Comparison between Polybutylcyanoacrylate Nanoparticles with Either Surface-Adsorbed or Encapsulated Brain-Derived Neurotrophic Factor on the Neural Differentiation of iPSCs
Abstract
1. Introduction
2. Results
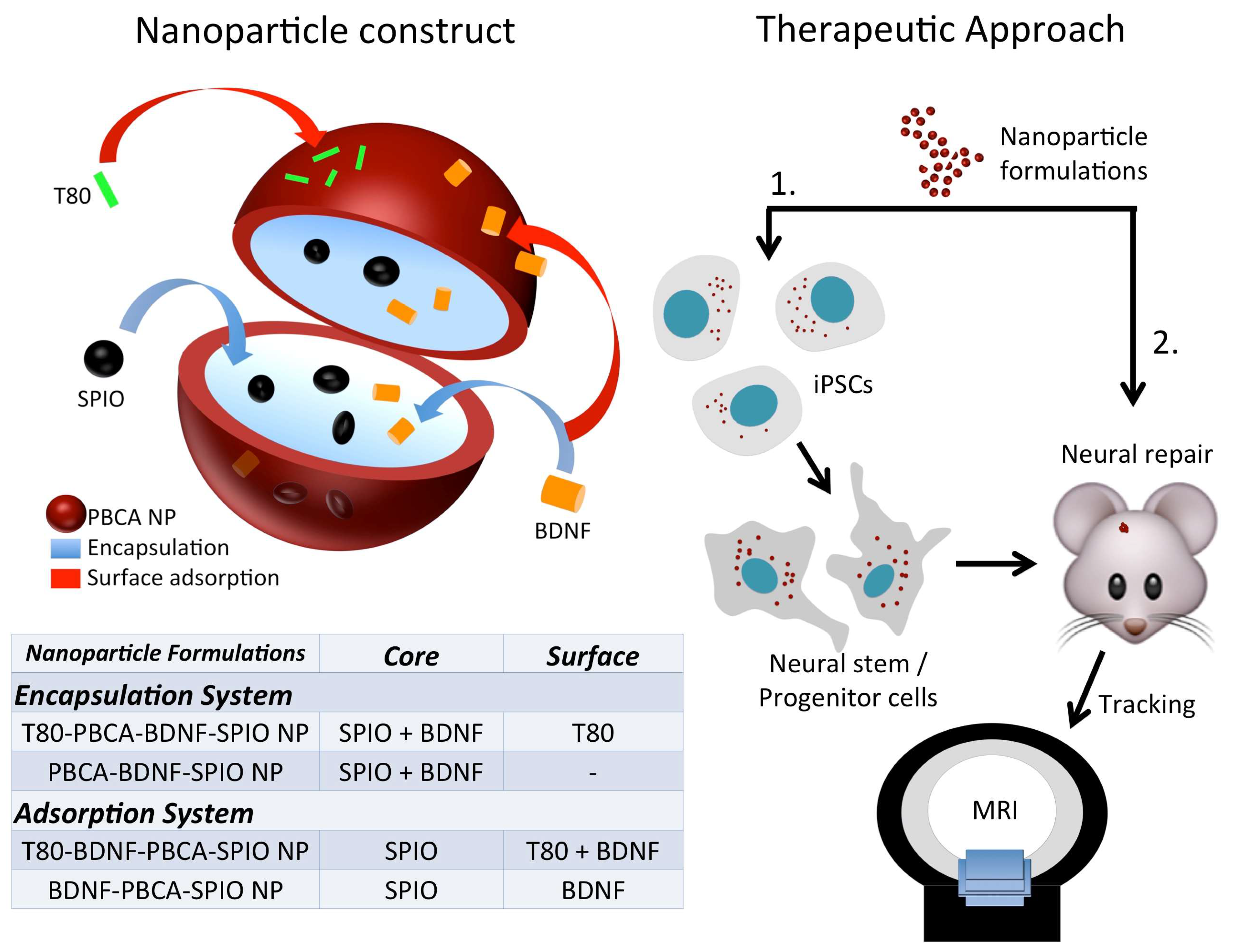
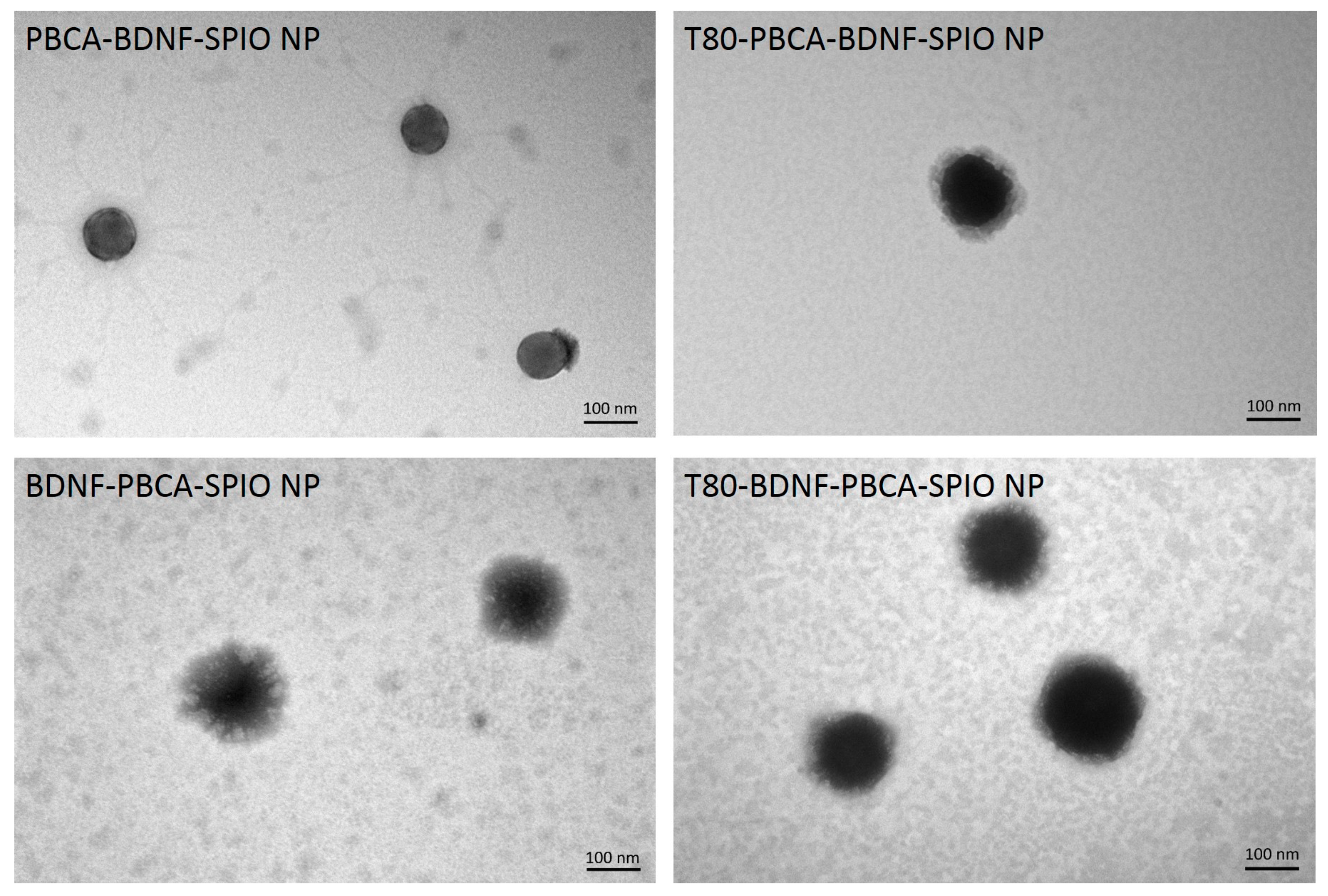
2.1. Characterization of the Nanoparticles
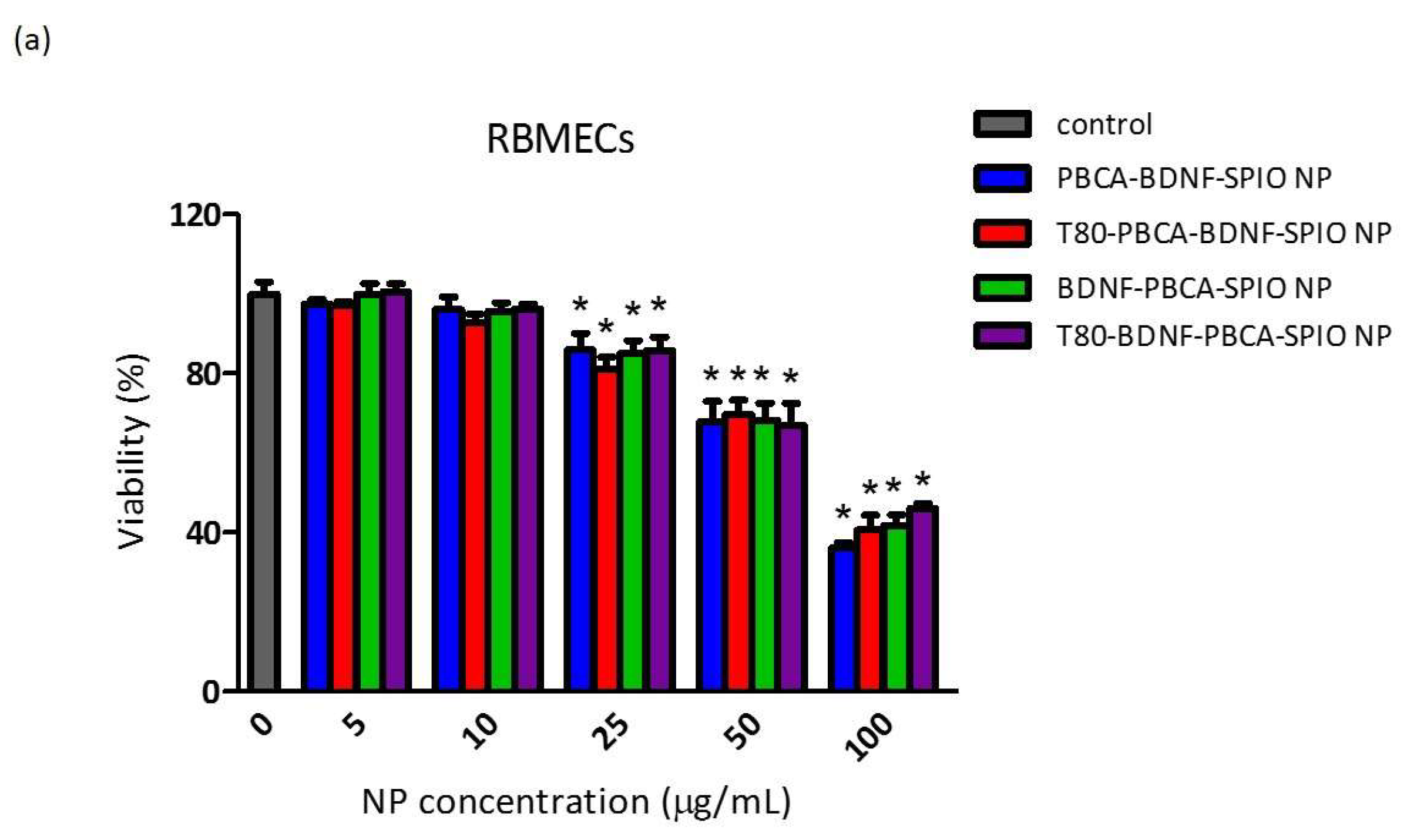
2.2. Cytotoxicity of the Nanoparticles on RBMECs, CTX TNA2, and iPSCs
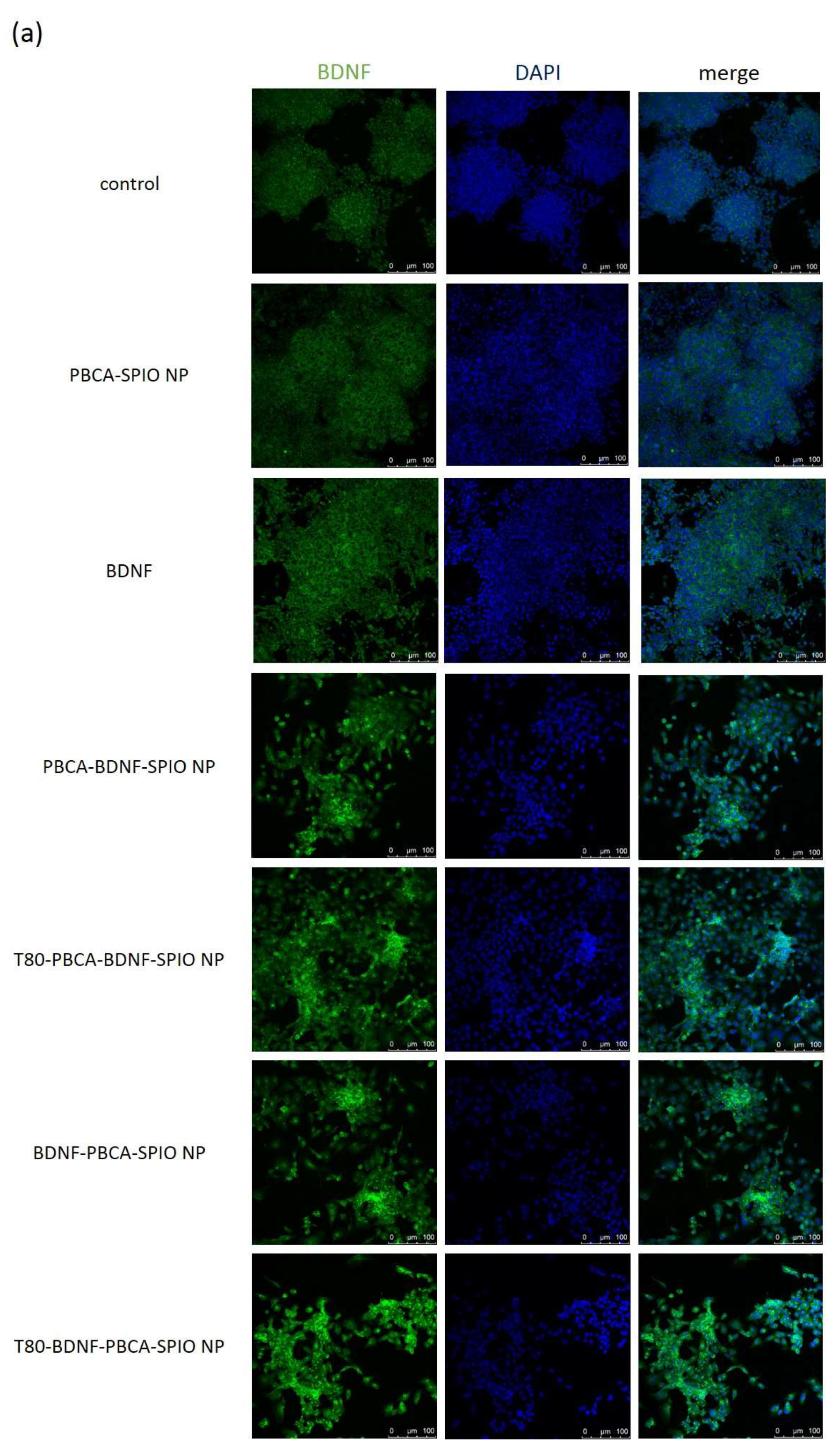
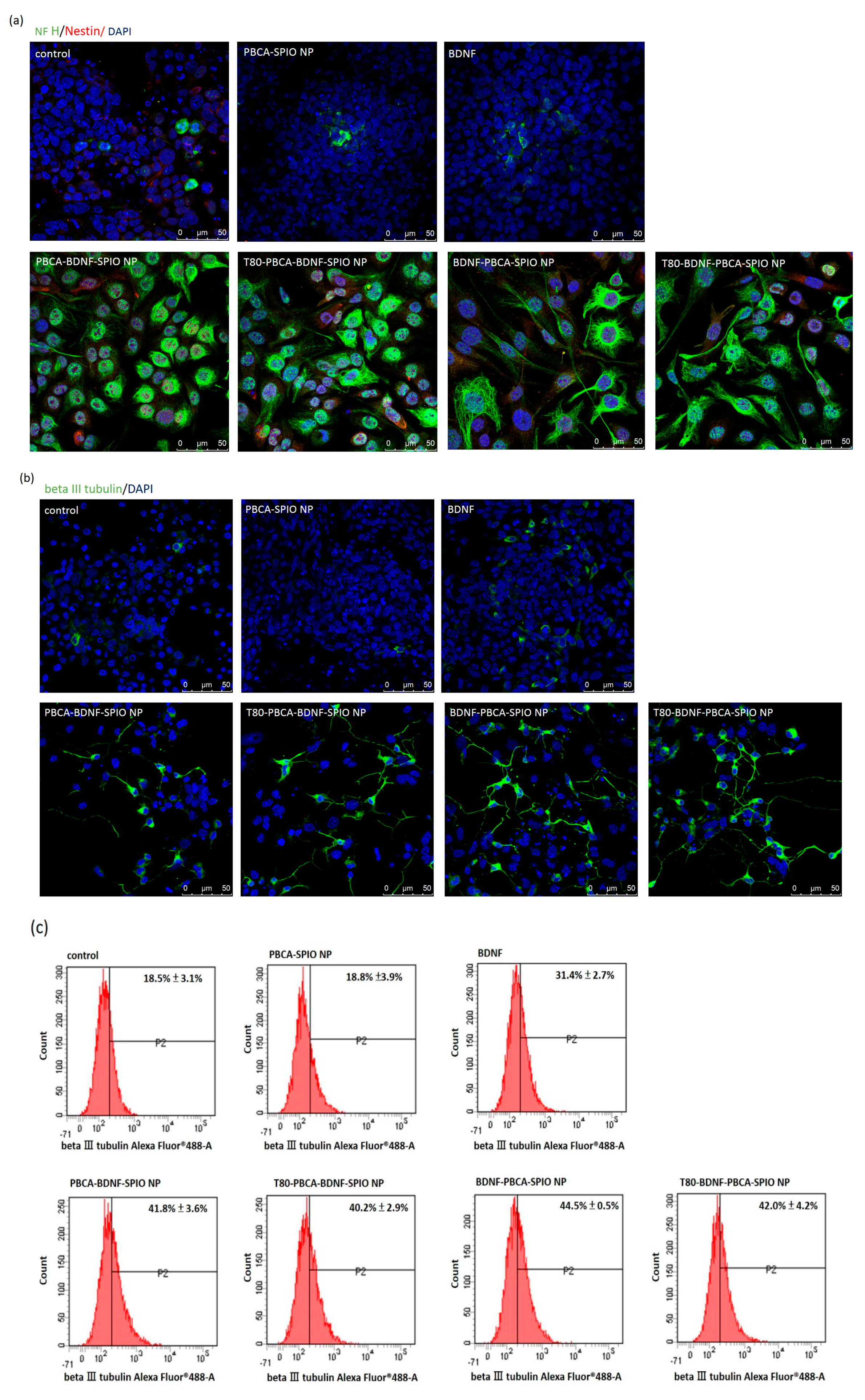
2.3. Neural Differentiation of iPSCs Treated with the Nanoparticles
2.4. The Effect on the Transendothelial Electrical Resistance and Permeability of the Nanoparticles across the In Vitro Blood–Brain Barrier Model
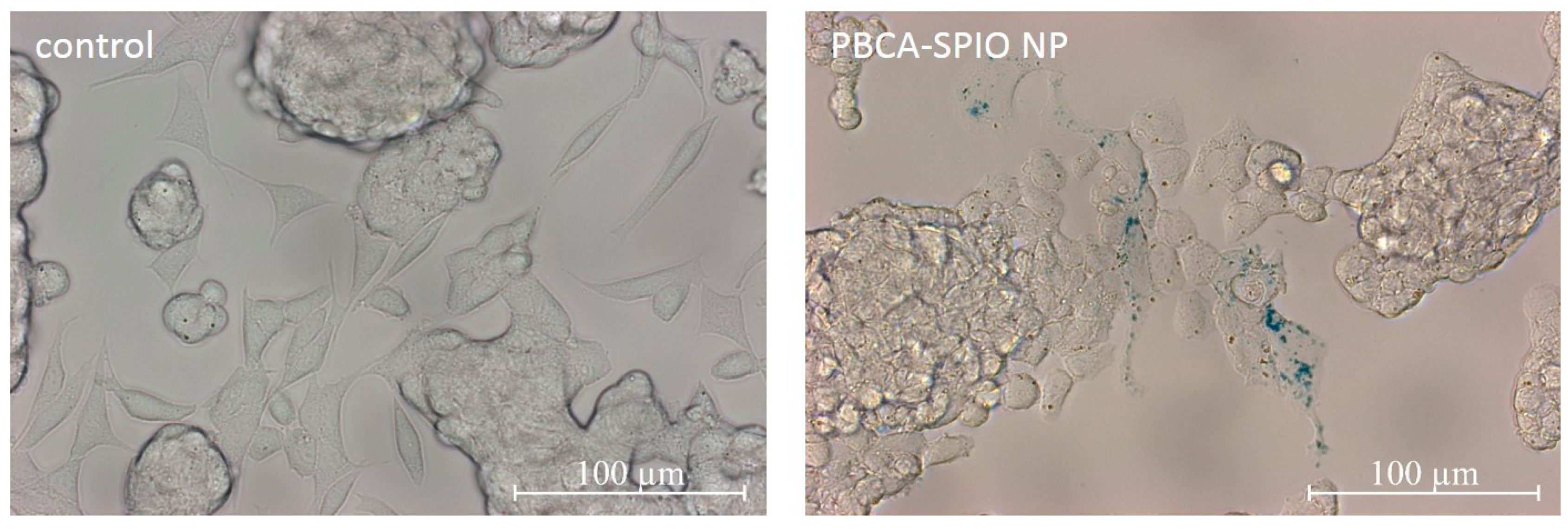
2.5. Prussian Blue Staining for Detection of SPIOs
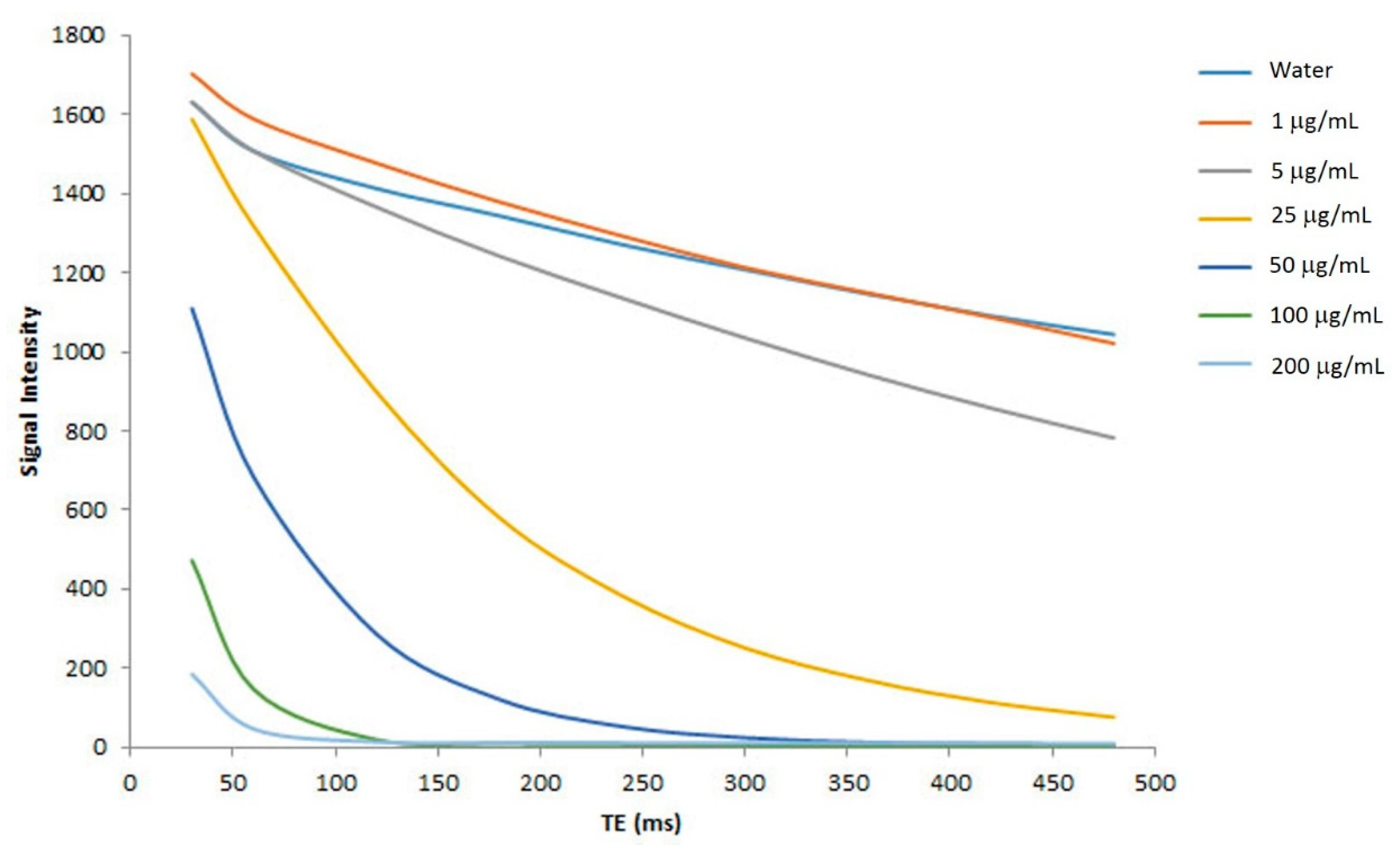
2.6. In Vitro MR Relaxitivities of the Nanoparticles
3. Discussion
4. Materials and Methods
4.1. Mouse iPSCs
4.2. Synthesis of SPIO
4.3. Fabrication of PBCA-BDNF-SPIO NP (BDNF Encapsulation System) and BDNF-PBCA-SPIO NP (BDNF Adsorption System) with and without T80 Coating
4.4. Characterization of Nanoparticles
4.5. Loading Efficiency of BDNF and SPIO
4.6. Cytotoxicity Assay
4.7. Immunofluorescence Staining and Flow Cytometry Analysis
4.7.1. In Vitro BBB Model by Co-Culturing Endothelial Cells (Upper Chamber) and Astrocytes (Opposite the Upper Chamber)
4.7.2. TEER of RBMECs/CTX TNA2 Monolayer after Treatment with the Nanoparticles
4.7.3. Permeability Across Co-Cultured RBMECs/CTX TNA2 System
4.8. Prussian Blue Staining for Detection of SPIOs
4.9. In Vitro MRI Signal Intensity of the Nanoparticles
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mimeault, M.; Batra, S.K. Great promise of tissue-resident adult stem/progenitor cells in transplantation and cancer therapies. Adv. Exp. Med. Biol. 2012, 741, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Bifari, F.; Berton, V.; Pino, A.; Kusalo, M.; Malpeli, G.; Di Chio, M.; Bersan, E.; Amato, E.; Scarpa, A.; Krampera, M.; et al. Meninges harbor cells expressing neural precursor markers during development and adulthood. Front. Cell. Neurosci. 2015, 9, 383. [Google Scholar] [CrossRef]
- Fiorelli, R.; Cebrian-Silla, A.; Garcia-Verdugo, J.M.; Raineteau, O. The adult spinal cord harbors a population of GFAP-positive progenitors with limited self-renewal potential. GLIA 2013, 61, 2100–2113. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.D.; Takahashi, J.; Gage, F.H. The adult rat hippocampus contains primordial neural stem cells. Mol. Cell. Neurosci. 1996, 8, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Gritti, A.; Bonfanti, L.; Doetsch, F.; Caille, I.; Alvarez-Buylla, A.; Lim, D.A.; Galli, R.; Verdugo, J.M.G.; Herrera, D.G.; Vescovi, A.L. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J. Neurosci. 2002, 22, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.; Roy, N.S.; Keyoung, H.M.; Goodman, R.R.; McKhann, G., 2nd; Jiang, L.; Kang, J.; Nedergaard, M.; Goldman, S.A. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat. Med. 2003, 9, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Steward, M.M.; Sridhar, A.; Meyer, J.S. Neural regeneration. Curr. Top. Microbiol. Immunol. 2013, 367, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Rolls, A.; Shechter, R.; Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195. [Google Scholar] [CrossRef] [PubMed]
- Sohur, U.S.; Emsley, J.G.; Mitchell, B.D.; Macklis, J.D. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2006, 361, 1477–1497. [Google Scholar] [CrossRef]
- Ekdahl, C.T.; Claasen, J.H.; Bonde, S.; Kokaia, Z.; Lindvall, O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA 2003, 100, 13632–13637. [Google Scholar] [CrossRef] [PubMed]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Bernal, G.M.; Peterson, D.A. Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging Cell 2011, 10, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Drago, D.; Basso, V.; Gaude, E.; Volpe, G.; Peruzzotti-Jametti, L.; Bachi, A.; Musco, G.; Andolfo, A.; Frezza, C.; Mondino, A.; et al. Metabolic determinants of the immune modulatory function of neural stem cells. J. Neuroinflamm. 2016, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Ravanidis, S.; Poulatsidou, K.N.; Lagoudaki, R.; Touloumi, O.; Polyzoidou, E.; Lourbopoulos, A.; Nousiopoulou, E.; Theotokis, P.; Kesidou, E.; Tsalikakis, D.; et al. Subcutaneous transplantation of neural precursor cells in experimental autoimmune encephalomyelitis reduces chemotactic signals in the central nervous system. Stem Cells Transl. Med. 2015, 4, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, C.; Li, Q.; Zhou, S.; Fu, J.; Wang, X.; Zhuge, Q. Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intracerebral haemorrhage stroke in mice. J. Cell. Mol. Med. 2011, 15, 2624–2633. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induced pluripotent stem cells in medicine and biology. Development 2013, 140, 2457–2461. [Google Scholar] [CrossRef]
- Rong, Z.; Wang, M.; Hu, Z.; Stradner, M.; Zhu, S.; Kong, H.; Yi, H.; Goldrath, A.; Yang, Y.-G.; Xu, Y.; et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell 2014, 14, 121–130. [Google Scholar] [CrossRef]
- Zhang, G.; Shang, B.; Yang, P.; Cao, Z.; Pan, Y.; Zhou, Q. Induced pluripotent stem cell consensus genes: Implication for the risk of tumorigenesis and cancers in induced pluripotent stem cell therapy. Stem Cells Dev. 2012, 21, 955–964. [Google Scholar] [CrossRef]
- Yamashita, T.; Kawai, H.; Tian, F.; Ohta, Y.; Abe, K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transpl. 2011, 20, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, H.; Feng, Q.-S.; Cai, M.-B.; Deng, W.; Qin, D.; Yun, J.-P.; Tsao, G.S.W.; Kang, T.; Esteban, M.A.; et al. The propensity for tumorigenesis in human induced pluripotent stem cells is related with genomic instability. Chin. J. Cancer 2013, 32, 205–212. [Google Scholar] [CrossRef]
- Tateno, H.; Onuma, Y.; Ito, Y.; Minoshima, F.; Saito, S.; Shimizu, M.; Aiki, Y.; Asashima, M.; Hirabayashi, J. Elimination of tumorigenic human pluripotent stem cells by a recombinant lectin-toxin fusion protein. Stem Cell Rep. 2015, 4, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Fukuda, K. Methods of induced pluripotent stem cells for clinical application. World J. Stem Cells 2015, 7, 116–125. [Google Scholar] [CrossRef] [PubMed]
- El Khatib, M.M.; Ohmine, S.; Jacobus, E.J.; Tonne, J.M.; Morsy, S.G.; Holditch, S.J.; Schreiber, C.A.; Uetsuka, K.; Fusaki, N.; Wigle, D.A.; et al. Tumor-Free Transplantation of Patient-Derived Induced Pluripotent Stem Cell Progeny for Customized Islet Regeneration. Stem Cells Transl. Med. 2016, 5, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Yu, J.; Rose, F.F.; Mattis, V.B.; Lorson, C.L.; Thomson, J.A.; Svendsen, C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009, 457, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.Y.; Weick, J.P.; Yu, J.; Ma, L.X.; Zhang, X.Q.; Thomson, J.A.; Zhang, S.C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc. Natl. Acad. Sci. USA 2010, 107, 4335–4340. [Google Scholar] [CrossRef]
- Lim, J.Y.; Park, S.I.; Oh, J.H.; Kim, S.M.; Jeong, C.H.; Jun, J.A.; Lee, K.S.; Oh, W.; Lee, J.K.; Jeun, S.S. Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J. Neurosci. Res. 2008, 86, 2168–2178. [Google Scholar] [CrossRef]
- Bolton, M.M.; Pittman, A.J.; Lo, D.C. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J. Neurosci. 2000, 20, 3221–3232. [Google Scholar] [CrossRef]
- Zheng, J.; Sun, J.; Lu, X.; Zhao, P.; Li, K.; Li, L. BDNF promotes the axonal regrowth after sciatic nerve crush through intrinsic neuronal capability upregulation and distal portion protection. Neurosci. Lett. 2016, 621, 1–8. [Google Scholar] [CrossRef]
- Ramge, P.; Unger, R.E.; Oltrogge, J.B.; Zenker, D.; Begley, D.; Kreuter, J.; Von Briesen, H. Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate (PBCA)-nanoparticles by human and bovine primary brain capillary endothelial cells. Eur. J. Neurosci. 2000, 12, 1931–1940. [Google Scholar] [CrossRef] [PubMed]
- Koffie, R.M.; Farrar, C.T.; Saidi, L.-J.; William, C.M.; Hyman, B.T.; Spires-Jones, T.L. Nanoparticles enhance brain delivery of blood–brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 18837–18842. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhang, Y.; Chen, W.; Shen, C.; Liao, M.; Pan, Y.; Wang, J.; Deng, X.; Zhao, J. Cationic polybutyl cyanoacrylate nanoparticles for DNA delivery. J. Biomed. Biotechnol. 2009, 2009, 149254. [Google Scholar] [CrossRef] [PubMed]
- Kurakhmaeva, K.B.; Djindjikhashvili, I.A.; Petrov, V.E.; Balabanyan, V.U.; Voronina, T.A.; Trofimov, S.S.; Kreuter, J.; Gelperina, S.; Begley, D.; Alyautdin, R.N. Brain targeting of nerve growth factor using poly(butyl cyanoacrylate) nanoparticles. J. Drug Target. 2009, 17, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Wang, W.F.; Liang, X.F.; Liu, Z.H.; Liu, Y.; Lin, L.; Zhu, X. Protective Effects of Poly (butyl) Cyanoacrylate Nanoparticles Containing Vasoactive Intestinal Peptide Against 6-Hydroxydopamine-Induced Neurotoxicity In Vitro. J. Mol. Neurosci. 2015, 55, 854–864. [Google Scholar] [CrossRef]
- Tian, X.-H.; Lin, X.-N.; Wei, F.; Feng, W.; Huang, Z.-C.; Wang, P.; Ren, L.; Diao, Y. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int. J. Nanomed. 2011, 6, 445–452. [Google Scholar] [CrossRef]
- Gao, S.; Xu, Y.; Asghar, S.; Chen, M.; Zou, L.; Eltayeb, S.; Huo, M.; Ping, Q.; Xiao, Y. Polybutylcyanoacrylate nanocarriers as promising targeted drug delivery systems. J. Drug Target. 2015, 23, 481–496. [Google Scholar] [CrossRef]
- Cabeza, L.; Ortiz, R.; Arias, J.L.; Prados, J.; Ruiz Martinez, M.A.; Entrena, J.M.; Luque, R.; Melguizo, C. Enhanced antitumor activity of doxorubicin in breast cancer through the use of poly(butylcyanoacrylate) nanoparticles. Int. J. Nanomed. 2015, 10, 1291–1306. [Google Scholar] [CrossRef]
- Melguizo, C.; Cabeza, L.; Prados, J.; Ortiz, R.; Caba, O.; Rama, A.R.; Delgado, Á.V.; Arias, J.L. Enhanced antitumoral activity of doxorubicin against lung cancer cells using biodegradable poly(Butylcyanoacrylate) nanoparticles. Drug Des. Dev. Therapy 2015, 9, 6433–6444. [Google Scholar] [CrossRef]
- Kolter, M.; Ott, M.; Hauer, C.; Reimold, I.; Fricker, G. Nanotoxicity of poly(n-butylcyano-acrylate) nanoparticles at the blood-brain barrier, in human whole blood and in vivo. J. Controll. Release 2015, 197, 165–179. [Google Scholar] [CrossRef]
- Kreuter, J.; Alyautdin, R.N.; Kharkevich, D.A.; Ivanov, A.A. Passage of peptides through the blood-brain barrier with colloidal polymer particles (nanoparticles). Brain Res. 1995, 674, 171–174. [Google Scholar] [CrossRef]
- Li, Y.J.; Barthes-Biesel, D.; Salsac, A.V. Polymerization kinetics of n-butyl cyanoacrylate glues used for vascular embolization. J. Mech. Behav. Biomed. Mater. 2017, 69, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Kakaei, F.; Seyyed Sadeghi, M.S.; Sanei, B.; Hashemzadeh, S.; Habibzadeh, A. A randomized clinical trial comparing the effect of different haemostatic agents for haemostasis of the liver after hepatic resection. HPB Surg. 2013, 2013, 587608. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.K.; Saydam, L. The use of cyanoacrylates for wound closure in head and neck surgery. Eur. Arch. Oto-Rhino-Laryngol. 2008, 265, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Tamatani, S.; Koike, T.; Ito, Y.; Tanaka, R. Embolization of Arteriovenous Malformation with Diluted Mixture of NBCA. Int. Neuroradiol. 2000, 6, 187–190. [Google Scholar] [CrossRef]
- Sulheim, E.; Baghirov, H.; von Haartman, E.; Boe, A.; Aslund, A.K.; Morch, Y.; Davies Cde, L. Cellular uptake and intracellular degradation of poly(alkyl cyanoacrylate) nanoparticles. J. Nanobiotechnol. 2016, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Birkinshaw, C. Hydrolysis of poly (n-butylcyanoacrylate) nanoparticles using esterase. Polym. Degrad. Stab. 2002, 78, 7–15. [Google Scholar] [CrossRef]
- Chaudhari, K.R.; Ukawala, M.; Manjappa, A.S.; Kumar, A.; Mundada, P.K.; Mishra, A.K.; Mathur, R.; Monkkonen, J.; Murthy, R.S. Opsonization, biodistribution, cellular uptake and apoptosis study of PEGylated PBCA nanoparticle as potential drug delivery carrier. Pharm. Res. 2012, 29, 53–68. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.; Paramakrishnan, N.; Suresh, B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer’s disease. Brain Res. 2008, 1200, 159–168. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Pulicherla, K.K.; Verma, M.K. Targeting Therapeutics Across the Blood Brain Barrier (BBB), Prerequisite Towards Thrombolytic Therapy for Cerebrovascular Disorders—An Overview and Advancements. AAPS PharmSciTech 2015, 16, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Forster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Huang, L.S.; Hou, L.B.; Jiang, L.; Yan, Z.T.; Wang, Y.L.; Chen, Z.L. Antitumor effects of polysorbate-80 coated gemcitabine polybutylcyanoacrylate nanoparticles in vitro and its pharmacodynamics in vivo on C6 glioma cells of a brain tumor model. Brain Res. 2009, 1261, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Bagad, M.; Khan, Z.A. Poly(n-butylcyanoacrylate) nanoparticles for oral delivery of quercetin: Preparation, characterization, and pharmacokinetics and biodistribution studies in Wistar rats. Int. J. Nanomed. 2015, 10, 3921–3935. [Google Scholar] [CrossRef]
- Reimold, I.; Domke, D.; Bender, J.; Seyfried, C.A.; Radunz, H.E.; Fricker, G. Delivery of nanoparticles to the brain detected by fluorescence microscopy. Eur. J. Pharm. Biopharm. 2008, 70, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Mulik, R.S.; Monkkonen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. ApoE3 mediated poly(butyl) cyanoacrylate nanoparticles containing curcumin: Study of enhanced activity of curcumin against beta amyloid induced cytotoxicity using in vitro cell culture model. Mol. Pharm. 2010, 7, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Arechabala, B.; Coiffard, C.; Rivalland, P.; Coiffard, L.J.; de Roeck-Holtzhauer, Y. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J. Appl. Toxicol. 1999, 19, 163–165. [Google Scholar] [CrossRef]
- Perriere, N.; Yousif, S.; Cazaubon, S.; Chaverot, N.; Bourasset, F.; Cisternino, S.; Decleves, X.; Hori, S.; Terasaki, T.; Deli, M.; et al. A functional in vitro model of rat blood-brain barrier for molecular analysis of efflux transporters. Brain Res. 2007, 1150, 1–13. [Google Scholar] [CrossRef]
- Molino, Y.; Jabes, F.; Lacassagne, E.; Gaudin, N.; Khrestchatisky, M. Setting-up an in vitro model of rat blood-brain barrier (BBB): A focus on BBB impermeability and receptor-mediated transport. J. Vis. Exp. 2014, 28, e51278. [Google Scholar] [CrossRef]
- Reichel, A.; Begley, D.J.; Abbott, N.J. An overview of in vitro techniques for blood-brain barrier studies. Methods Mol. Med. 2003, 89, 307–324. [Google Scholar] [CrossRef]
- Butt, A.M.; Jones, H.C.; Abbott, N.J. Electrical resistance across the blood-brain barrier in anaesthetized rats: A developmental study. J. Physiol. 1990, 429, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, E.S.; Al-Ahmad, A.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014, 4, 4160. [Google Scholar] [CrossRef] [PubMed]
- Rempe, R.; Cramer, S.; Huwel, S.; Galla, H.J. Transport of Poly(n-butylcyano-acrylate) nanoparticles across the blood-brain barrier in vitro and their influence on barrier integrity. Biochem. Biophys. Res. Commun. 2011, 406, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J.; Ramge, P.; Petrov, V.; Hamm, S.; Gelperina, S.E.; Engelhardt, B.; Alyautdin, R.; von Briesen, H.; Begley, D.J. Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drug to the nanoparticles. Pharm. Res. 2003, 20, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, Z. CNS Drug Design: Balancing Physicochemical Properties for Optimal Brain Exposure. J. Med. Chem. 2015, 58, 2584–2608. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.D.; Ye, M.; Levy, A.F.; Rothstein, J.D.; Bergles, D.E.; Searson, P.C. The blood-brain barrier: An engineering perspective. Front. Neuroeng. 2013, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, C.; Praca, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Controll. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Wang, C.C. Electrophoresis of human brain microvascular endothelial cells with uptake of cationic solid lipid nanoparticles: Effect of surfactant composition. Colloids Surf. B 2010, 76, 286–291. [Google Scholar] [CrossRef]
- Patil, S.; Sandberg, A.; Heckert, E.; Self, W.; Seal, S. Protein adsorption and cellular uptake of cerium oxide nanoparticles as a function of zeta potential. Biomaterials 2007, 28, 4600–4607. [Google Scholar] [CrossRef]
- Zabara, M.; Vasheghani-Farahani, E.; Hosseinkhani, H.; Shojaosadati, S.; Soleimani, M. Fabrication and characterization of a new MRI contrast agent based on a magnetic dextran–spermine nanoparticle system. Iran. Polym. J. 2012, 21, 239–251. [Google Scholar]
- Huang, J.; Zhong, X.; Wang, L.; Yang, L.; Mao, H. Improving the magnetic resonance imaging contrast and detection methods with engineered magnetic nanoparticles. Theranostics 2012, 2, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Estelrich, J.; Sánchez-Martín, M.J.; Busquets, M.A. Nanoparticles in magnetic resonance imaging: From simple to dual contrast agents. Int. J. Nanomed. 2015, 10, 1727–1741. [Google Scholar] [CrossRef]
- Wu, P.-C.; Shieh, D.-B.; Hsiao, H.-T.; Wang, J.C.-F.; Lin, Y.-C.; Liu, Y.-C. Magnetic field distribution modulation of intrathecal delivered ketorolac iron-oxide nanoparticle conjugates produce excellent analgesia for chronic inflammatory pain. J. Nanobiotechnol. 2018, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Nadri, S.; Ghollasi, M.; Khajeh, K.; Soleimani, M. Comparison of different protocols for neural differentiation of human induced pluripotent stem cells. Mol. Biol. Rep. 2014, 41, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Sun, G.; Herszfeld, D.; Sylvain, A.; Campanale, N.V.; Hirst, C.E.; Caine, S.; Parkington, H.C.; Tonta, M.A.; Coleman, H.A.; et al. Neural differentiation of patient specific iPS cells as a novel approach to study the pathophysiology of multiple sclerosis. Stem Cell Res. 2012, 8, 259–273. [Google Scholar] [CrossRef]
- Cao, S.-Y.; Hu, Y.; Chen, C.; Yuan, F.; Xu, M.; Li, Q.; Fang, K.-H.; Chen, Y.; Liu, Y. Enhanced derivation of human pluripotent stem cell-derived cortical glutamatergic neurons by a small molecule. Sci. Rep. 2017, 7, 3282. [Google Scholar] [CrossRef] [PubMed]
- Juopperi, T.A.; Kim, W.R.; Chiang, C.H.; Yu, H.; Margolis, R.L.; Ross, C.A.; Ming, G.L.; Song, H. Astrocytes generated from patient induced pluripotent stem cells recapitulate features of Huntington’s disease patient cells. Mol. Brain 2012, 5, 17. [Google Scholar] [CrossRef]
- Chung, S.Y.; Kishinevsky, S.; Mazzulli, J.R.; Graziotto, J.; Mrejeru, A.; Mosharov, E.V.; Puspita, L.; Valiulahi, P.; Sulzer, D.; Milner, T.A.; et al. Parkin and PINK1 Patient iPSC-Derived Midbrain Dopamine Neurons Exhibit Mitochondrial Dysfunction and alpha-Synuclein Accumulation. Stem Cell Rep. 2016, 7, 664–677. [Google Scholar] [CrossRef]
- Wattanapanitch, M.; Klincumhom, N.; Potirat, P.; Amornpisutt, R.; Lorthongpanich, C.; U-pratya, Y.; Laowtammathron, C.; Kheolamai, P.; Poungvarin, N.; Issaragrisil, S. Dual small-molecule targeting of SMAD signaling stimulates human induced pluripotent stem cells toward neural lineages. PLoS ONE 2014, 9, e106952. [Google Scholar] [CrossRef]
- Du, Z.-W.; Chen, H.; Liu, H.; Lu, J.; Qian, K.; Huang, C.-L.; Zhong, X.; Fan, F.; Zhang, S.-C. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat. Commun. 2015, 6, 6626. [Google Scholar] [CrossRef]
- Zhu, Y.; Wan, S.; Zhan, R.Y. Inducible pluripotent stem cells for the treatment of ischemic stroke: Current status and problems. Rev. Neurosci. 2012, 23, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Frega, M.; van Gestel, S.H.C.; Linda, K.; van der Raadt, J.; Keller, J.; Van Rhijn, J.-R.; Schubert, D.; Albers, C.A.; Nadif Kasri, N. Rapid Neuronal Differentiation of Induced Pluripotent Stem Cells for Measuring Network Activity on Micro-electrode Arrays. J. Vis. Exp. 2017, 119, 54900. [Google Scholar] [CrossRef] [PubMed]
- Busskamp, V.; Lewis, N.E.; Guye, P.; Ng, A.H.; Shipman, S.L.; Byrne, S.M.; Sanjana, N.E.; Murn, J.; Li, Y.; Li, S.; et al. Rapid neurogenesis through transcriptional activation in human stem cells. Mol. Syst. Biol. 2014, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pak, C.; Han, Y.; Ahlenius, H.; Zhang, Z.; Chanda, S.; Marro, S.; Patzke, C.; Acuna, C.; Covy, J.; et al. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 2013, 78, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Bibel, M.; Barde, Y.A. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000, 14, 2919–2937. [Google Scholar] [CrossRef]
- Chung, C.Y.; Lin, M.H.; Lee, I.N.; Lee, T.H.; Lee, M.H.; Yang, J.T. Brain-Derived Neurotrophic Factor Loaded PS80 PBCA Nanocarrier for In Vitro Neural Differentiation of Mouse Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2017, 18, 663. [Google Scholar] [CrossRef]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Cheng, P.-L.; Song, A.-H.; Wong, Y.-H.; Wang, S.; Zhang, X.; Poo, M.-M. Self-amplifying autocrine actions of BDNF in axon development. Proc. Natl. Acad. Sci. USA 2011, 108, 18430–18435. [Google Scholar] [CrossRef]
- Kumari, A.; Singla, R.; Guliani, A.; Yadav, S.K. Nanoencapsulation for drug delivery. EXCLI J. 2014, 13, 265–286. [Google Scholar]
- Kuo, Y.C. Loading efficiency of stavudine on polybutylcyanoacrylate and methylmethacrylate- sulfopropylmethacrylate copolymer nanoparticles. Int. J. Pharm. 2005, 290, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Yang, J.T.; Kuo, Y.C. Polybutylcyanoacrylate nanoparticles for delivering hormone response element-conjugated neurotrophin-3 to the brain of intracerebral hemorrhagic rats. Biomaterials 2013, 34, 9717–9727. [Google Scholar] [CrossRef] [PubMed]




| Sample | Dav (nm) | Zeta Potential (mV) | PDI | Loading Efficiency (%) | |
|---|---|---|---|---|---|
| SPIO | BDNF | ||||
| PBCA-BDNF-SPIO NP | 125.2 ± 3.2 | −1.3 ± 0.3 | 0.17 ± 0.01 | 10.96 | 100 |
| T80-PBCA-BDNF-SPIO NP | 124.5 ± 0.8 | −0.2 ± 1.0 | 0.13 ± 0.04 | 15.32 | 100 |
| BDNF-PBCA-SPIO NP | 148.7 ± 0.4 | −0.4 ± 0.2 | 0.12 ± 0.02 | 7.78 | 98.29 ± 0.16 |
| T80-BDNF-PBCA-SPIO NP | 146.7 ± 1.1 | −1.4 ± 0.1 | 0.13 ± 0.02 | 14.16 | 98.96 ± 0.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.H.-C.; Chung, C.-Y.; Chen, K.-T.; Yeh, J.-C.; Lee, T.-H.; Lee, M.-H.; Lee, I.-N.; Huang, W.-C.; Yang, J.-T. Comparison between Polybutylcyanoacrylate Nanoparticles with Either Surface-Adsorbed or Encapsulated Brain-Derived Neurotrophic Factor on the Neural Differentiation of iPSCs. Int. J. Mol. Sci. 2019, 20, 182. https://doi.org/10.3390/ijms20010182
Lin MH-C, Chung C-Y, Chen K-T, Yeh J-C, Lee T-H, Lee M-H, Lee I-N, Huang W-C, Yang J-T. Comparison between Polybutylcyanoacrylate Nanoparticles with Either Surface-Adsorbed or Encapsulated Brain-Derived Neurotrophic Factor on the Neural Differentiation of iPSCs. International Journal of Molecular Sciences. 2019; 20(1):182. https://doi.org/10.3390/ijms20010182
Chicago/Turabian StyleLin, Martin Hsiu-Chu, Chiu-Yen Chung, Kuo-Tai Chen, Jih-Chao Yeh, Tsong-Hai Lee, Ming-Hsueh Lee, I-Neng Lee, Wei-Chao Huang, and Jen-Tsung Yang. 2019. "Comparison between Polybutylcyanoacrylate Nanoparticles with Either Surface-Adsorbed or Encapsulated Brain-Derived Neurotrophic Factor on the Neural Differentiation of iPSCs" International Journal of Molecular Sciences 20, no. 1: 182. https://doi.org/10.3390/ijms20010182
APA StyleLin, M. H.-C., Chung, C.-Y., Chen, K.-T., Yeh, J.-C., Lee, T.-H., Lee, M.-H., Lee, I.-N., Huang, W.-C., & Yang, J.-T. (2019). Comparison between Polybutylcyanoacrylate Nanoparticles with Either Surface-Adsorbed or Encapsulated Brain-Derived Neurotrophic Factor on the Neural Differentiation of iPSCs. International Journal of Molecular Sciences, 20(1), 182. https://doi.org/10.3390/ijms20010182





