Periostin Links Skin Inflammation to Melanoma Progression in Humans and Mice
Abstract
1. Introduction
2. Results
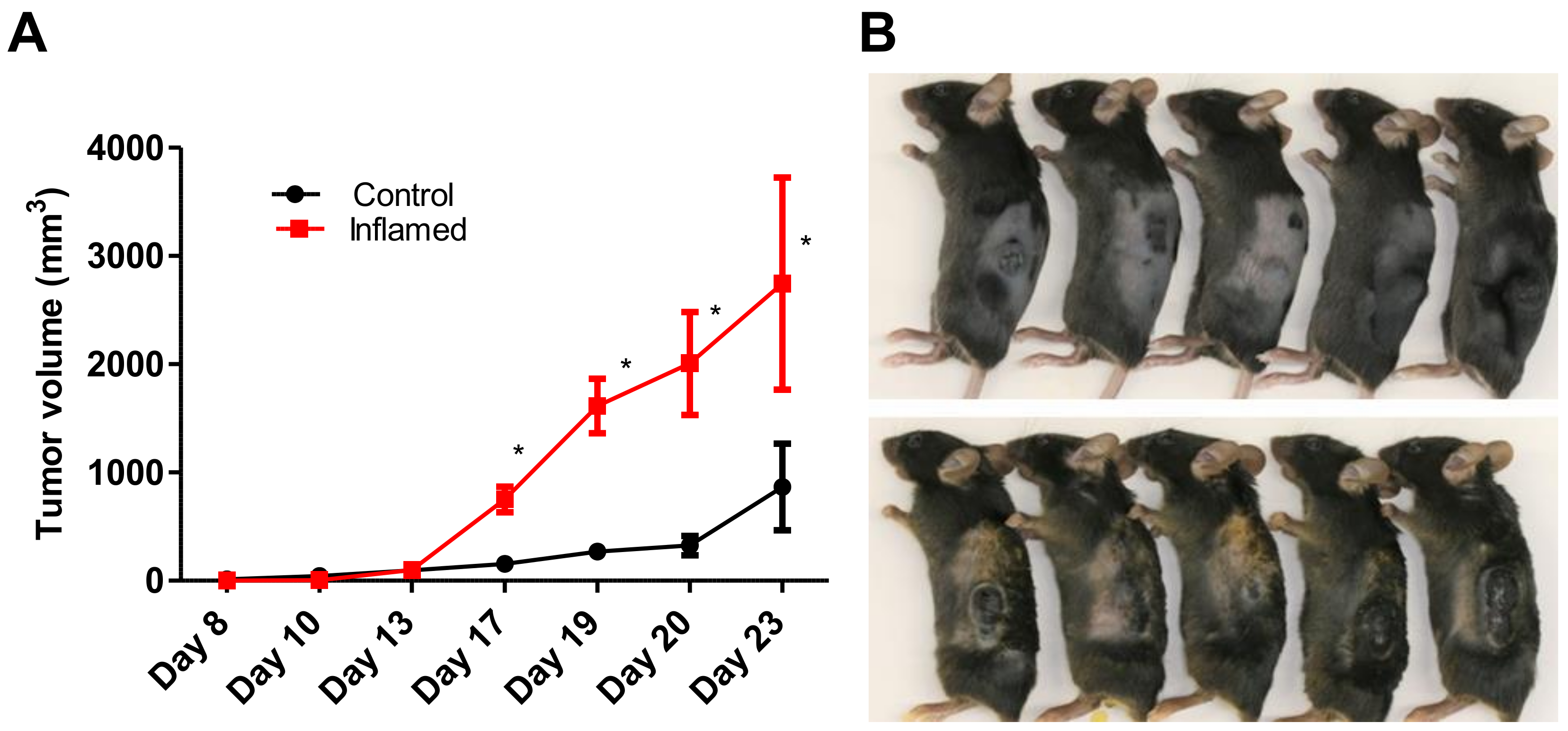
2.1. Chronic Inflammation Significantly Accelerates Murine Melanoma Progression
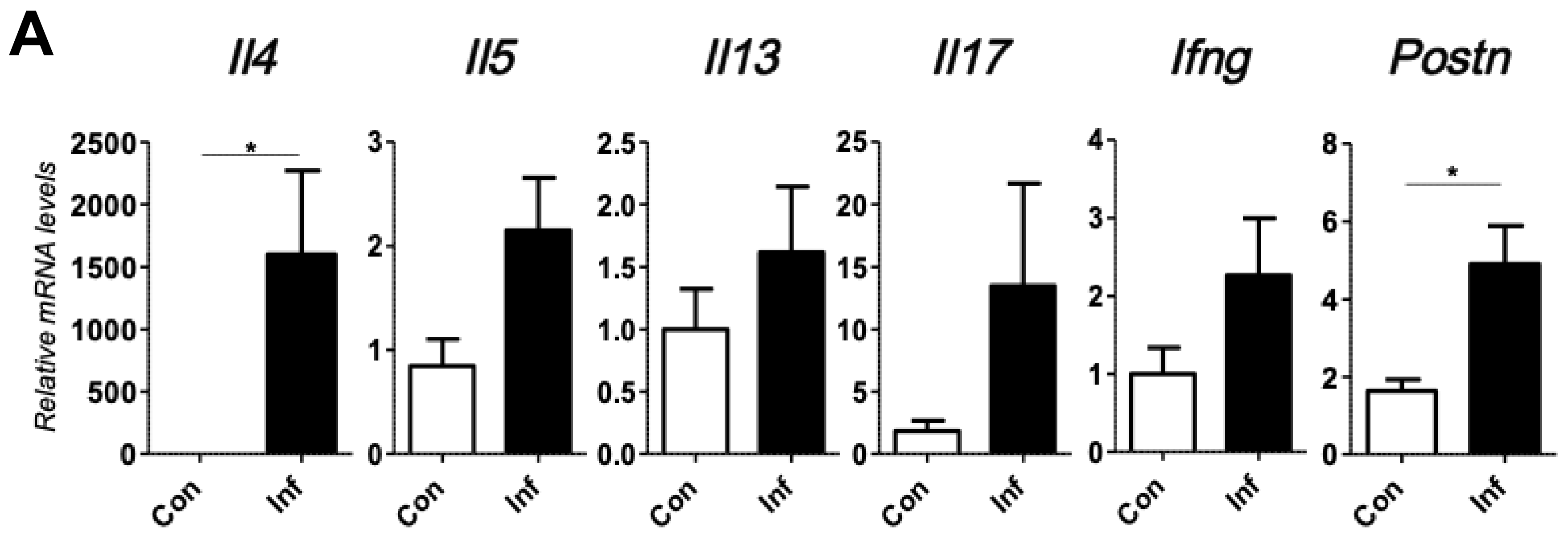
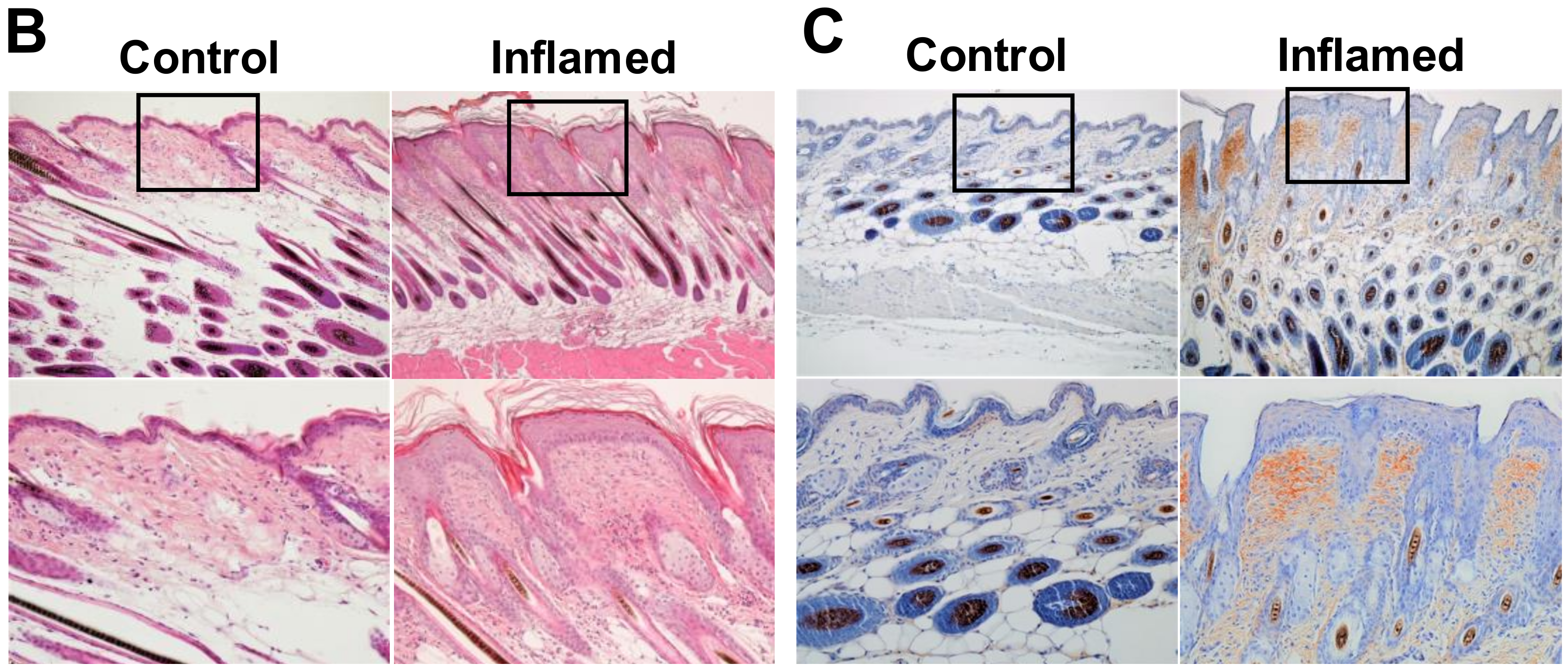
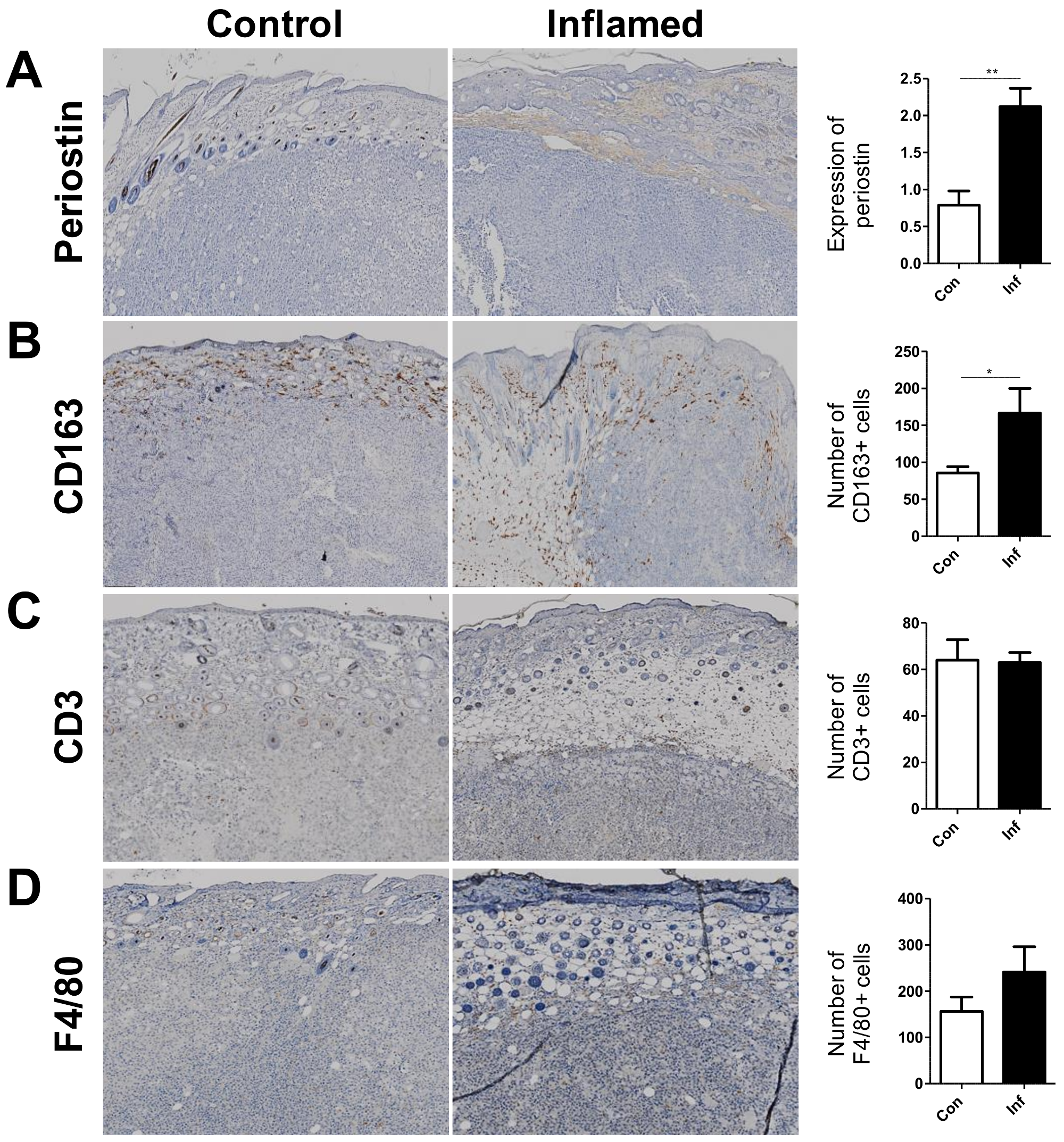
2.2. Stromal Periostin Expression and the Number of CD163+ M2 Macrophages Are Significantly Elevated in Melanomas in Inflamed Mice
2.3. High Periostin Expression and a Large Number of Infiltrated CD163+ M2 Macrophages Are Significantly Correlated with Poor Prognosis in Patients with Melanoma
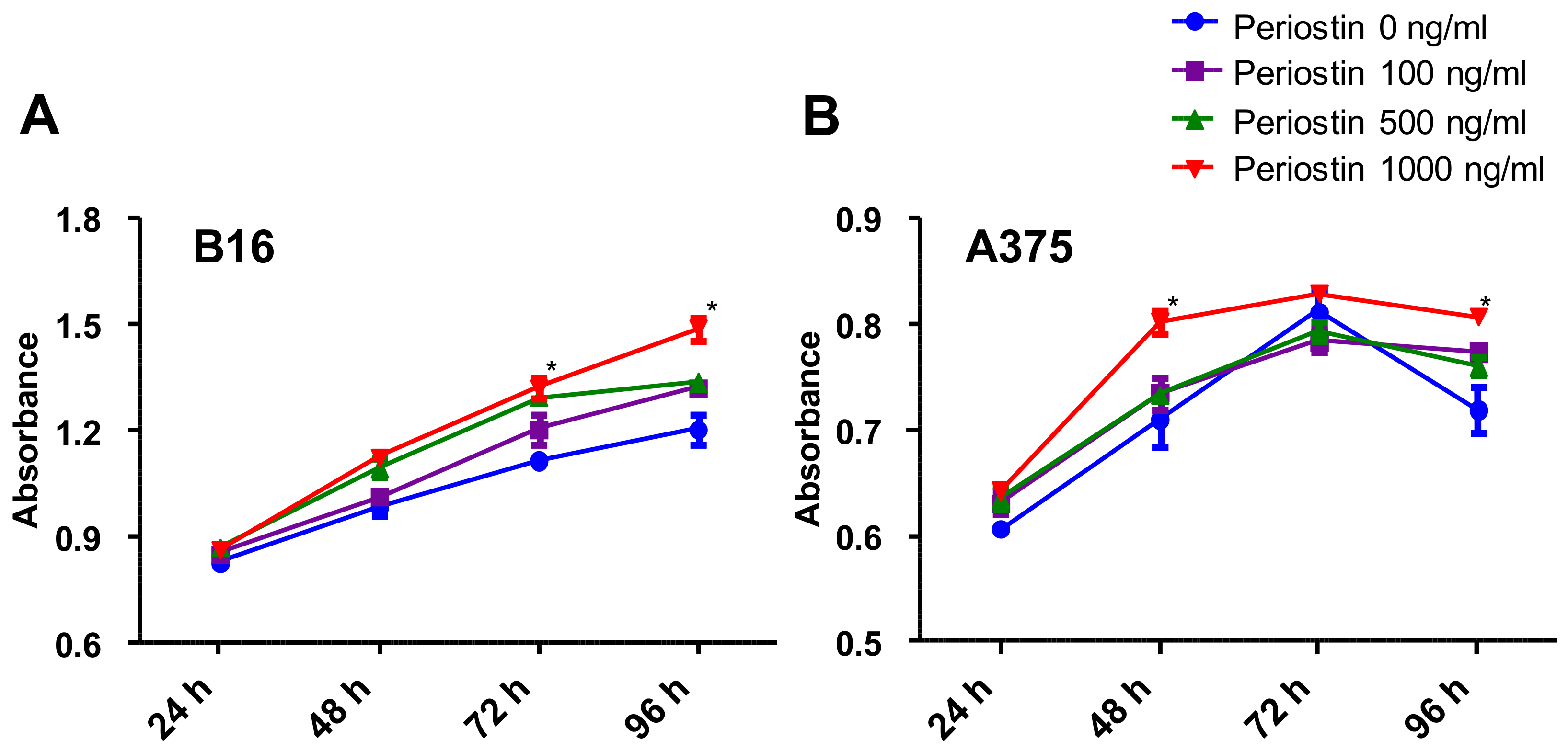
2.4. Periostin Promotes the Proliferation of Murine and Human Melanoma Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. In Vivo Experiments
4.3. Quantitative RT-PCR Analysis
4.4. Immunohistochemistry and Scoring
4.5. Patients and Tissue Samples
4.6. Cell Culture
4.7. Proliferation Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TGF | transforming growth factor |
| IL | Interleukin |
| PDGF | platelet-derived growth factor |
| CCN | connective tissue growth factor |
| DFS | disease-free survival |
References
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, 493–503. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Shalapour, S.; Karin, M. Immunity, inflammation, and cancer: An eternal fight between good and evil. J. Clin. Investig. 2015, 125, 3347–3355. [Google Scholar] [CrossRef]
- Hawryluk, E.B.; Tsao, H. Melanoma: Clinical features and genomic insights. Cold Spring Harb. Perspect. Med. 2014, 4, a015388. [Google Scholar] [CrossRef]
- Ito, T.; Wada, M.; Nagae, K.; Nakano-Nakamura, M.; Nakahara, T.; Hagihara, A.; Furue, M.; Uchi, H. Acral lentiginous melanoma: Who benefits from sentinel lymph node biopsy? J. Am. Acad. Dermatol. 2015, 72, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kulichová, D.; Dáňová, J.; Kunte, C.; Ruzicka, T.; Celko, A.M. Risk factors for malignant melanoma and preventive methods. Cutis 2014, 94, 241–248. [Google Scholar] [PubMed]
- Wada, M.; Ito, T.; Tsuji, G.; Nakahara, T.; Hagihara, A.; Furue, M.; Uchi, M. Acral lentiginous melanoma versus other melanoma: A single-center analysis in Japan. J. Dermatol. 2017, 44, 932–938. [Google Scholar] [CrossRef]
- Donatella, L.; Marika, C.; Lorenzo, G.; Vito, A.; Cathryn, A.S.; Giovanna, D.M.; Stefano, P.; Carla, D.L.; Fabio, P.; Gianpiero, F.; et al. Tumour-infiltrating lymphocytes, programmed death ligand 1 and cyclooxygenase-2 expression in skin melanoma of elderly patients: Clinicopathological correlations. Melanoma Res. 2018, 28, 547–554. [Google Scholar]
- Isabella, V.; Maria, V.; Cinzia, F.; Manuela, R.; Simona, R.; Maria, C.; Gerardo, C.; Concetta, A.; Francesco, T.; Diana, T.; Mario, V. PGE2 induces interleukin-8 depression in human astrocytoma through coordinated DNA demethylation and histone hyperacethlation. Epigenetics 2012, 7, 1315–1330. [Google Scholar]
- Gomes, M.; Teixeira, A.L.; Coelho, A.; Araújo, A.; Medeiros, R. The role of inflammation in lung cancer. Adv. Exp. Med. Biol. 2014, 816, 1–23. [Google Scholar]
- Maru, G.B.; Gandhi, K.; Ramchandani, A.; Kumar, G. The role of inflammation in skin cancer. Adv. Exp. Med. Biol. 2014, 816, 437–469. [Google Scholar] [PubMed]
- Izuhara, K.; Conway, S.J.; Moore, B.B.; Matsumoto, H.; Holweg, C.T.; Matthews, J.G.; Arron, J.R. Roles of periostin in respiratory disorders. Am. J. Respir. Crit. Care Med. 2016, 193, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Laura, G.; Javier, A. Periostin: A matricellular protein with multiple functions in cancer development and progression. Front. Oncol. 2018, 8, 225. [Google Scholar]
- Fukuda, K.; Sugihara, E.; Ohta, S.; Izuhara, K.; Funakoshi, T.; Amagai, M.; Saya, H. Periostin is a key niche component for wound metastasis of melanoma. PLoS ONE 2015, 10, e0129704. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Arima, K.; Masuoka, M.; Ohta, S.; Shiraishi, H.; Ontsuka, K.; Suzuki, S.; Inamitsu, M.; Yamamoto, K.I.; Simmons, O.; et al. Periostin controls keratinocyte proliferation and differentiation by interacting with the paracrine IL-1α/IL-6 loop. J. Investig. Dermatol. 2014, 134, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Ouyang, G.; Bai, X.; Huang, Z.; Ma, C.; Liu, M.; Shao, R.; Anderson, R.M.; Rich, J.N.; Wang, X.F. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 2004, 5, 329–339. [Google Scholar] [CrossRef]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for αVβ3 and αVβ5 integrins and promotes cell motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar]
- Tilman, G.; Mattiussi, M.; Brasseur, F.; van Baren, N.; Decottignies, A. Human periostin gene expression in normal tissues, tumors and melanoma: Evidences for periostin production by both stromal and melanoma cells. Mol. Cancer 2007, 6, 80. [Google Scholar] [CrossRef]
- Kim, C.J.; Yoshioka, N.; Tambe, Y.; Kushima, R.; Okada, Y.; Inoue, H. Periostin is down-regulated in high grade human bladder cancers and suppresses in vitro cell invasiveness and in vivo metastasis of cancer cells. Int. J. Cancer 2005, 117, 51–58. [Google Scholar] [CrossRef]
- Yoshioka, N.; Fuji, S.; Shimakage, M.; Kodama, K.; Hakura, A.; Yutsudo, M.; Inoue, H.; Nojima, H. Suppression of anchorage-independent growth of human cancer cell lines by the TRIF52/periostin/OSF-2 gene. Exp. Cell Res. 2002, 279, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Kotobuki, Y.; Yang, L.; Serada, S.; Tanemura, A.; Yang, F.; Nomura, S.; Kudo, A.; Izuhara, K.; Murota, H.; Fujimoto, M.; et al. Periostin accelerates human malignant melanoma progression by modifying the melanoma microenvironment. Pigment Cell Melanoma Res. 2014, 27, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; Mclendon, R.E.; Li, X.; et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Hutchenreuther, J.; Vincent, K.M.; Carter, D.E.; Postovit, L.M.; Leask, A. CCN2 expression by tumor stroma is required for melanoma metastasis. J. Investig. Dermatol. 2015, 135, 2805–2813. [Google Scholar] [CrossRef] [PubMed]
- Falleni, M.; Savi, F.; Tosi, D.; Agape, E.; Cerri, A.; Moneghini, L.; Bulfamante, G.P. M1 and M2 macrophages’ clinicopathological significance in cutaneous melanoma. Melanoma Res. 2017, 27, 200–210. [Google Scholar] [CrossRef] [PubMed]
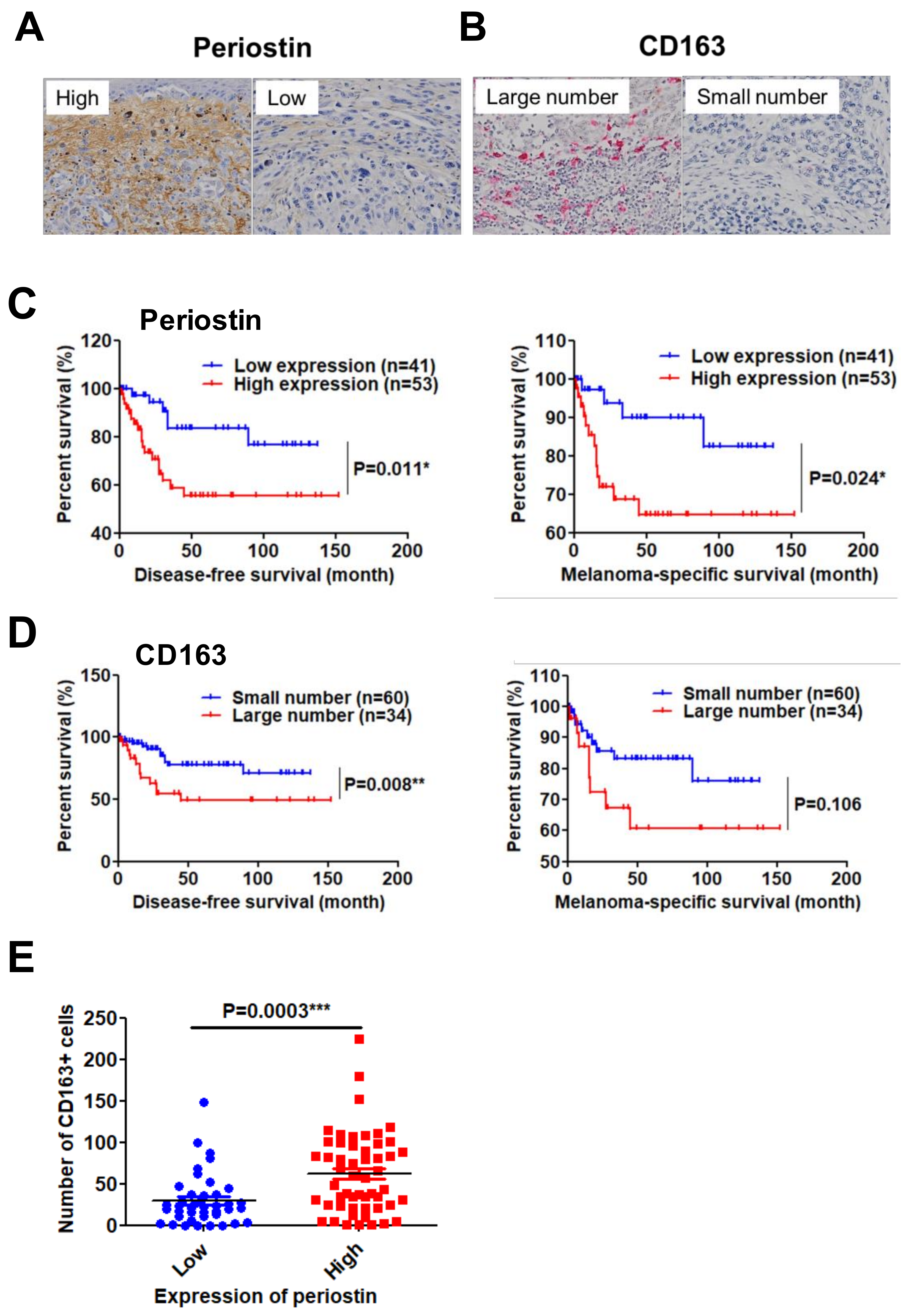
| Parameters | Expression of Periostin | Number of CD163+ Cells | ||||
|---|---|---|---|---|---|---|
| Low | High | p-Value | Small | Large | p-Value | |
| Age (y) | ||||||
| <60 | 15 | 10 | 0.053 ¶ | 18 | 5 | 0.13 ∫ |
| ≥60 | 26 | 43 | 42 | 29 | ||
| Gender | ||||||
| Male | 16 | 24 | 0.54 ¶ | 23 | 17 | 0.27 ¶ |
| Female | 25 | 29 | 37 | 17 | ||
| Breslow Thickness § | ||||||
| Tis | 20 ** | 4 †† | <0.0001 ‡¶ | 23 ** | 1 †† | 0.0001 ‡¶ |
| T1 | 8 | 9 | 14 | 3 | ||
| T2 | 5 | 2 | 4 | 3 | ||
| T3 | 6 | 15 | 8 †† | 13 ** | ||
| T4 | 2 †† | 22 ** | 10 † | 14 * | ||
| TNM Stage | ||||||
| 0 | 20 ** | 4 †† | <0.0001 ‡¶ | 23 ** | 1 †† | <0.0001 ‡¶ |
| I | 11 | 10 | 17 | 4 | ||
| II | 4 †† | 22 ** | 13 | 23 | ||
| III | 6 | 12 | 5†† | 13 ** | ||
| IV | 0 † | 5 * | 2 | 3 | ||
| Total No. | 41 | 53 | 60 | 34 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohno, F.; Nakahara, T.; Kido-Nakahara, M.; Ito, T.; Nunomura, S.; Izuhara, K.; Furue, M. Periostin Links Skin Inflammation to Melanoma Progression in Humans and Mice. Int. J. Mol. Sci. 2019, 20, 169. https://doi.org/10.3390/ijms20010169
Ohno F, Nakahara T, Kido-Nakahara M, Ito T, Nunomura S, Izuhara K, Furue M. Periostin Links Skin Inflammation to Melanoma Progression in Humans and Mice. International Journal of Molecular Sciences. 2019; 20(1):169. https://doi.org/10.3390/ijms20010169
Chicago/Turabian StyleOhno, Fumitaka, Takeshi Nakahara, Makiko Kido-Nakahara, Takamichi Ito, Satoshi Nunomura, Kenji Izuhara, and Masutaka Furue. 2019. "Periostin Links Skin Inflammation to Melanoma Progression in Humans and Mice" International Journal of Molecular Sciences 20, no. 1: 169. https://doi.org/10.3390/ijms20010169
APA StyleOhno, F., Nakahara, T., Kido-Nakahara, M., Ito, T., Nunomura, S., Izuhara, K., & Furue, M. (2019). Periostin Links Skin Inflammation to Melanoma Progression in Humans and Mice. International Journal of Molecular Sciences, 20(1), 169. https://doi.org/10.3390/ijms20010169






