Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes
Abstract
1. Introduction
2. Results
2.1. Effect of High Salt on Viability in Pre-Adipocytes and Adipocytes
2.2. Salt Induced SIK2, Na+K+-ATPase, and RAAS Signals
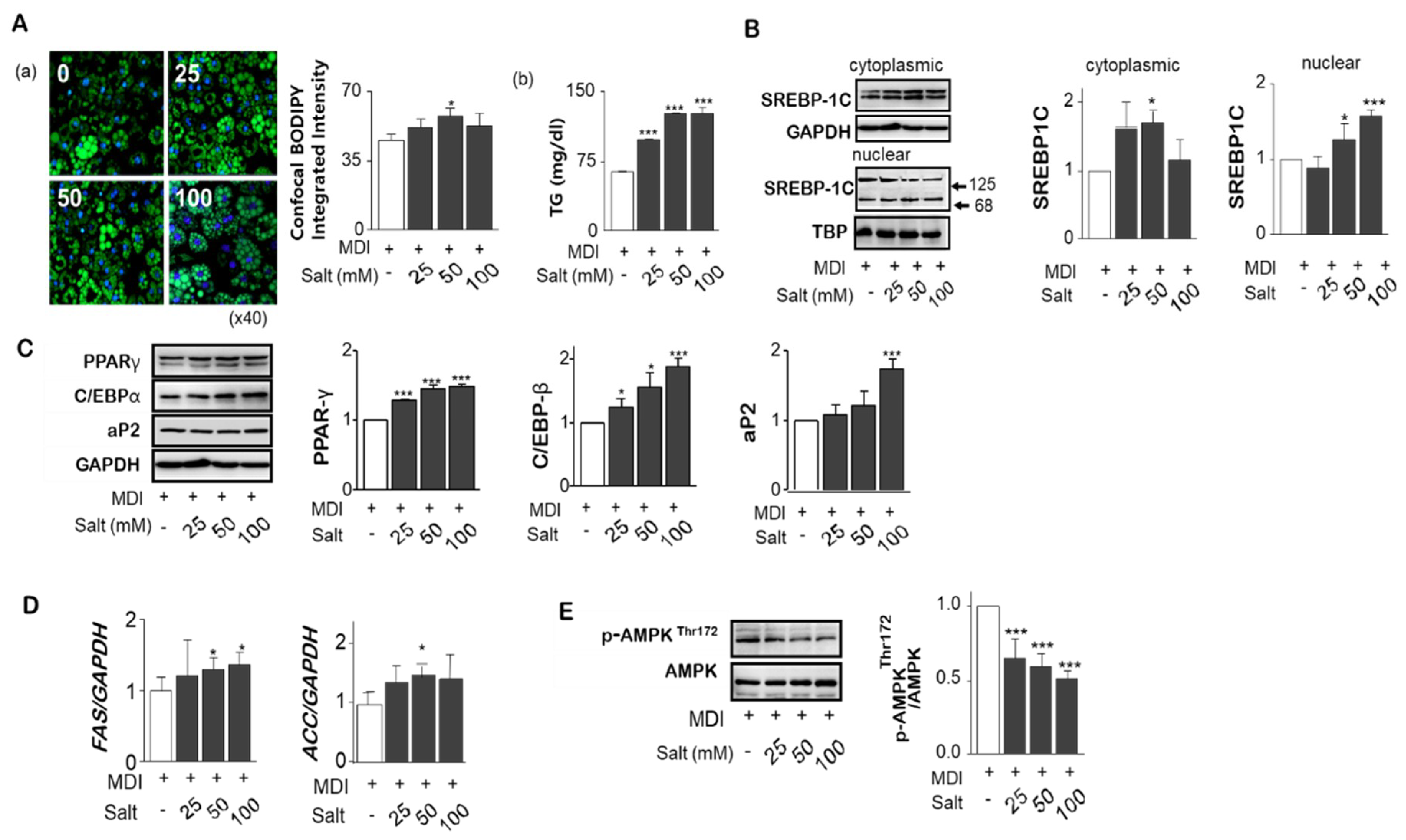
2.3. Salt Stimulates Adipogenesis/Lipogesesis Genes in Adipocytes
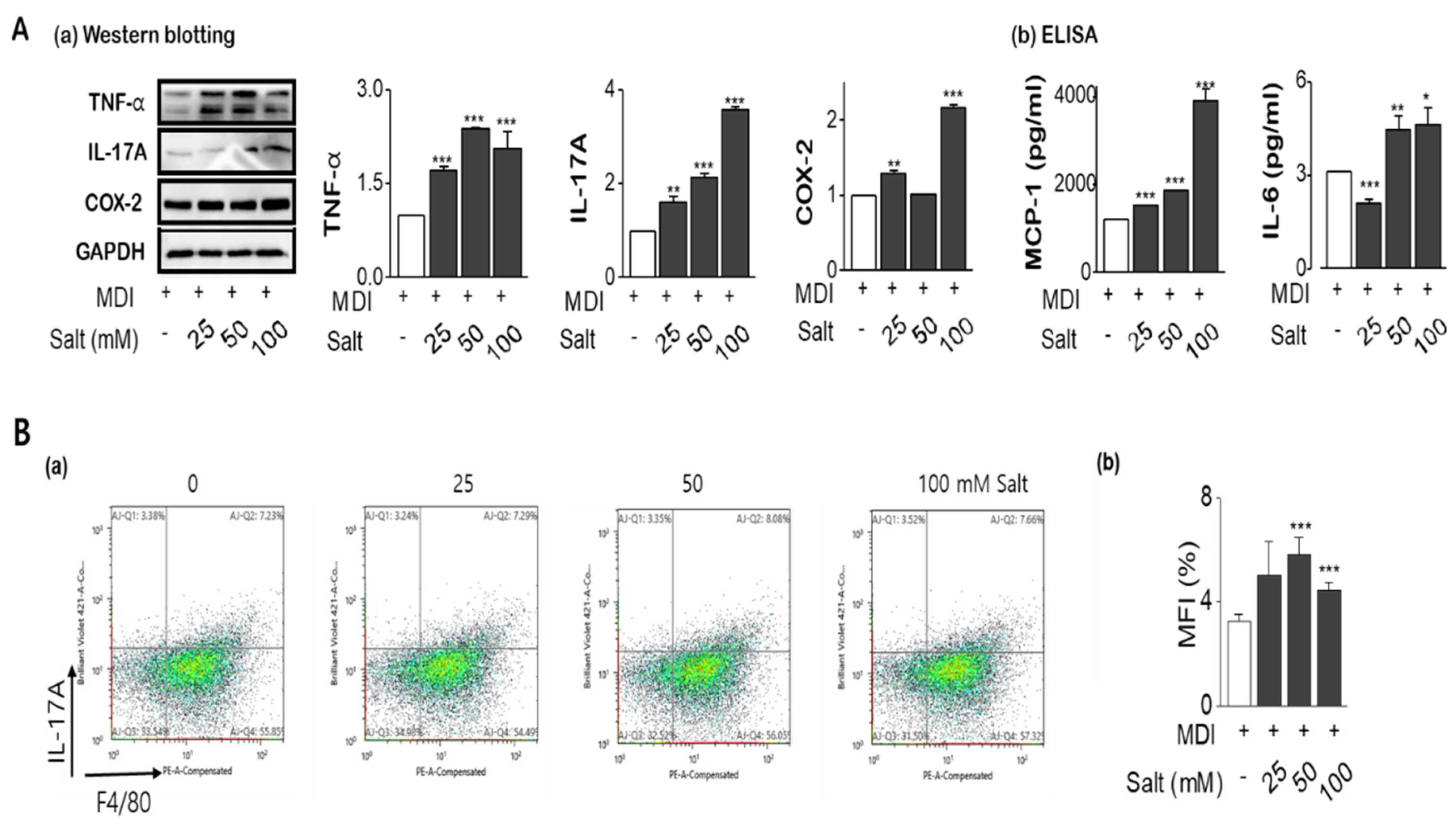
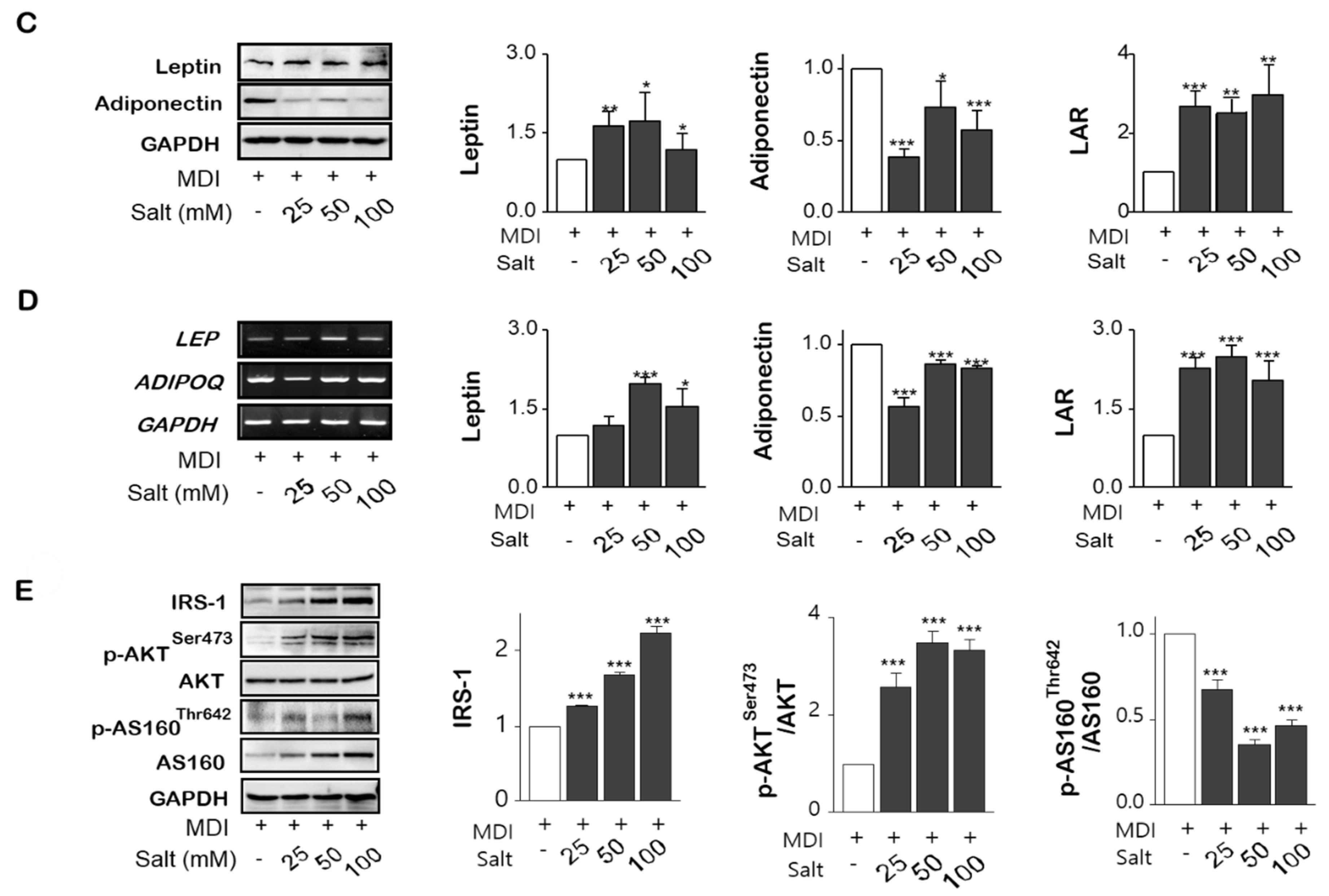
2.4. Salt Enhances the Tolerance of Inflammatory Cytokines with Insulin Resistance
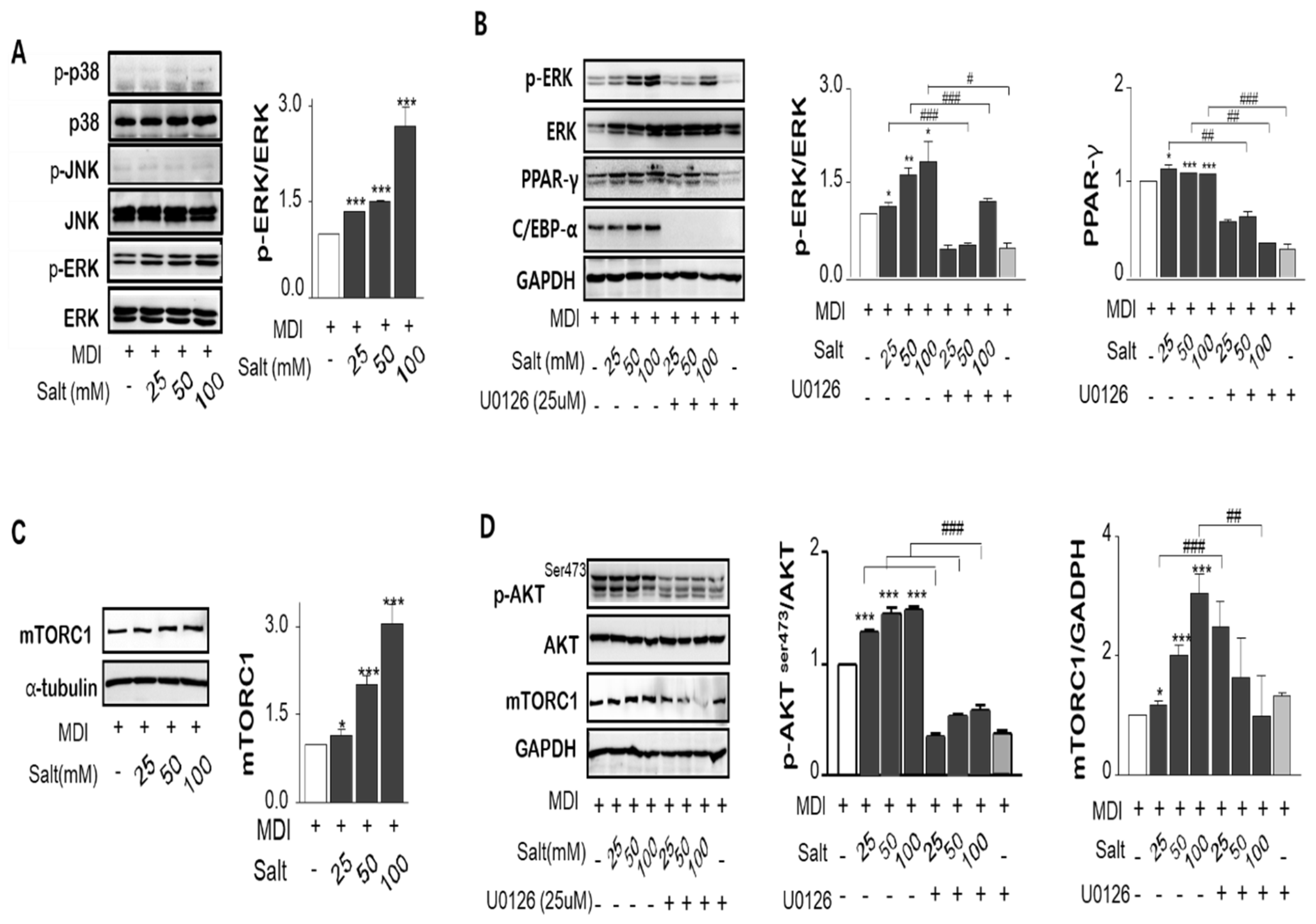
2.5. Salt Activates MAPK/ERK and Akt Ser473-mTOR Dependent Mechanisms on the Adipogenesis
3. Discussion
4. Materials/Subjects and Methods
4.1. Materials
4.2. Experimental Design for the Cell Culture
4.3. Cell Viability and Accumulation of Lipid Droplets
4.4. Quantitation Polymerase Chain Reaction (PCR) and Western Blotting Analysis
4.5. ELISA and Intracellular Cytokines Staining
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, S.K.; Kim, Y.J.; Yang, S.J.; Lee, M. Nutrigenomic functions of PPARs in obesogenic environments. PPAR Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Fulgoni, V.; Spence, L.; Samuel, P. Sodium intake status in United States and potential reduction modelling: An NHANES 2007-2010 analysis. Food. Sci. Nutr. 2015, 3, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Republic of Korea: Ministry of Health & Welfare 2010 Korean National Health and Nutrition Examination Survey (KNHANES). 2010. Available online: https://knhanes.cdc.go.kr (accessed on 3 December 2018).
- Usukura, M.; Zhu, A.; Yoneda, T.; Karashima, S.; Yagi, K.; Yamagishi, M.; Takeda, Y. Effects of high salt diet on adipocyte glucocorticoid receptor and 11-hydroxysteroid dehydrogenase 1 in salt sensitive hypertensive rats. Steroids 2009, 74, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Underwood, P.C.; Adler, G.K. The renin agiotensin aldosterone system and insulin resistance in human. Curr. Hypertens. Rep. 2013, 15, 59–70. [Google Scholar] [CrossRef]
- Thethi, T.; Kamiyama, M.; Kobori, H. The link between the renin-angiotensin-aldosterone system and renal injury in obesity and the metabolic syndrome. Curr. Hypertens. Rep. 2012, 14, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Weiland, F.; Verpohl, E.J. Varity of angiotensin receptors in 3T3-L1 preadipose cells and differentiated adipocytes. Horm. Metab. Res. 2008, 40, 760–766. [Google Scholar] [CrossRef]
- Lu, H.; Boustany-Kari, C.M.; Daugherty, A.; Cassis, L.A. Angiotensin II increases adipose angiotensinogen expression. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1280–1287. [Google Scholar] [CrossRef]
- Shibata, S.; Mu, S.; Kawarazaki, H.; Muraoka, K.; Ishizawa, K.; Yoshida, S.; Kawarazaki, W.; Takeuchi, M.; Ayuzawa, N.; Miyoshi, J.; et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J. Clin. Invest. 2011, 121, 3233–3243. [Google Scholar] [CrossRef]
- Katoh, Y.; Takemori, H.; Horike, N.; Doi, J.; Muraokaa, M.; Mina, L.; Okamoto, M. Salt-inducible kinase (SIK) isoforms: Their involvement in steroidogenesis and adipogenesis. Mol. Cell. Endocrinol. 2004, 217, 109–112. [Google Scholar] [CrossRef]
- Horike, N.; Takemori, H.; Katoh, Y.; Doi, J.; Min, L.; Asano, T.; Sun, X.J.; Yamamoto, H.; Kasayama, S.; Muraoka, M.; et al. Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. J. Biol. Chem. 2003, 278, 18440–18447. [Google Scholar] [CrossRef]
- Weng, L.P.; Smith, W.M.; Brown, J.L.; Eng, C. PTEN inhibits insulin-stimulated MEK/MAPK activation and cell growth by blocking IRS-1 phosphorylation and IRS-1/Grb-2/Sos complex formation in a breast cancer model. Hum. Mol. Genet. 2001, 10, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Yecies, J.L.; Zhang, H.H.; Menon, S.; Liu, S.; Yecies, D.; Lipovsky, A.I.; Gorgun, C.; Kwiatkowski, D.J.; Hotamisligil, G.S.; Lee, C.-H.; et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011, 14, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, T.; Asano, T.; Ando, K.; Sakoda, H.; Anai, M.; Shojima, N.; Ono, H.; Onishi, Y.; Fujishiro, M.; Abe, M.; et al. High-salt diet enhances insulin signaling and induces insulin resistance in Dahl salt-sensitive rats. Hypertension 2002, 40, 83–89. [Google Scholar] [CrossRef]
- Cai, H.; Dong, L.Q.; Lie, F. Recent Advances in Adipose mTOR Signaling and Function: Therapeutic Prospects. Trends Pharmacol. Sci. 2016, 37, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Cornu, M.; Albert, V.; Hall, M.N. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet Dev. 2013, 23, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Cao, H. Adipocytokines in obesity and metabolic disease. Themat. Rev. 2014, 220, T47–T59. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Ouchi, N.; Ohashi, K.; Shibata, R.; Murohara, T. Adipocytokines and obesity-linked disorders. Nagoya J. Med. Sci. 2012, 74, 19–30. [Google Scholar]
- Fazolini, N.P.B.; Cruz, A.L.S.; Werneck, M.B.F.; Viola, J.P.B.; Maya-Monteiro, C.M.; Bozza, P.T. Leptin activation of mTOR pathway in intestinal epithelial cell triggers lipid droplet formation, cytokine production and increased cell proliferation. Cell Cycle 2015, 14, 2667–2676. [Google Scholar] [CrossRef]
- Sun, G.; Shan, M.H.; Ma, B.L.; Geng, G.L.; Alibiyati, A.; Zhong, H.; Wang, J.; Ren, G.H.; Li, H.T.; Dong, C. Identifying crosstalk of mTOR signaling pathway of lobular breast carcinomas. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1355–1361. [Google Scholar]
- Cassis, L.A.; Police, S.B.; Yiannikouris, F.; Thatcher, S.E. Local adipose tissue renin-angiotensin system. Curr. Hypertens. Rep. 2008, 10, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y. Effects of eplerenone, a selective mineratlcorticoid receptor antagonist, on clinical and experimental salt-sensitive hypertension. Hypertens. Res. 2009, 32, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.I.; Whaley-Connell, A.; DeMarco, V.G.; Pulakat, L.; Habibi, J.; Sowers, J.R. High salt diet induces SIK2 and Akt-mediated ventricular hypertrophy in female (mRen2)27 Ren2 TG rats. Hypertension 2010, 56, e79. [Google Scholar]
- Dünner, N.; Quezada, C.; Berndt, F.A.; Cánovas JRojas, C.V. Angiotensin II signaling in human preadipose cells: Participation of ERK1,2-dependent modulation of Akt. PLoS ONE 2013, 8, e75440. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Chang, S.C.; Wen, H.C.; Chen, C.Y.; Lia, J.F.; Chang, C.H. Plumbagin activates ERK1/2 and Akt via superoxide, Src and PI3-kinase in 3T3-L1 Cells. Eur. J. Pharmacol. 2010, 638, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Bannergee, S.; Schwartz, J.H.; Lieberthal, W.; Levine, J.S. Inhibition of ligand-independent ERK1/2 activity in kidney proximal tubular cells deprived of soluble survival factors up-regulates Akt and prevents apoptosis. J. Biol. Chem. 2004, 279, 10962–10972. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Ullrich, A. Endothelin-1 inhibits adipogenesis: Role of phosphorylation of Akt and ERK1/2. FEBS Lett. 2006, 580, 7765–7771. [Google Scholar] [CrossRef]
- Prusty, D.; Park, B.H.; Davis, K.E.; Farmer, S.R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 2002, 277, 46226–46232. [Google Scholar] [CrossRef]
- Zick, Y. Insulin resistance: A phosphorylation-based uncoupling of insulin signaling. Trends Cell Biol. 2001, 11, 437–441. [Google Scholar] [CrossRef]
- Wu, J.J.; Roth, R.J.; Anderson, E.J.; Hong, E.-G.; Lee, M.-K.; Choi, C.S.; Neufer, P.D.; Shulman, G.I.; Kim, J.K.; Bennett, A.M. Mice lacking MAP kinase phosphatase-1 have enhanced MAP kinase activity and resistance to diet-induced obesity. Cell Metab. 2006, 4, 61–73. [Google Scholar] [CrossRef]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Emrah, E.; Er Blenis, J. The Ras-ERK and PI3K-mTOR Pathways: Cross-talk and Compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Dokladda, K.; Green, K.A.; Pan, D.A.; Hardie, D.G. PD98059 and U0126 activate AMP-activated protein kinase by increasing the cellular AMP: ATP ratio and not via inhibition of the MAP kinase pathway. FEBS Lett. 2005, 579, 236–240. [Google Scholar] [CrossRef]
- Soltoff, S.P.; Hedden, L. Regulation of ERK1/2 by ouabain and Na+/K+ -ATPase-dependent energy utilization and AMPK activation in parotid acinar cells”. Am. J. Physiol. Cell Physiol. 2008, 295, C590–C599. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Bue, G.R.; Blenis, J. Nutrient Regulation of the mTOR Complex 1 Signaling Pathway. Mol. Cells 2013, 35, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Espenshade, P.J. Expanding roles for SREBP in metabolism. Cell Metab. 2012, 16, 414–419. [Google Scholar] [CrossRef]
- Norrmén, C.; Suter, U. Akt/mTOR signaling in myelination. Biochem. Soc. Trans. 2013, 41, 944–950. [Google Scholar] [CrossRef]
- Middelbeek, R.J.W.; A Chambers, M.; Tantiwong, P.; Treebak, J.; Hirshman, M.F.; Musi, N.; Goodyear, L.J.; An, D. Insulin stimulation regulates AS160 and TBC1D1 phosphorylation sites in human skeletal muscle. Nutr. Diabetes 2013, 3, e74. [Google Scholar] [CrossRef]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. Nutrient excess in AMPK down-regulation and insulin resistance. J. Endocrinol. Diabetes Obes. 2013, 1, 1008–1021. [Google Scholar]
- Yamaguchi, S.; Katahira, H.; Ozawa, S.; Nakamichi, Y.; Tanaka, T.; Shimoyama, T.; Takahashi, K.; Yoshimoto, K.; Imaizumi, M.O.; Nagamatsu, S.; et al. Activators of AMP-activated protein kinase enhance GLUT4 translocation and its glucose transport activity in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E643–E649. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Daval, M.; Foufelle, F.; Ferr, P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006, 574, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Zhang, Q.; Ma, S.; Chen, Z.; Mo, Z.; You, Z. Interleukin-17A differentially induces inflammatory and metabolic gene expression in the adipose tissues of lean and obese mice. Int. J. Mol. Sci. 2016, 17, 522. [Google Scholar] [CrossRef] [PubMed]
- Hajer, C.R.; Van Haeften, T.W.; Visseren, F.L.J. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef] [PubMed]
- Daikoku, T.; Terakawa, J.; Hossain, M.; Yoshie, M.; Cappelletti, M.; Yang, P.; Ellenson, L.H.; Dey, S.K. Mammalian target of rapamycin complex 1 and cyclooxygenase 2 pathways cooperatively exacerbate endometrial cancer. Am. J. Pathol. 2014, 184, 2390–2402. [Google Scholar] [CrossRef]
- Chan, P.C.; Hsiao, F.C.; Chang, H.M.; Wabitsch, M.; Hsieh, P.S. Importance of adipocyte cyclooxygenase-2 and prostaglandin E2–prostaglandin E receptor 3 signaling in the development of obesity-induced adipose tissue inflammation and insulin resistance. FASEB J. 2016, 30, 2282–2297. [Google Scholar] [CrossRef]
- Maccallini, C.; Mollica, A.; Amoroso, R. The positive regulation of eNOS signaling by PPAR agonists in cardiovascular diseases. Am. J. Cardio Drugs 2017, 17, 273–281. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Sorn, S.R.; Lee, Y.; Kang, I. Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes. Int. J. Mol. Sci. 2019, 20, 160. https://doi.org/10.3390/ijms20010160
Lee M, Sorn SR, Lee Y, Kang I. Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes. International Journal of Molecular Sciences. 2019; 20(1):160. https://doi.org/10.3390/ijms20010160
Chicago/Turabian StyleLee, Myoungsook, Sungbin Richard Sorn, Yunkyoung Lee, and Inhae Kang. 2019. "Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes" International Journal of Molecular Sciences 20, no. 1: 160. https://doi.org/10.3390/ijms20010160
APA StyleLee, M., Sorn, S. R., Lee, Y., & Kang, I. (2019). Salt Induces Adipogenesis/Lipogenesis and Inflammatory Adipocytokines Secretion in Adipocytes. International Journal of Molecular Sciences, 20(1), 160. https://doi.org/10.3390/ijms20010160






