Identification and In Vivo Characterisation of Cardioactive Peptides in Drosophila melanogaster
Abstract
1. Introduction
2. Results
2.1. The Larval Drosophila Heart Is Highly Responsive to Peptide Application
2.2. Peptidergic Signalling Affects Proper Heart Function In Vivo
3. Discussion
4. Materials and Methods
4.1. Fly Strains
4.2. Peptide Application Assay and Dose–Response Analysis
4.3. In Vivo Measurement of Heart Parameters
4.4. Video Analysis and Calculation of Cardiac Parameters
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouwman, J.; Spijker, S.; Schut, D.; Wachtler, B.; Ylstra, B.; Smit, A.B.; Verhage, M. Reduced expression of neuropeptide genes in a genome-wide screen of a secretion-deficient mouse. J. Neurochem. 2006, 99, 84–96. [Google Scholar] [CrossRef]
- Hewes, R.S.; Taghert, P.H. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 2001, 11, 1126–1142. [Google Scholar] [CrossRef] [PubMed]
- Hokfelt, T. Neuropeptides in perspective: the last ten years. Neuron 1991, 7, 867–879. [Google Scholar] [CrossRef]
- Husson, S.J.; Landuyt, B.; Nys, T.; Baggerman, G.; Boonen, K.; Clynen, E.; Lindemans, M.; Janssen, T.; Schoofs, L. Comparative peptidomics of Caenorhabditis elegans versus C. briggsae by LC-MALDI-TOF MS. Peptides 2009, 30, 449–457. [Google Scholar] [CrossRef]
- Li, B.; Predel, R.; Neupert, S.; Hauser, F.; Tanaka, Y.; Cazzamali, G.; Williamson, M.; Arakane, Y.; Verleyen, P.; Schoofs, L.; et al. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res. 2008, 18, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nässel, D.R.; Winther, A.M. Drosophila neuropeptides in regulation of physiology and behaviour. Prog. Neurobiol. 2010, 92, 42–104. [Google Scholar] [CrossRef]
- Anctil, M. Chemical transmission in the sea anemone Nematostella vectensis: A genomic perspective. Comp. Biochem. Physiol. Part D Genom. Proteom. 2009, 4, 268–289. [Google Scholar] [CrossRef]
- Grimmelikhuijzen, C.J.; Leviev, I.; Carstensen, K. Peptides in the nervous systems of cnidarians: Structure, function, and biosynthesis. Int. Rev. Cytol. 1996, 167, 37–89. [Google Scholar] [PubMed]
- Hansen, G.N.; Williamson, M.; Grimmelikhuijzen, C.J. A new case of neuropeptide coexpression (RGamide and LWamides) in Hydra, found by whole-mount, two-color double-labeling in situ hybridization. Cell Tissue Res. 2002, 308, 157–165. [Google Scholar] [CrossRef]
- Takahashi, T.; Hayakawa, E.; Koizumi, O.; Fujisawa, T. Neuropeptides and their functions in Hydra. Acta Boil. Hung. 2008, 59, 227–235. [Google Scholar] [CrossRef]
- Schoofs, L.; De Loof, A.; Van Hiel, M.B. Neuropeptides as regulators of behaviour in insects. Annu. Rev. Èntomol. 2017, 62, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Anderson, K.M.; Chang, C.L.; Makarewich, C.A.; Nelson, B.R.; McAnally, J.R.; Kasaragod, P.; Shelton, J.M.; Liou, J.; Bassel-Duby, R.; et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 2015, 160, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Makarewich, C.A.; Anderson, K.M.; Shelton, J.M.; Bezprozvannaya, S.; Bassel-Duby, R.; Olson, E.N. Widespread control of calcium signalling by a family of SERCA-inhibiting micropeptides. Sci. Signal. 2016, 9, ra119. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.R.; Makarewich, C.A.; Anderson, D.M.; Winders, B.R.; Troupes, C.D.; Wu, F.; Reese, A.L.; McAnally, J.R.; Chen, X.; Kavalali, E.T.; et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science 2016, 351, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Zanet, J.; Chanut-Delalande, H.; Plaza, S.; Payre, F. Small peptides as newcomers in the control of Drosophila development. Curr. Top. Dev. Biol. 2016, 117, 199–219. [Google Scholar] [PubMed]
- Baraban, S.C.; Tallent, M.K. Interneuron Diversity series: Interneuronal neuropeptides—Endogenous regulators of neuronal excitability. Trends Neurosci. 2004, 27, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hokfelt, T.; Broberger, C.; Xu, Z.Q.; Sergeyev, V.; Ubink, R.; Diez, M. Neuropeptides—An overview. Neuropharmacology 2000, 39, 1337–1356. [Google Scholar] [CrossRef]
- Zupanc, G.K. Peptidergic transmission: From morphological correlates to functional implications. Micron 1996, 27, 35–91. [Google Scholar] [CrossRef]
- Keller, R. Crustacean neuropeptides: Structures, functions and comparative aspects. Experientia 1992, 48, 439–448. [Google Scholar] [CrossRef]
- Moroz, L.L.; Edwards, J.R.; Puthanveettil, S.V.; Kohn, A.B.; Ha, T.; Heyland, A.; Knudsen, B.; Sahni, A.; Yu, F.; Liu, L.; et al. Neuronal transcriptome of Aplysia: Neuronal compartments and circuitry. Cell 2006, 127, 1453–1467. [Google Scholar] [CrossRef]
- Nusbaum, M.P.; Blitz, D.M.; Swensen, A.M.; Wood, D.; Marder, E. The roles of co-transmission in neural network modulation. Trends Neurosci. 2001, 24, 146–154. [Google Scholar] [CrossRef]
- O’Shea, M.; Schaffer, M. Neuropeptide function: The invertebrate contribution. Annu. Rev. Neurosci. 1985, 8, 171–198. [Google Scholar] [CrossRef] [PubMed]
- Predel, R.; Nachman, R.J.; Gäde, G. Myostimulatory neuropeptides in cockroaches: Structures, distribution, pharmacological activities, and mimetic analogs. J. Insect Physiol. 2001, 47, 311–324. [Google Scholar] [CrossRef]
- Predel, R.; Neupert, S.; Garczynski, S.F.; Crim, J.W.; Brown, M.R.; Russell, W.K.; Kahnt, J.; Russell, D.H.; Nachman, R.J. Neuropeptidomics of the mosquito Aedes aegypti. J. Proteome Res. 2010, 9, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Schoofs, L.; Vanden Broeck, J.; De Loof, A. The myotropic peptides of Locusta migratoria: Structures, distribution, functions and receptors. Insect Biochem. Mol. Biol. 1993, 23, 859–881. [Google Scholar] [CrossRef]
- Dietzl, G.; Chen, D.; Schnorrer, F.; Su, K.C.; Barinova, Y.; Fellner, M.; Gasser, B.; Kinsey, K.; Oppel, S.; Scheiblauer, S.; et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007, 448, 151–156. [Google Scholar] [CrossRef]
- Jones, W.D. The expanding reach of the GAL4/UAS system into the behavioural neurobiology of Drosophila. BMB Rep. 2009, 42, 705–712. [Google Scholar] [CrossRef]
- Luo, L.; Callaway, E.M.; Svoboda, K. Genetic dissection of neural circuits. Neuron 2008, 57, 634–660. [Google Scholar] [CrossRef]
- McNabb, S.L.; Baker, J.D.; Agapite, J.; Steller, H.; Riddiford, L.M.; Truman, J.W. Disruption of a behavioural sequence by targeted death of peptidergic neurons in Drosophila. Neuron 1997, 19, 813–823. [Google Scholar] [CrossRef]
- Pfeiffer, B.D.; Jenett, A.; Hammonds, A.S.; Ngo, T.T.; Misra, S.; Murphy, C.; Scully, A.; Carlson, J.W.; Wan, K.H.; Laverty, T.R.; et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. (PNAS) 2008, 105, 9715–9720. [Google Scholar] [CrossRef]
- Predel, R.; Eckert, M.; Pollak, E.; Molnar, L.; Scheibner, O.; Neupert, S. Peptidomics of identified neurons demonstrates a highly differentiated expression pattern of FXPRLamides in the neuroendocrine system of an insect. J. Comp. Neurol. 2007, 500, 498–512. [Google Scholar] [CrossRef]
- Predel, R.; Wegener, C.; Russell, W.K.; Tichy, S.E.; Russell, D.H.; Nachman, R.J. Peptidomics of CNS-associated neurohemal systems of adult Drosophila melanogaster: A mass spectrometric survey of peptides from individual flies. J. Comp. Neurol. 2004, 474, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Wegener, C.; Reinl, T.; Jänsch, L.; Predel, R. Direct mass spectrometric peptide profiling and fragmentation of larval peptide hormone release sites in Drosophila melanogaster reveals tagma-specific peptide expression and differential processing. J. Neurochem. 2006, 96, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Brogiolo, W.; Stocker, H.; Ikeya, T.; Rintelen, F.; Fernandez, R.; Hafen, E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr. Biol. CB 2001, 11, 213–221. [Google Scholar] [CrossRef]
- Liu, H.; Kubli, E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl. Acad. Sci. (PNAS) 2003, 100, 9929–9933. [Google Scholar] [CrossRef] [PubMed]
- Rulifson, E.J.; Kim, S.K.; Nusse, R. Ablation of insulin-producing neurons in flies: Growth and diabetic phenotypes. Science 2002, 296, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Wigby, S.; Chapman, T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr. Biol. CB 2005, 15, 316–321. [Google Scholar] [CrossRef]
- Yapici, N.; Kim, Y.J.; Ribeiro, C.; Dickson, B.J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature 2008, 451, 33–37. [Google Scholar] [CrossRef]
- Chen, J.; Reiher, W.; Hermann-Luibl, C.; Sellami, A.; Cognigni, P.; Kondo, S.; Helfrich-Forster, C.; Veenstra, J.A.; Wegener, C. Allatostatin A signalling in Drosophila regulates feeding and sleep and is modulated by PDF. PLoS Genet. 2016, 12, e1006346. [Google Scholar]
- Hallier, B.; Schiemann, R.; Cordes, E.; Vitos-Faleato, J.; Walter, S.; Heinisch, J.J.; Malmendal, A.; Paululat, A.; Meyer, H. Drosophila neprilysins control insulin signalling and food intake via cleavage of regulatory peptides. eLife 2016, 5, e19430. [Google Scholar] [CrossRef]
- Wang, Y.; Pu, Y.; Shen, P. Neuropeptide-gated perception of appetitive olfactory inputs in Drosophila larvae. Cell Rep. 2013, 3, 820–830. [Google Scholar] [CrossRef] [PubMed]
- DeZazzo, J.; Xia, S.; Christensen, J.; Velinzon, K.; Tully, T. Developmental expression of an amn(+) transgene rescues the mutant memory defect of amnesiac adults. J. Neurosci. 1999, 19, 8740–8746. [Google Scholar] [CrossRef] [PubMed]
- Feany, M.B.; Quinn, W.G. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science 1995, 268, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Waddell, S.; Armstrong, J.D.; Kitamoto, T.; Kaiser, K.; Quinn, W.G. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell 2000, 103, 805–813. [Google Scholar] [CrossRef]
- Cavey, M.; Collins, B.; Bertet, C.; Blau, J. Circadian rhythms in neuronal activity propagate through output circuits. Nat. Neurosci. 2016, 19, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Dubowy, C.M.; Cavanaugh, D.J. Sleep: A neuropeptidergic wake-up call for flies. Curr. Biol. CB 2014, 24, R1092-4. [Google Scholar] [CrossRef] [PubMed]
- Hermann-Luibl, C.; Yoshii, T.; Senthilan, P.R.; Dircksen, H.; Helfrich-Forster, C. The ion transport peptide is a new functional clock neuropeptide in the fruit fly Drosophila melanogaster. J. Neurosci. 2014, 34, 9522–9536. [Google Scholar] [CrossRef] [PubMed]
- Isaac, R.E.; Johnson, E.C.; Audsley, N.; Shirras, A.D. Metabolic inactivation of the circadian transmitter, pigment dispersing factor (PDF), by neprilysin-like peptidases in Drosophila. J. Exp. Biol. 2007, 210 Pt 24, 4465–4470. [Google Scholar] [CrossRef]
- Shang, Y.; Donelson, N.C.; Vecsey, C.G.; Guo, F.; Rosbash, M.; Griffith, L.C. Short neuropeptide F is a sleep-promoting inhibitory modulator. Neuron 2013, 80, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Panz, M.; Zmojdzian, M.; Jagla, K.; Paululat, A. Neprilysin 4, a novel endopeptidase from Drosophila melanogaster, displays distinct substrate specificities and exceptional solubility states. J. Exp. Biol. 2009, 212 Pt 22, 3673–3683. [Google Scholar] [CrossRef]
- Baggerman, G.; Boonen, K.; Verleyen, P.; De Loof, A.; Schoofs, L. Peptidomic analysis of the larval Drosophila melanogaster central nervous system by two-dimensional capillary liquid chromatography quadrupole time-of-flight mass spectrometry. J. Mass Spectrom. 2005, 40, 250–260. [Google Scholar] [CrossRef]
- Yew, J.Y.; Wang, Y.; Barteneva, N.; Dikler, S.; Kutz-Naber, K.K.; Li, L.; Kravitz, E.A. Analysis of neuropeptide expression and localisation in adult Drosophila melanogaster central nervous system by affinity cell-capture mass spectrometry. J. Proteome Res. 2009, 8, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Halpern, M.E.; Keshishian, H. Identification of the neuropeptide transmitter proctolin in Drosophila larvae: Characterisation of muscle fiber-specific neuromuscular endings. J. Neurosci. 1988, 8, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.T.; Chang, C.Y.; Su, M.T.; Kuo, W.C. Necessity of angiotensin-converting enzyme-related gene for cardiac functions and longevity of Drosophila melanogaster assessed by optical coherence tomography. J. Biomed. Opt. 2014, 19, 011014. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Levine, R.B.; Ewer, J. Role of the neuropeptide CCAP in Drosophila cardiac function. J. Neurobiol. 2005, 64, 259–274. [Google Scholar] [CrossRef]
- Nichols, R.; Kaminski, S.; Walling, E.; Zornik, E. Regulating the activity of a cardioacceleratory peptide. Peptides 1999, 20, 1153–1158. [Google Scholar] [CrossRef]
- Johnson, E.; Ringo, J.; Dowse, H. Native and heterologous neuropeptides are cardioactive in Drosophila melanogaster. J. Insect Physiol. 2000, 46, 1229–1236. [Google Scholar] [CrossRef]
- Nichols, R.; McCormick, J.; Cohen, M.; Howe, E.; Jean, C.; Paisley, K.; Rosario, C. Differential processing of neuropeptides influences Drosophila heart rate. J. Neurogenet. 1999, 13, 89–104. [Google Scholar] [CrossRef]
- Ormerod, K.G.; LePine, O.K.; Bhutta, M.S.; Jung, J.; Tattersall, G.J.; Mercier, A.J. Characterizing the physiological and behavioural roles of proctolin in Drosophila melanogaster. J. Neurophysiol. 2016, 115, 568–580. [Google Scholar] [CrossRef]
- Taylor, C.A.; Winther, A.M.; Siviter, R.J.; Shirras, A.D.; Isaac, R.E.; Nässel, D.R. Identification of a proctolin preprohormone gene (Proct) of Drosophila melanogaster: Expression and predicted prohormone processing. J. Neurobiol. 2004, 58, 379–391. [Google Scholar] [CrossRef]
- Zornik, E.; Paisley, K.; Nichols, R. Neural transmitters and a peptide modulate Drosophila heart rate. Peptides 1999, 20, 45–51. [Google Scholar] [CrossRef]
- Fink, M.; Callol-Massot, C.; Chu, A.; Ruiz-Lozano, P.; Izpisua Belmonte, J.C.; Giles, W.; Bodmer, R.; Ocorr, K. A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. BioTechniques 2009, 46, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Capote, L.A.; Mendez Perez, R.; Lymperopoulos, A. GPCR signaling and cardiac function. Eur. J. Pharmacol. 2015, 763, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Merkely, B.; Bauer, A.; Geller, L.; Fazekas, L.; Freigang, K.D.; Voss, F.; Senges, J.C.; Kuebler, W.; Schoels, W. Ventricular arrhythmias induced by endothelin-1 or by acute ischemia: A comparative analysis using three-dimensional mapping. Cardiovasc. Res. 2000, 45, 310–320. [Google Scholar] [CrossRef]
- Garg, S.; Narula, J.; Marelli, C.; Cesario, D. Role of angiotensin receptor blockers in the prevention and treatment of arrhythmias. Am. J. Cardiol. 2006, 97, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Gondo, N.; Kumagai, K.; Nakashima, H.; Saku, K. Angiotensin II provokes cesium-induced ventricular tachyarrhythmias. Cardiovasc. Res. 2001, 49, 381–390. [Google Scholar] [CrossRef]
- Reid, A.C.; Brazin, J.A.; Morrey, C.; Silver, R.B.; Levi, R. Targeting cardiac mast cells: Pharmacological modulation of the local renin-angiotensin system. Curr. Pharm. Des. 2011, 17, 3744–3752. [Google Scholar] [CrossRef] [PubMed]
- Von Lewinski, D.; Kockskämper, J.; Rübertus, S.U.; Zhu, D.; Schmitto, J.D.; Schöndube, F.A.; Hasenfuss, G.; Pieske, B. Direct pro-arrhythmogenic effects of angiotensin II can be suppressed by AT1 receptor blockade in human atrial myocardium. Eur. J. Heart Fail. 2008, 10, 1172–1176. [Google Scholar] [CrossRef]
- Yahiro, E.; Ideishi, M.; Wang, L.X.; Urata, H.; Kumagai, K.; Arakawa, K.; Saku, K. Reperfusion-induced arrhythmias are suppressed by inhibition of the angiotensin II type 1 receptor. Cardiology 2003, 99, 61–67. [Google Scholar] [CrossRef]
- Takata, Y.; Hirayama, Y.; Kiyomi, S.; Ogawa, T.; Iga, K.; Ishii, T.; Nagai, Y.; Ibukiyama, C. The beneficial effects of atrial natriuretic peptide on arrhythmias and myocardial high-energy phosphates after reperfusion. Cardiovasc. Res. 1996, 32, 286–293. [Google Scholar] [CrossRef]
- Zois, N.E.; Bartels, E.D.; Hunter, I.; Kousholt, B.S.; Olsen, L.H.; Goetze, J.P. Natriuretic peptides in cardiometabolic regulation and disease. Nat. Rev. Cardiol. 2014, 11, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Dulcis, D.; Levine, R.B. Innervation of the heart of the adult fruit fly, Drosophila melanogaster. J. Comp. Neurol. 2003, 465, 560–578. [Google Scholar] [CrossRef]
- Cook, B.J.; Holman, G.M.; Wagner, R.M.; Nachman, R.J. Pharmacological actions of a new class of neuropeptides, the Leucokinins-I-IV, on the visceral muscles of Leucophaea maderae. Comp. Biochem. Physiol. C 1989, 93, 257–262. [Google Scholar] [CrossRef]
- Cook, B.J.; Holman, G.M.; Wagner, R.M.; Nachman, R.J. Comparative pharmacological actions of Leucokinins-V-VIII on the visceral muscles of Leucophaea maderae. Comp. Biochem. Physiol. C 1990, 95, 19–24. [Google Scholar] [CrossRef]
- Poels, J.; Birse, R.T.; Nachman, R.J.; Fichna, J.; Janecka, A.; Vanden Broeck, J.; Nässel, D.R. Characterization and distribution of NKD, a receptor for Drosophila tachykinin-related peptide 6. Peptides 2009, 30, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Birse, R.T.; Johnson, E.C.; Taghert, P.H.; Nässel, D.R. Widely distributed Drosophila G-protein-coupled receptor (CG7887) is activated by endogenous tachykinin-related peptides. J. Neurobiol. 2006, 66, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.J.; Burton, K.J.; Zantello, M.R.; Smith, V.G.; Lowery, D.L.; Kubiak, T.M. Type A allatostatins from Drosophila melanogaster and Diplotera puncata activate two Drosophila allatostatin receptors, DAR-1 and DAR-2, expressed in CHO cells. Biochem. Biophys. Res. Commun. 2001, 286, 895–901. [Google Scholar] [CrossRef]
- McGaw, I.J.; Wilkens, J.L.; McMahon, B.R.; Airriess, C.N. Crustacean cardioexcitatory peptides may inhibit the heart in vivo. J. Exp. Biol. 1995, 198 Pt 12, 2547–2550. [Google Scholar]
- Vogler, G.; Ocorr, K. Visualizing the beating heart in Drosophila. J. Vis. Exp. 2009. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ocorr, K.; Vogler, G.; Bodmer, R. Methods to assess Drosophila heart development, function and aging. Methods 2014, 68, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Bradski, G. The OpenCV library. Dr. Dobbs J. 2000, 120, 122–125. [Google Scholar]
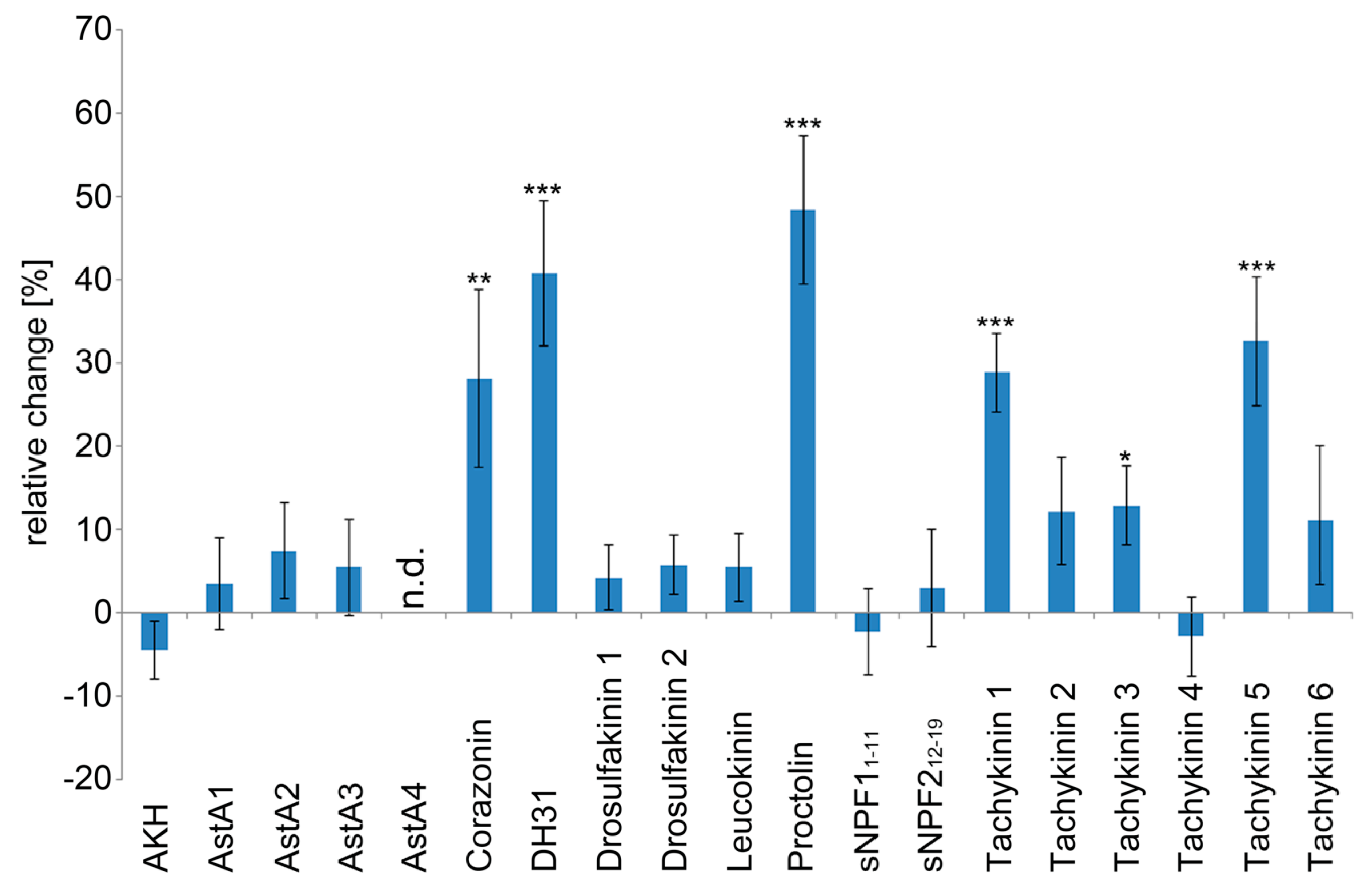
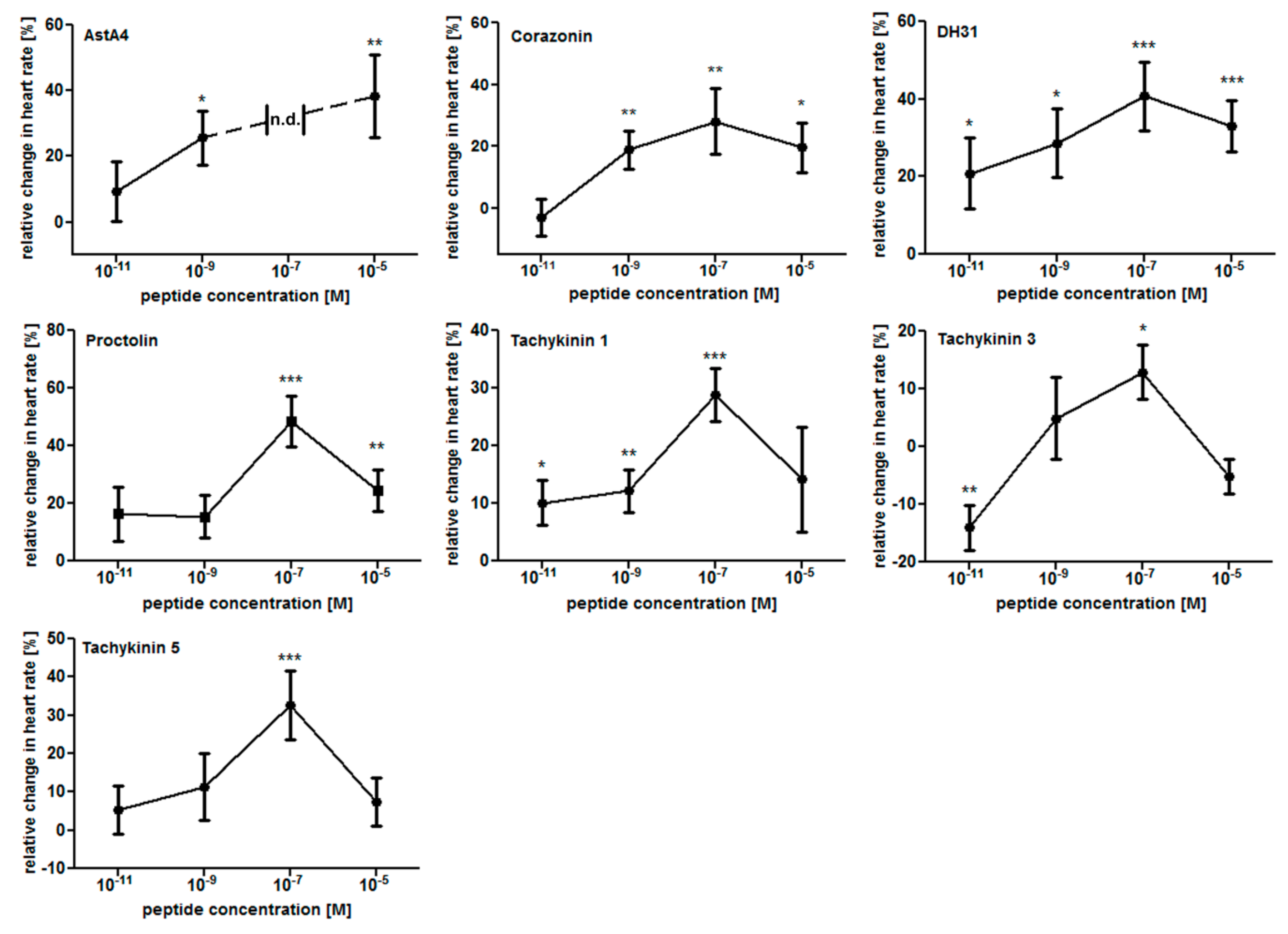
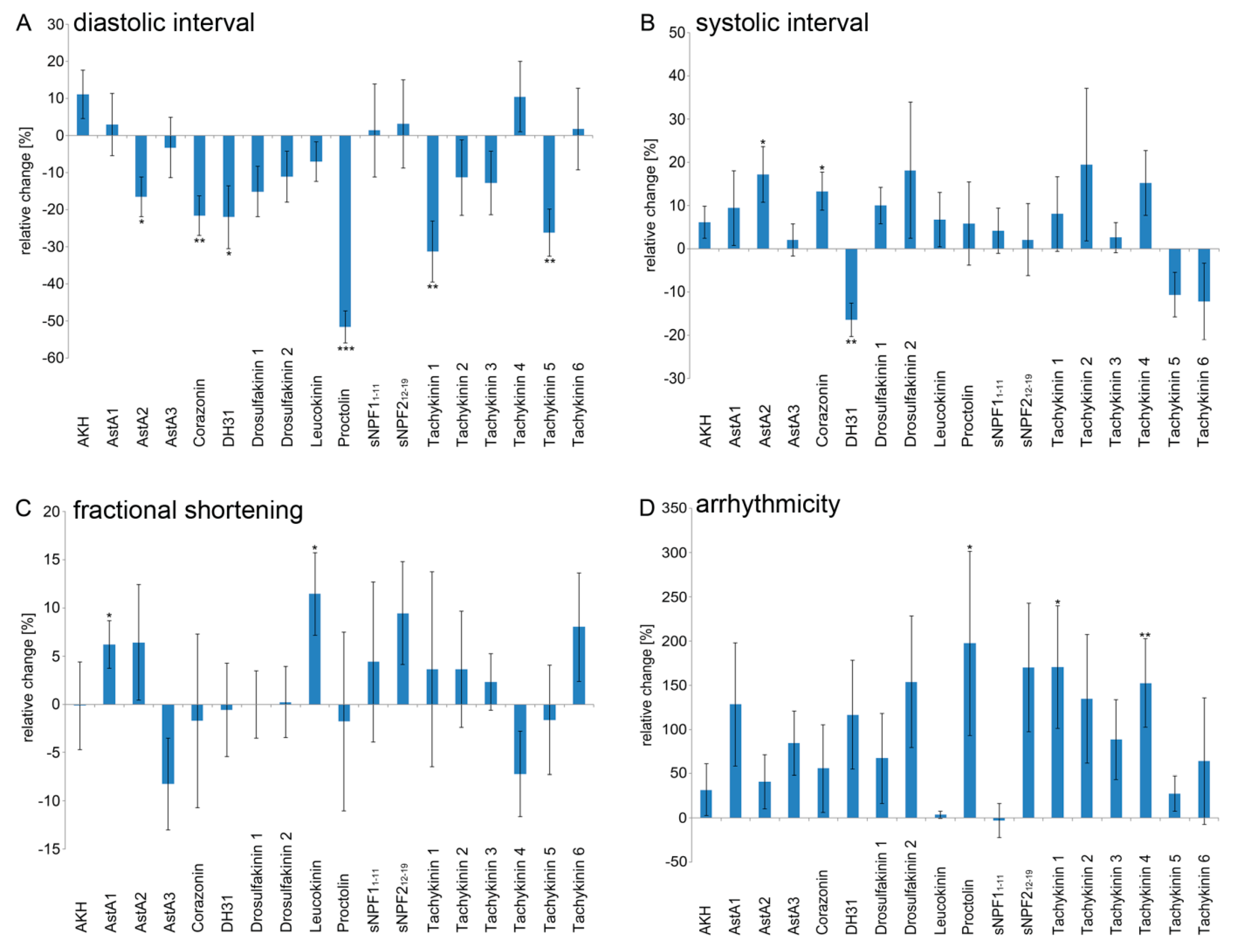

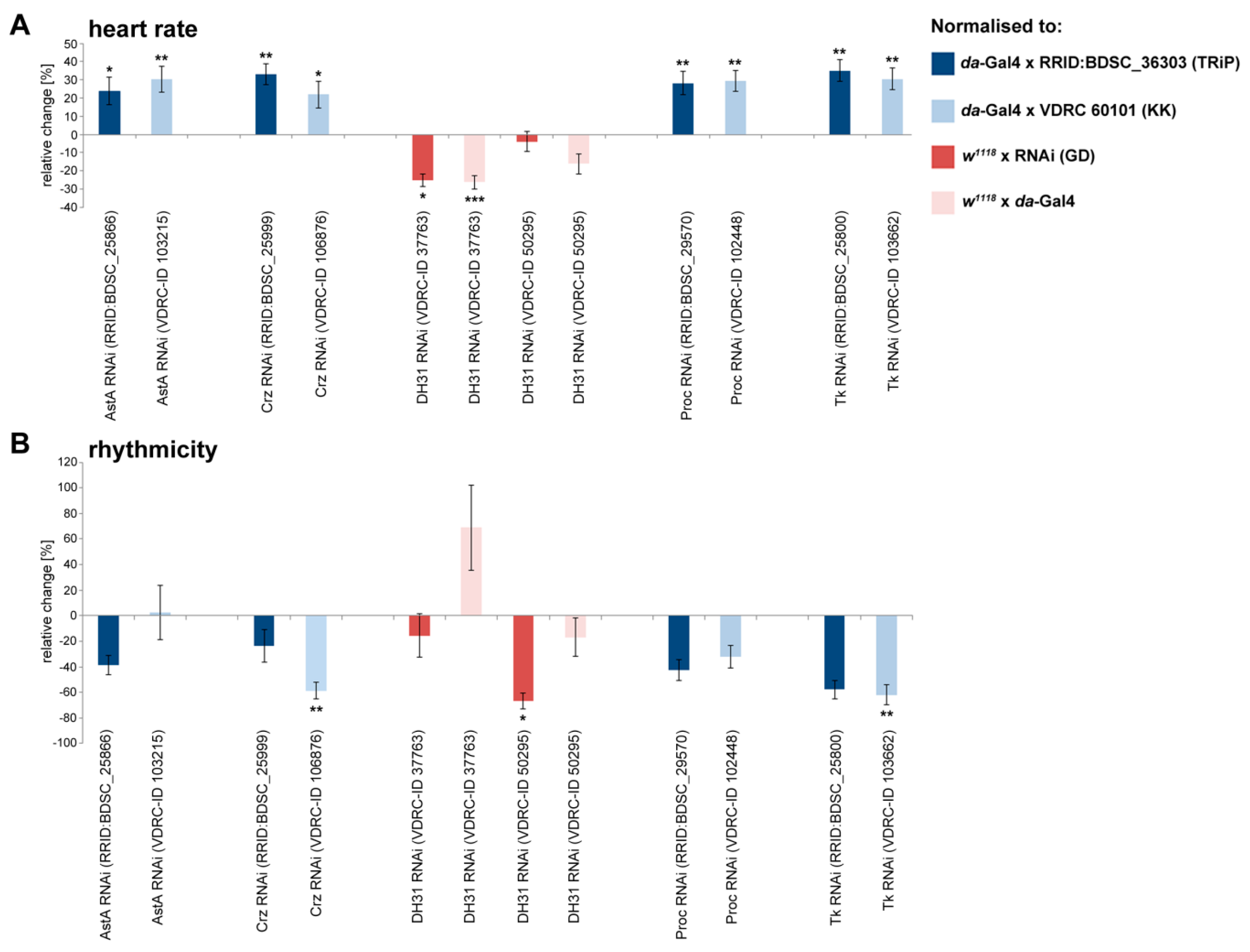
| Peptide | Sequence | Heart Rate | Diastolic Interval | Systolic Interval | Fractional Shortening | Arrhythmicity Index |
|---|---|---|---|---|---|---|
| AKH | QLTFSPDWa | / | / | / | / | / |
| AstA1 | VERYAFGLa | / | / | / | + | / |
| AstA2 | LPVYNFGLa | / | - | + | / | / |
| AstA3 | SRPYSFGLa | / | / | / | / | / |
| AstA4 | TTRPQPFNFGLa | Θ | Θ | Θ | Θ | Θ |
| Corazonin | QTFQYSRGWTNa | ++ | -- | + | / | / |
| DH31 | TVDFGLARGYSGTQEAKHRMGLAAANFAGGPa | +++ | - | -- | / | / |
| DSK1 | FDDYGHMRFa | / | / | / | / | / |
| DSK2 | GGDDQFDDYGHMRFa | / | / | / | / | / |
| Leucokinin | NSVVLGKKQRFHSWGa | / | / | / | + | / |
| Proctolin | RYLPT | +++ | --- | / | / | + |
| sNPF11-11 | AQRSPSLRLRFa | / | / | / | / | / |
| sNPF14-11 sNPF212-19 | SPSLRLRFa | / | / | / | / | / |
| Tachykinin 1 | APTSSFIGMRa | +++ | -- | / | / | + |
| Tachykinin 2 | APLAFVGLRa | / | / | / | / | / |
| Tachykinin 3 | APTGFTGMRa | + | / | / | / | / |
| Tachykinin 4 | APVNSFVGMRa | / | / | / | / | ++ |
| Tachykinin 5 | APNGFLGMRa | +++ | -- | / | / | / |
| Tachykinin 6 | AALSDSYDLRGKQQRFADFNSKFVAVRa | / | / | / | / | / |
| Provided by | Collection | Identifier | Target Gene |
|---|---|---|---|
| VDRC | KK | VDRC-ID 103215 | CG13633; Allatostatin A |
| VDRC | GD | VDRC-ID 50295 | CG13094; Diuretic hormone 31 |
| VDRC | GD | VDRC-ID 37763 | CG13094; Diuretic hormone 31 |
| VDRC | KK | VDRC-ID 106876 | CG3302; Corazonin |
| VDRC | KK | VDRC-ID 102488 | CG7105; Proctolin |
| VDRC | KK | VDRC-ID 103662 | CG14734; Tachykinin |
| BDSC | TRiP | RRID:BDSC_25800 | CG14734; Tachykinin |
| BDSC | TRiP | RRID:BDSC_25866 | CG13633; Allatostatin A |
| BDSC | TRiP | RRID:BDSC_25999 | CG3302; Corazonin |
| BDSC | TRiP | RRID:BDSC_29570 | CG7105; Proctolin |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiemann, R.; Lammers, K.; Janz, M.; Lohmann, J.; Paululat, A.; Meyer, H. Identification and In Vivo Characterisation of Cardioactive Peptides in Drosophila melanogaster. Int. J. Mol. Sci. 2019, 20, 2. https://doi.org/10.3390/ijms20010002
Schiemann R, Lammers K, Janz M, Lohmann J, Paululat A, Meyer H. Identification and In Vivo Characterisation of Cardioactive Peptides in Drosophila melanogaster. International Journal of Molecular Sciences. 2019; 20(1):2. https://doi.org/10.3390/ijms20010002
Chicago/Turabian StyleSchiemann, Ronja, Kay Lammers, Maren Janz, Jana Lohmann, Achim Paululat, and Heiko Meyer. 2019. "Identification and In Vivo Characterisation of Cardioactive Peptides in Drosophila melanogaster" International Journal of Molecular Sciences 20, no. 1: 2. https://doi.org/10.3390/ijms20010002
APA StyleSchiemann, R., Lammers, K., Janz, M., Lohmann, J., Paululat, A., & Meyer, H. (2019). Identification and In Vivo Characterisation of Cardioactive Peptides in Drosophila melanogaster. International Journal of Molecular Sciences, 20(1), 2. https://doi.org/10.3390/ijms20010002





