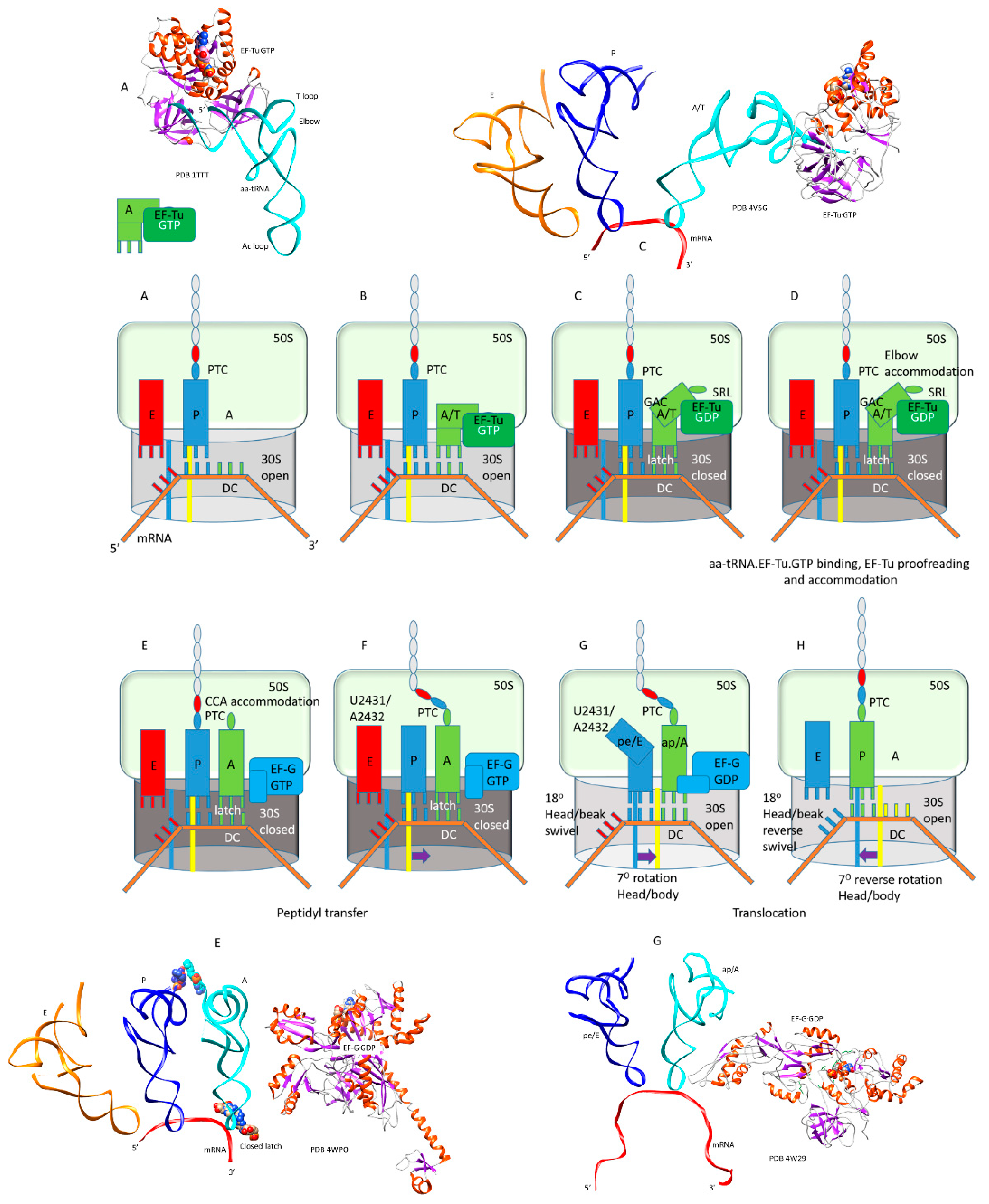
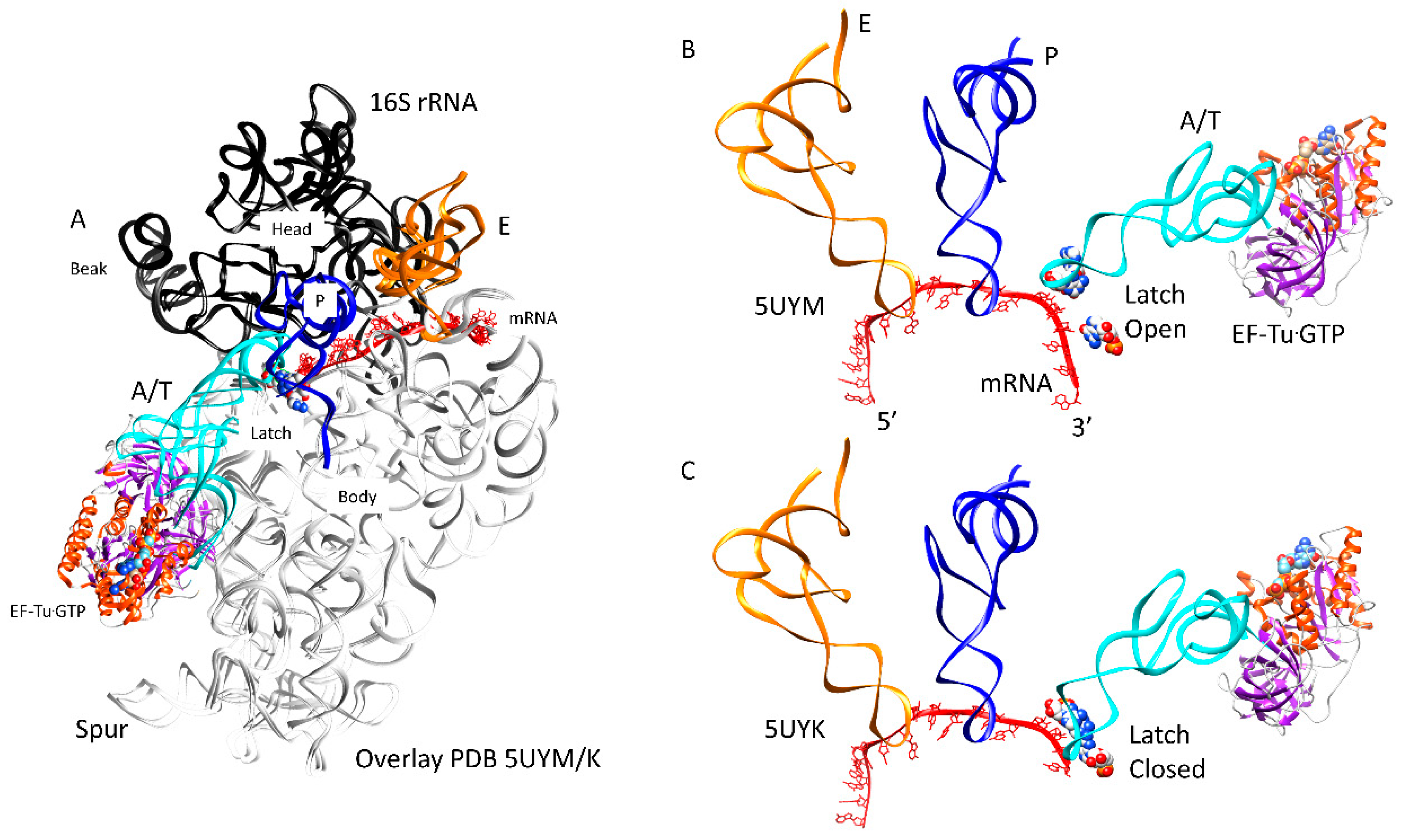
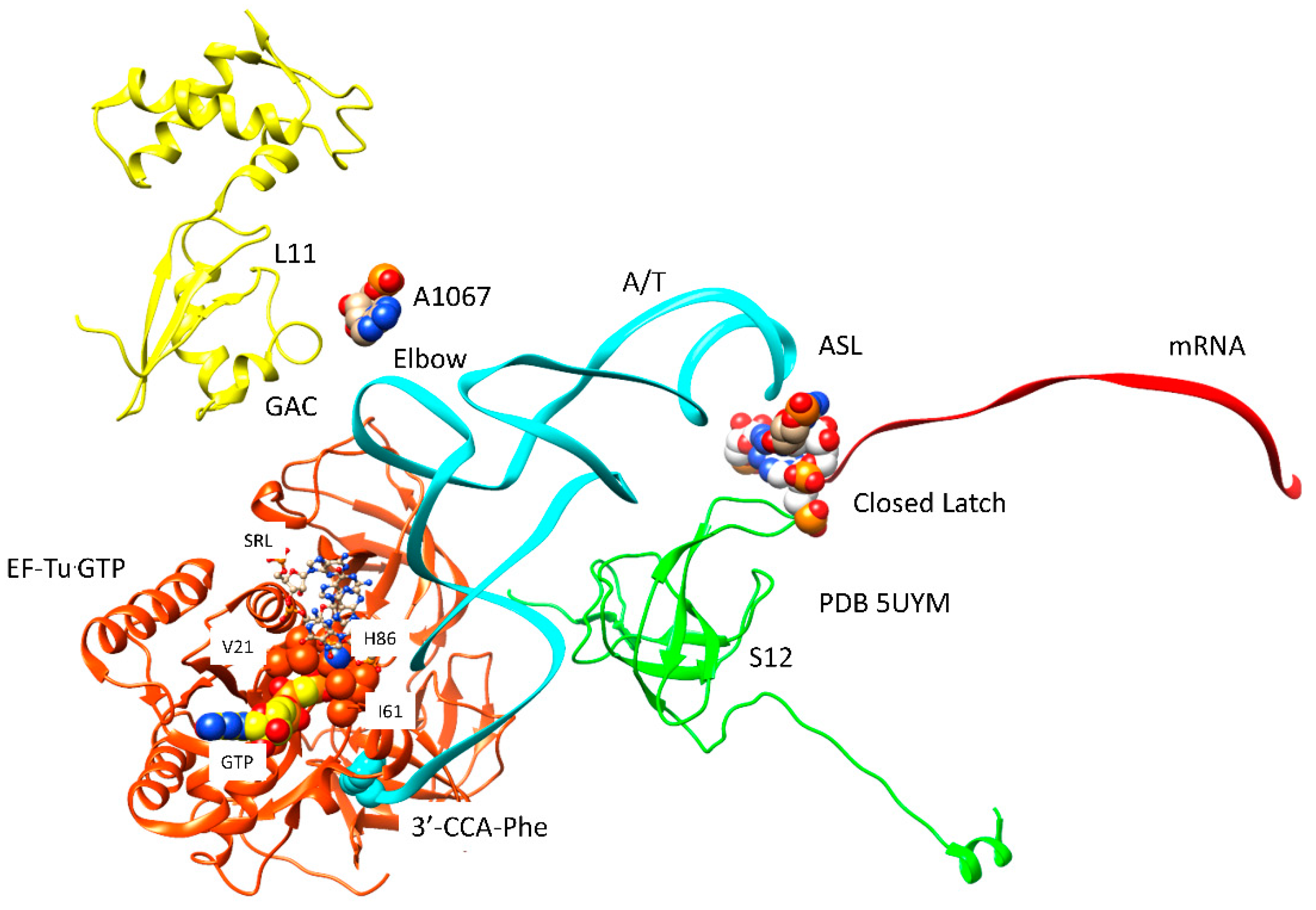
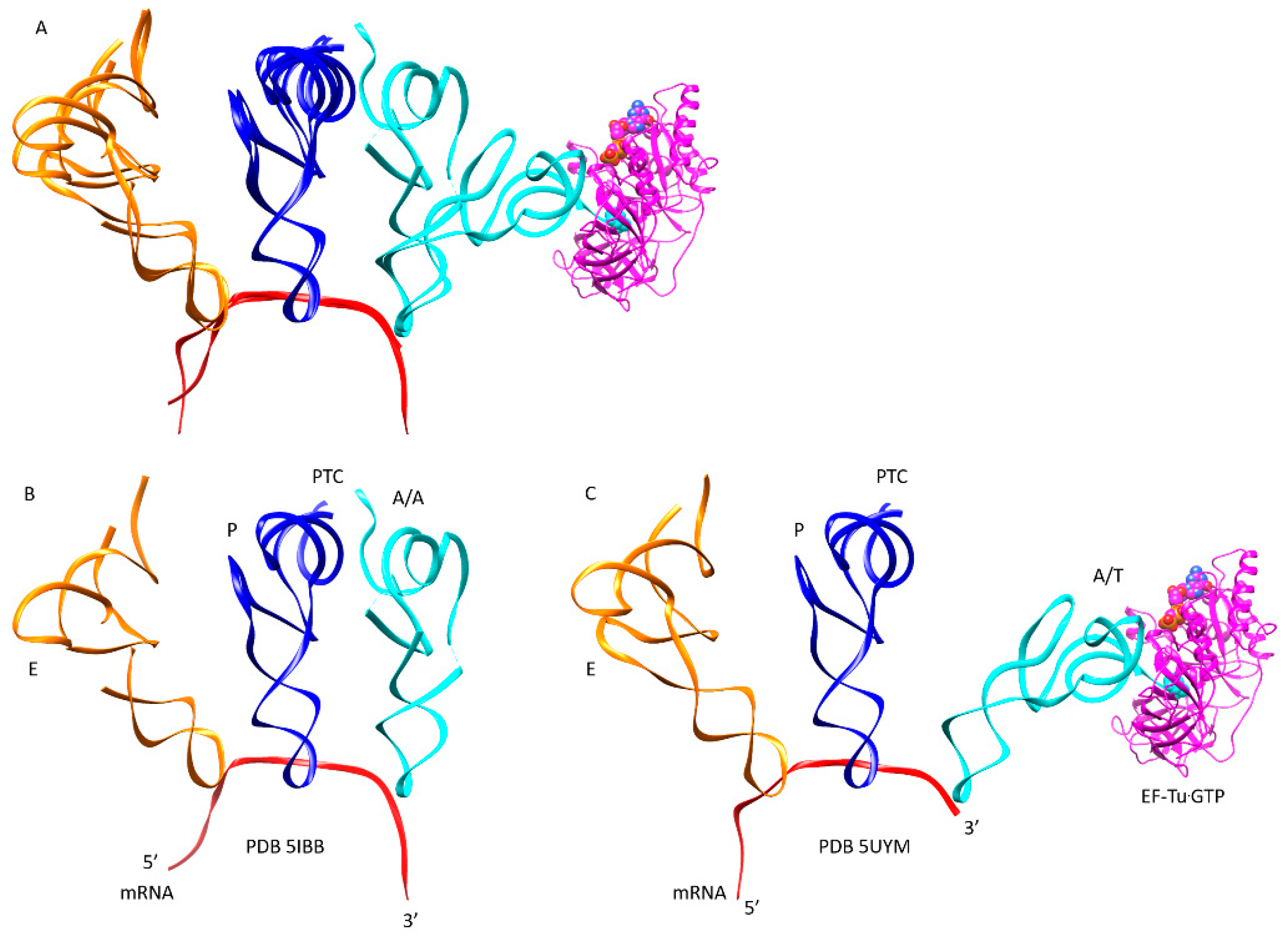
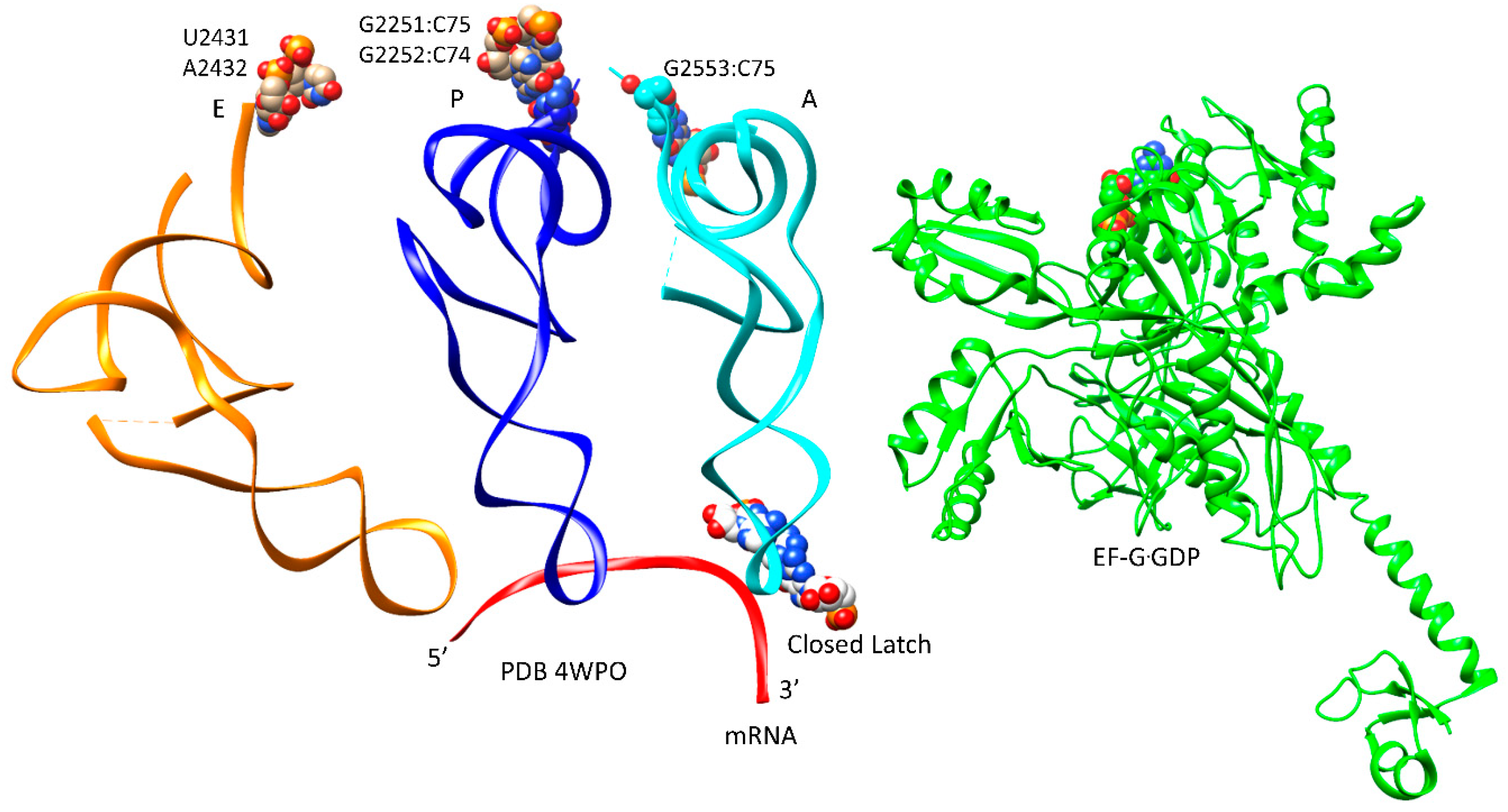
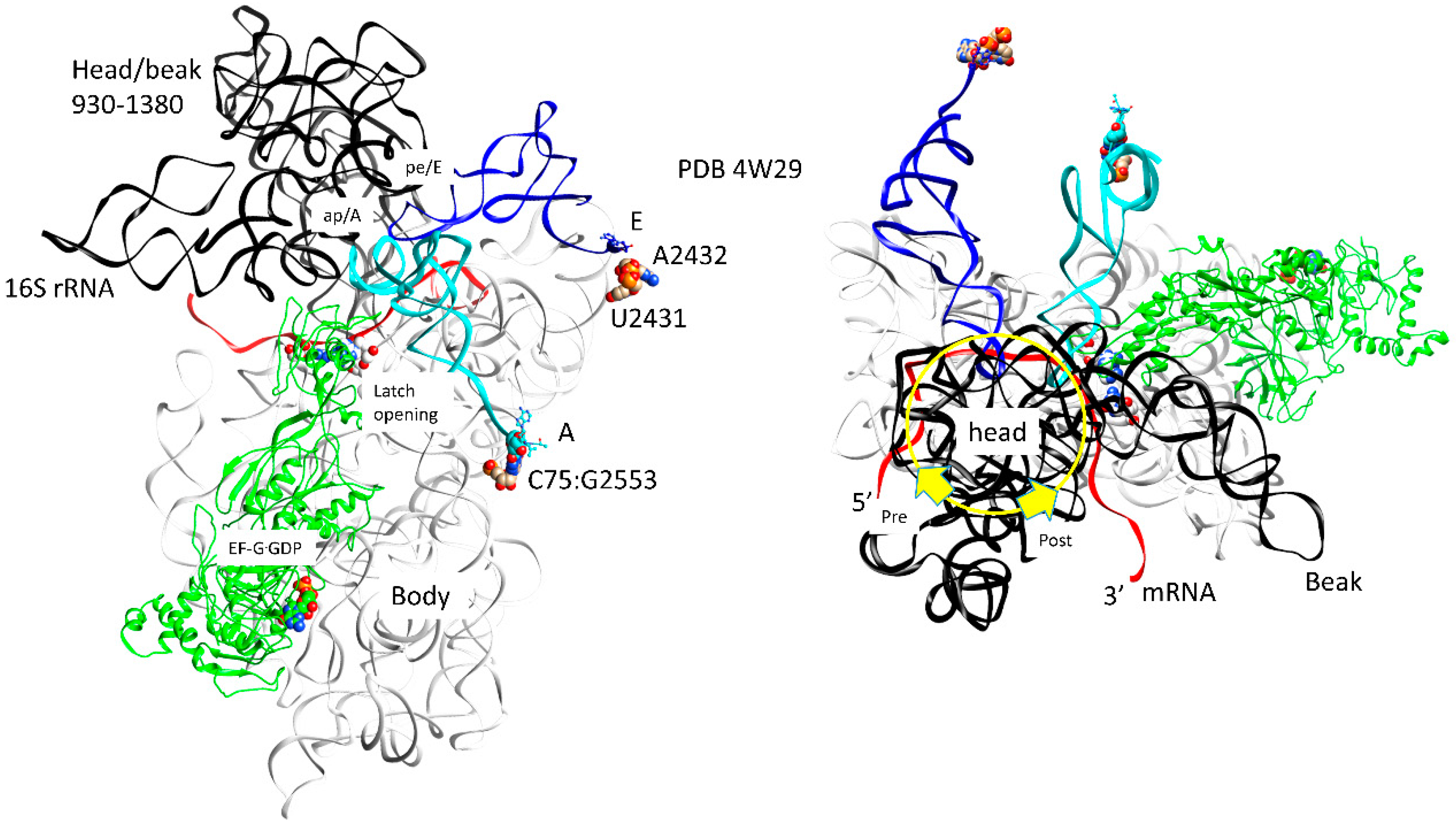
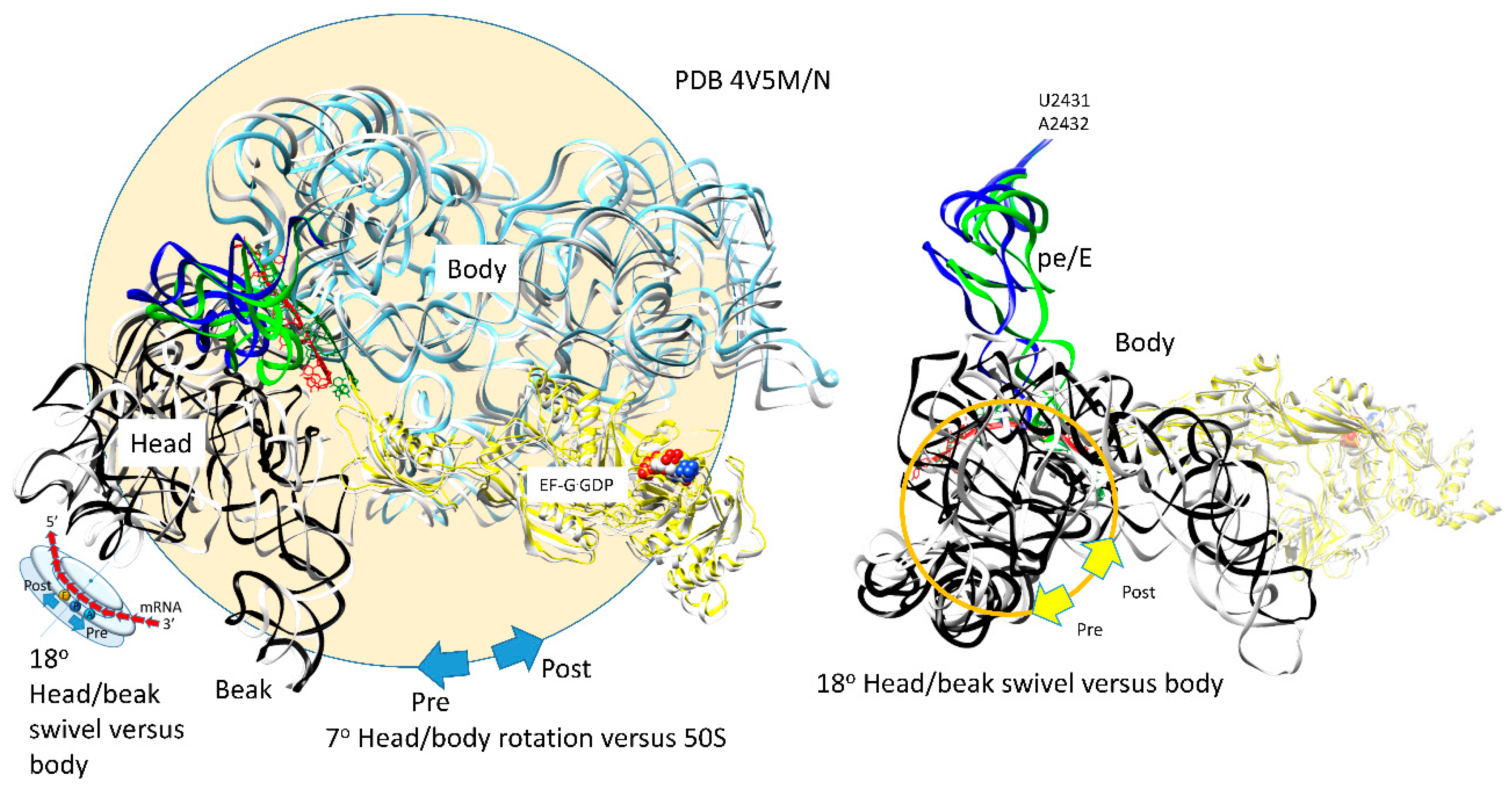
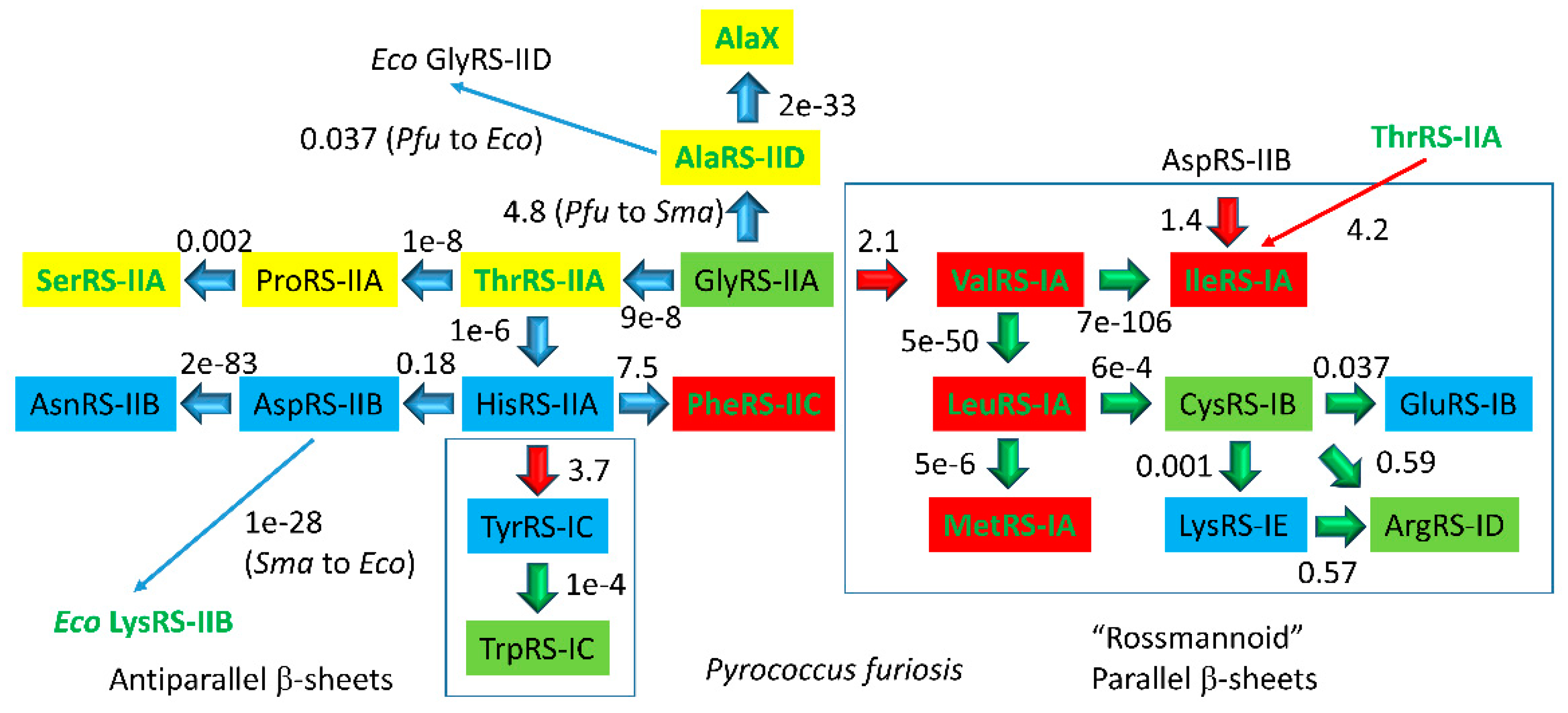
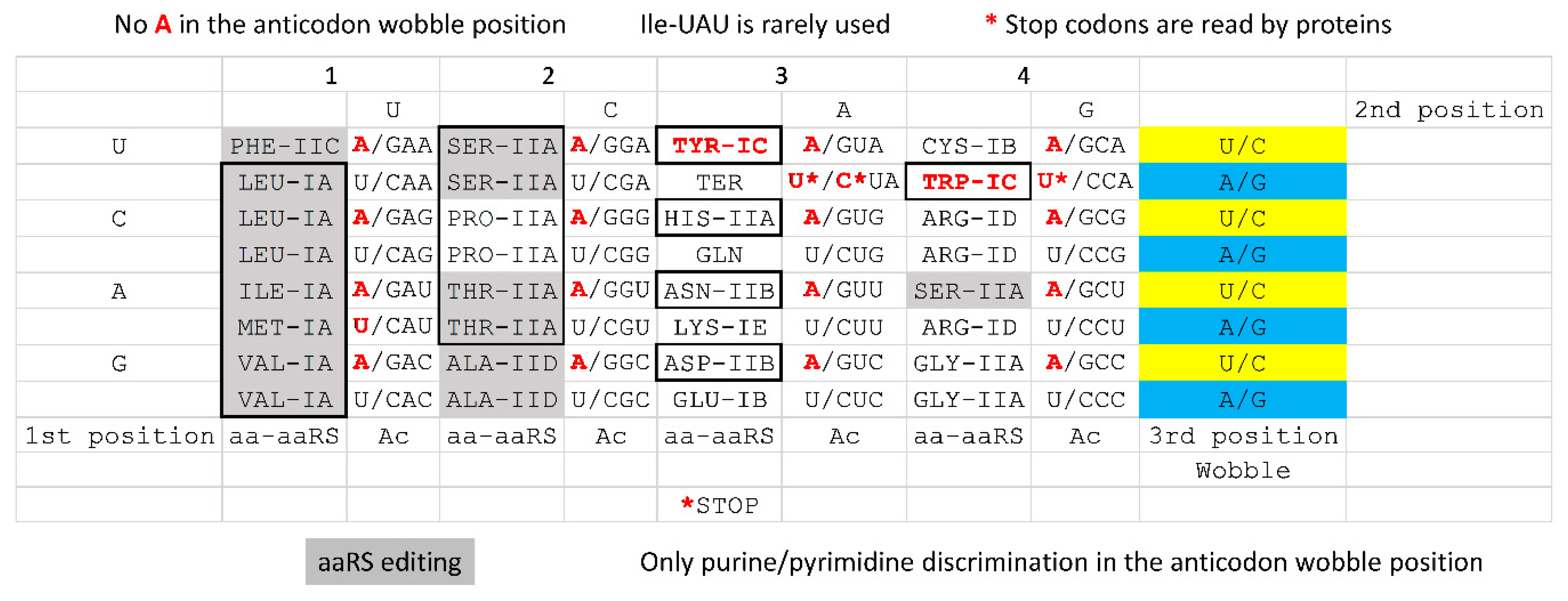
| Intermediate | Stage | Ternary Complex | EF-G | A Site | P Site | E Site | PDB | Proofreading | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Figure 1) | ASL | CCA/PTC | ASL | CCA/PTC | ASL | CCA | |||||
| A | aa-tRNA.EF-Tu.GTP (free) | empty | empty | P site | A2451, C2452, U2585, G2252:C74, G2251:C75, CCA-peptide | E site | E site U2431, A2432 | 1TTT | |||
| B | Cplx 1 | aa-tRNA.EF-Tu.GTP (bound) | A/T, open | empty | P site | A2451, C2452, U2585, G2252:C74, G2251:C75, CCA-peptide | E site | E site U2431, A2432 | 5UYK | ||
| Cplx 2 | aa-tRNA.EF-Tu.GTP (bound) | A/T, latched | empty | P site | A2451, C2452, U2585, G2252:C74, G2251:C75, CCA-peptide | E site | E site U2431, A2432 | 5UYL | !! | ||
| C | Cplx 3 | aa-tRNA.EF-Tu.GTP (bound) | A/T, latched | empty | P site | A2451, C2452, U2585, G2252:C74, G2251:C75, CCA-peptide | E site | E site U2431, A2432 | 5UYM | !! | |
| D | Elbow | aa-tRNA.EF-Tu.GDP (bound) | EA, latched | empty | P site | A2451, C2452, U2585, G2252:C74, G2251:C75, CCA-peptide | E site | E site U2431, A2432 | !!!! | ||
| E | CCA | EF-G.GTP (binds) | A, latched | CCA-aa, C75:G2553 | P site | A2451, C2452, U2585, G2252:C74, G2251:C75, CCA-peptide | E site | E site U2431, A2432 | 5IBB, 4WPO | ||
| F | EF-G.GTP | A, latched | CCA-peptide, C75:G2553 | P site | A2451, C2452, U2585, G2252:C74, G2251:C75, CCA | E site | E site U2431, A2432 | ||||
| G | pre | EF-G.GDP | ap, open | (CCA-peptide, C75:G2553) | empty | (ap/A<-->ap/ap tRNA-peptide) | pe | pe/E tRNA: U2431, A2432 | 4W29, 4V5M | ||
| pre/post | EF-G.GDP | ap, open | (CCA-peptide, C75:G2553) | empty | (ap/A<-->ap/ap tRNA-peptide) | pe | pe/E tRNA: U2431, A2432 | ||||
| H | post | EF-G.GDP | ap, open | (CCA-peptide, C75:G2553) | empty | (ap/A<-->ap/ap tRNA-peptide) | pe | pe/E tRNA: U2431, A2432 | 4V5N, 5OT7 | ||
| latch: 30S: S12, 16S: G530~A1492, A1493; 23S: A1913 | |||||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opron, K.; Burton, Z.F. Ribosome Structure, Function, and Early Evolution. Int. J. Mol. Sci. 2019, 20, 40. https://doi.org/10.3390/ijms20010040
Opron K, Burton ZF. Ribosome Structure, Function, and Early Evolution. International Journal of Molecular Sciences. 2019; 20(1):40. https://doi.org/10.3390/ijms20010040
Chicago/Turabian StyleOpron, Kristopher, and Zachary F. Burton. 2019. "Ribosome Structure, Function, and Early Evolution" International Journal of Molecular Sciences 20, no. 1: 40. https://doi.org/10.3390/ijms20010040
APA StyleOpron, K., & Burton, Z. F. (2019). Ribosome Structure, Function, and Early Evolution. International Journal of Molecular Sciences, 20(1), 40. https://doi.org/10.3390/ijms20010040






