Targeting Multiple Receptors to Increase Checkpoint Blockade Efficacy
Abstract
1. Introduction
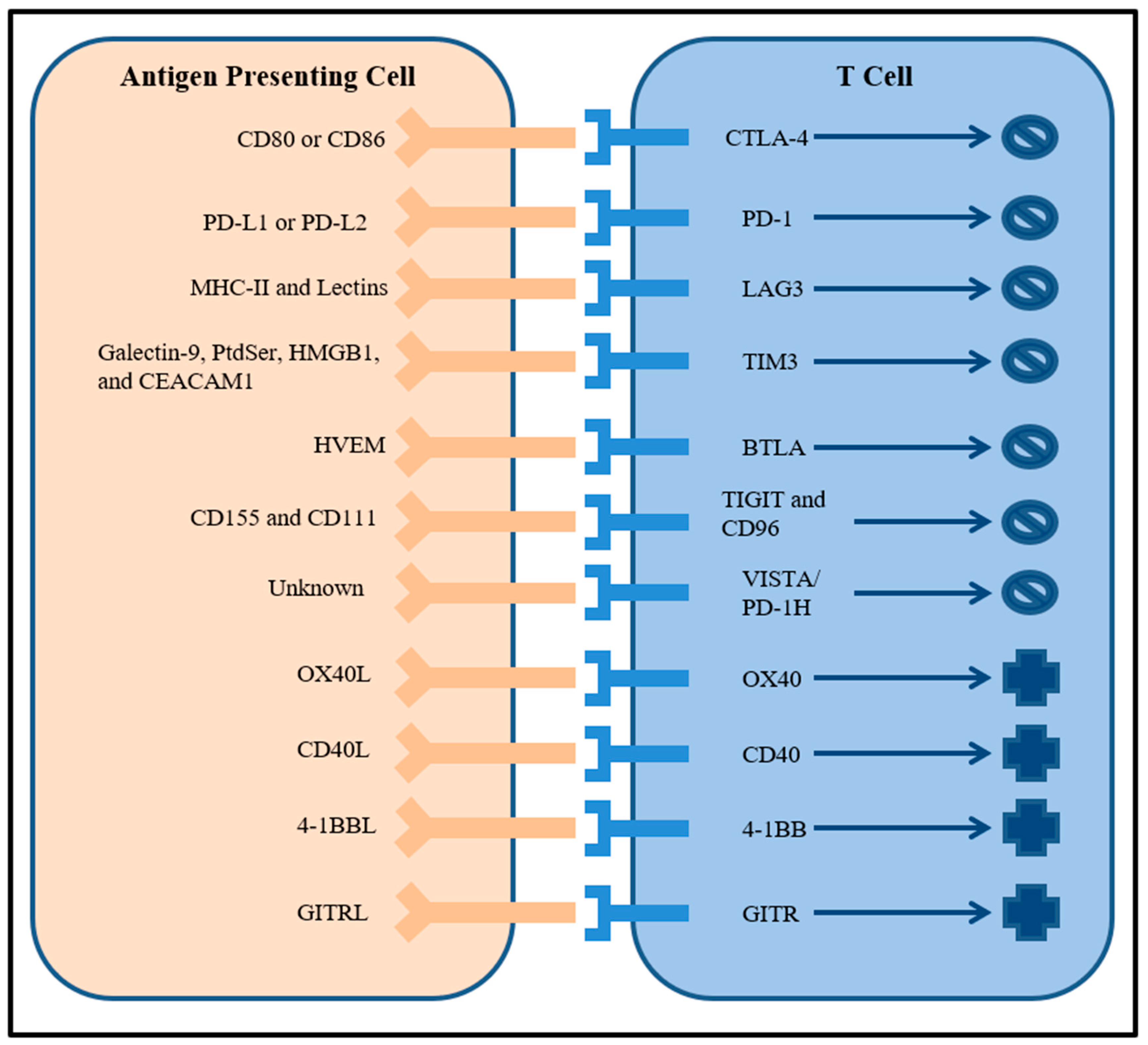
2. Checkpoint Blockade
3. Targeting Multiple Immune Checkpoints
4. Combining Checkpoint Blockade with Other Receptor Targets
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hargadon, K.; Johnson, C.; Williams, C. Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.; Hodi, F.; Kaufman, H.; Wigginton, J.; Wolchok, J. Combination Immunotherapy: A Road Map. J. Immunother. Cancer 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Karamouzis, M.; Papavassiliou, A. Combination of Checkpoint Inhibitors with Other Agents as a Strategy to Improve Anti-Cancer Effect—A Glimpse to the Future. Expert Opin. Investig. Drugs 2018, 27, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Intlekofer, A.; Thompson, C. At the Bench: Preclinical Rationale for CTLA-4 and PD-1 Blockade as Cancer Immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, O.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.; Baker, J.; Jeffery, L.; Kaur, S.; Briggs, Z.; et al. Trans-Endocytosis of CD80 and CD86: A Molecular Basis for the Cell-Extrinsic Function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.; Allison, J. CTLA-4 Engagement Inhibits IL-2 Accumulation and Cell Cycle Progression Upon Activation of Resting T Cells. J. Exp. Med. 1996, 183, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.; Sullivan, T.; Truong, T.; Allison, J. Secondary but Not Primary T Cell Responses Are Enhanced in CTLA-4-Deficient CD8+ T Cells. Eur. J. Immunol. 1998, 28, 3137–3143. [Google Scholar] [CrossRef]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 Control Over Foxp3+ Regulatory T Cell Function. Science 2008, 322, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.; Krummel, M.; Allison, J. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Hodi, F.; O’Day, S.; McDermott, D.; Weber, R.; Sosman, J.; Haanen, J.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Topalian, S.; Drake, C.; Pardoll, D. Immune Checkpoint Blockade: A Common Denominator Approach to Cancer Therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Page, D.; Postow, M.; Callahan, M.; Allison, J.; Wolchok, J. Immune Modulation in Cancer with Antibodies. Annu. Rev. Med. 2014, 65, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-Like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Topalian, S.; Hodi, F.; Brahmer, J.; Gettinger, S.; Smith, D.; McDermott, D.; Powderly, J.; Carvajal, R.; Sosman, J.; Atkins, M.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Sznol, M.; Chen, L. Antagonist Antibodies to PD-1 and B7-H1 (PD-L1) in the Treatment of Advanced Human Cancer—Response. Clin. Cancer Res. 2013, 19, 5542. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; D’Angelo, S.; Minor, D.; Hodi, F.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.; Miller, W.; Lao, C.; et al. Nivolumab Versus Chemotherapy in Patients with Advanced Melanoma Who Progressed after Anti-CTLA-4 Treatment (Checkmate 037): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.; Baas, P.; Crinò, L.; Eberhardt, W.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.; Holgado, E.; et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Escudier, B.; McDermott, D.; George, S.; Hammers, H.; Srinivas, S.; Tykodi, S.; Sosman, J.; Procopio, G.; Plimack, E.; et al. Nivolumab Versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.; Schachter, J.; Pavlick, A.; Lewis, K.; et al. Pembrolizumab Versus Investigator-Choice Chemotherapy for Ipilimumab-Refractory Melanoma (KEYNOTE-002): A Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Fuchs, C.; Doi, T.; Jang, R.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.; Shah, M.; Metges, J.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Abdin, S.; Zaher, D.; Arafa, E.; Omar, H. Tackling Cancer Resistance by Immunotherapy: Updated Clinical Impact and Safety of PD-1/PD-L1 Inhibitors. Cancers 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Huard, B.; Gaulard, P.; Faure, F.; Hercend, T.; Triebel, F. Cellular Expression and Tissue Distribution of the Human LAG-3-Encoded Protein, an MHC Class II Ligand. Immunogenetics 1994, 39, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Liu, J.; Liu, D.; Liu, B.; Wang, M.; Hu, Z.; Du, X.; Tang, L.; He, F. Lsectin Expressed on Melanoma Cells Promotes Tumor Progression by Inhibiting Antitumor T-Cell Responses. Cancer Res. 2014, 74, 3418–3428. [Google Scholar] [CrossRef] [PubMed]
- Sierro, S.; Romero, P.; Speiser, D. The CD4-Like Molecule LAG-3, Biology and Therapeutic Applications. Expert Opin. Ther. Targets 2010, 15, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Duffy, C.; Allison, J. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.; Blazar, B.; Kuchroo, V.; Anderson, A. Targeting Tim-3 and PD-1 Pathways to Reverse T Cell Exhaustion and Restore Anti-Tumor Immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Ndhlovu, L.; Lopez-Verges, S.; Barbour, J.; Jones, R.; Jha, A.; Long, B.; Schoeffler, E.; Fujita, T.; Nixon, D.; Lanier, L. Tim-3 Marks Human Natural Killer Cell Maturation and Suppresses Cell-Mediated Cytotoxicity. Blood 2012, 119, 3734–3743. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.; Clark, H.; et al. The Surface Protein TIGIT Suppresses T Cell Activation by Promoting the Generation of Mature Immunoregulatory Dendritic Cells. Nat. Immunol. 2008, 10, 48–57. [Google Scholar] [CrossRef]
- Dougall, W.; Kurtulus, S.; Smyth, M.; Anderson, A. TIGIT and CD96: New Checkpoint Receptor Targets for Cancer Immunotherapy. Immunol. Rev. 2017, 276, 112–120. [Google Scholar] [CrossRef]
- Blake, S.; Stannard, K.; Liu, J.; Allen, S.; Yong, M.; Mittal, D.; Aguilera, A.; Miles, J.; Lutzky, V.; de Andrade, L.; et al. Suppression of Metastases Using a New Lymphocyte Checkpoint Target for Cancer Immunotherapy. Cancer Discov. 2016, 6, 446–459. [Google Scholar] [CrossRef]
- Zhu, Y.; Paniccia, A.; Schulick, A.; Chen, W.; Koenig, M.; Byers, J.; Yao, S.; Bevers, S.; Edil, B. Identification of CD112R as a Novel Checkpoint for Human T Cells. J. Exp. Med. 2016, 213, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Sunderland, A.; Zhou, Y.; Schulick, R.; Edil, B.; Zhu, Y. Blockade of CD112R and TIGIT Signaling Sensitizes Human Natural Killer Cell Functions. Cancer Immunol. Immunother. 2017, 66, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Nelson, C.; Šedý, J. Balancing Co-Stimulation and Inhibition with BTLA and HVEM. Nat. Rev. Immunol. 2006, 6, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Dong, C. New Checkpoints in Cancer Immunotherapy. Immunol. Rev. 2017, 276, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, M.; Shah, U.; Liu, W.; Zhao, A.; Schoenberg, M.; Zang, X. The Third Group of the B7-CD28 Immune Checkpoint Family: HHLA2, TMIGD2, B7x, and B7-H3. Immunol. Rev. 2017, 276, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.; Vonderheide, R. CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer. Cell Rep. 2016, 15, 2719–2732. [Google Scholar] [CrossRef]
- Korman, A.; Chen, B.; Wang, C.; Wu, L.; Cardarelli, P.; Selby, M. Activity of anti-PD-1 in murine tumor models: Role of “host” PD-L1 and synergistic effect of anti-PD-1 and anti-CTLA-4. J. Immunol. 2007, 178, 48.1–48.40. [Google Scholar]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.; Cowey, C.; Lao, C.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Postow, M.; Chesney, J.; Pavlick, A.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.; Meyer, N.; Giguere, J.; Agarwala, S.; et al. Nivolumab and Ipilimumab Versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Nolan, E.; Savas, P.; Policheni, A.; Darcy, P.; Vaillant, F.; Mintoff, C.; Dushyanthen, S.; Mansour, M.; Pang, J.; Fox, S.; et al. Combined Immune Checkpoint Blockade as a Therapeutic Strategy for BRCA1-Mutated Breast Cancer. Sci. Transl. Med. 2017, 9, eaal4922. [Google Scholar] [CrossRef]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A.; et al. Safety Profiles of Anti-CTLA-4 and Anti-PD-1 Antibodies Alone and in Combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8 + T Cell Effector Function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, Y.; Chen, W.; Putra, J.; Suriawinata, A.; Schenk, A.; Miller, H.; Guleria, I.; Barth, R.; Huang, Y.; et al. Immune-Checkpoint Proteins VISTA and PD-1 Nonredundantly Regulate Murine T-Cell Responses. Proc. Natl. Acad. Sci. USA 2015, 112, 6682–6687. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.; Kim, M.; Montalvo, W.; Al-Shamkhani, A.; Allison, J. Combination CTLA-4 Blockade and 4-1BB Activation Enhances Tumor Rejection by Increasing T-Cell Infiltration, Proliferation, and Cytokine Production. PLoS ONE 2011, 6, e19499. [Google Scholar] [CrossRef] [PubMed]
- Shuford, W.; Klussman, K.; Tritchler, D.; Loo, D.; Chalupny, J.; Siadak, A.; Brown, T.; Emswiler, J.; Raecho, H.; Larsen, C.; et al. 4-1BB Costimulatory Signals Preferentially Induce CD8+T Cell Proliferation and Lead to the Amplification In Vivo of Cytotoxic T Cell Responses. J. Exp. Med. 1997, 186, 47–55. [Google Scholar] [CrossRef]
- Kohrt, H.; Houot, R.; Goldstein, M.; Weiskopf, K.; Alizadeh, A.; Brody, J.; Muller, A.; Pachynski, R.; Czerwinski, D.; Coutre, S.; et al. CD137 Stimulation Enhances the Antilymphoma Activity of Anti-CD20 Antibodies. Blood 2010, 117, 2423–2432. [Google Scholar] [CrossRef]
- Shimizu, J.; Yamazaki, S.; Takahashi, T.; Ishida, Y.; Sakaguchi, S. Stimulation of CD25+CD4+ Regulatory T Cells Through GITR Breaks Immunological Self-Tolerance. Nat. Immunol. 2002, 3, 135–142. [Google Scholar] [CrossRef]
- Siu, L.; Steeghs, N.; Meniawy, T.; Joerger, M.; Spratlin, J.; Rottey, S.; Nagrial, A.; Cooper, A.; Meier, R.; Guan, X.; et al. Preliminary Results of a Phase I/Iia Study of BMS-986156 (Glucocorticoid-Induced Tumor Necrosis Factor Receptor–Related Gene [GITR] Agonist), Alone and in Combination with Nivolumab in Pts with Advanced Solid Tumors. J. Clin. Oncol. 2017, 35, 104. [Google Scholar] [CrossRef]
- Melero, I.; Berman, D.; Aznar, M.; Korman, A.; Gracia, J.; Haanen, J. Evolving Synergistic Combinations of Targeted Immunotherapies to Combat Cancer. Nat. Rev. Cancer 2015, 15, 457–472. [Google Scholar] [CrossRef]
- Leone, R.; Sun, I.; Oh, M.; Sun, I.; Wen, J.; Englert, J.; Powell, J. Inhibition of the Adenosine A2a Receptor Modulates Expression of T Cell Coinhibitory Receptors and Improves Effector Function for Enhanced Checkpoint Blockade and ACT in Murine Cancer Models. Cancer Immunol. Immunother. 2018, 67, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Klepsch, V.; Hermann-Kleiter, N.; Do-Dinh, P.; Jakic, B.; Offermann, A.; Efremova, M.; Sopper, S.; Rieder, D.; Krogsdam, A.; Gamerith, G.; et al. Nuclear Receptor NR2F6 Inhibition Potentiates Responses to PD-L1/PD-1 Cancer Immune Checkpoint Blockade. Nat. Commun. 2018, 9, 1538. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. Tgfβ in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, R.; Schaer, D.; Li, Y.; Castaneda, S.; Murphy, M.; Xu, X.; Inigo, I.; Dobkin, J.; Manro, J.; Iversen, P.; et al. Targeting the Tgfβ Pathway with Galunisertib, a Tgfβri Small Molecule Inhibitor, Promotes Anti-Tumor Immunity Leading to Durable, Complete Responses, as Monotherapy and in Combination with Checkpoint Blockade. J. Immunother. Cancer 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Umemura, N.; Saio, M.; Suwa, T.; Kitoh, Y.; Bai, J.; Nonaka, K.; Ouyang, G.; Okada, M.; Balazs, M.; Adany, R.; et al. Tumor-Infiltrating Myeloid-Derived Suppressor Cells are Pleiotropic-Inflamed Monocytes/Macrophages That Bear M1- and M2-Type Characteristics. J. Leukoc. Biol. 2008, 83, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, R.; Brachfeld, A.; Gasmi, B.; Jones, D.; Mattar, M.; Doman, T.; Murphy, M.; Schaer, D.; Wolchok, J.; Merghoub, T. Timing of CSF-1/CSF-1R Signaling Blockade Is Critical to Improving Responses to CTLA-4 Based Immunotherapy. OncoImmunology 2016, 5, e1151595. [Google Scholar] [CrossRef] [PubMed]
- Kandalaft, L.; Motz, G.; Busch, J.; Coukos, G. Angiogenesis and the Tumor Vasculature as Antitumor Immune Modulators: The Role of Vascular Endothelial Growth Factor and Endothelin. Curr. Top. Microbiol. Immunol. 2010, 129–148. [Google Scholar] [CrossRef]
- Ohm, J.; Carbone, D. VEGF as a Mediator of Tumor-Associated Immunodeficiency. Immunol. Res. 2001, 23, 263–272. [Google Scholar] [CrossRef]
- Ott, P.; Hodi, F.; Buchbinder, E. Inhibition of Immune Checkpoints and Vascular Endothelial Growth Factor as Combination Therapy for Metastatic Melanoma: An Overview of Rationale, Preclinical Evidence, and Initial Clinical Data. Front. Oncol. 2015, 5, 202. [Google Scholar] [CrossRef]
- Läubli, H.; Müller, P.; D’Amico, L.; Buchi, M.; Kashyap, A.; Zippelius, A. The Multi-Receptor Inhibitor Axitinib Reverses Tumor-Induced Immunosuppression and Potentiates Treatment with Immune-Modulatory Antibodies in Preclinical Murine Models. Cancer Immunol. Immunother. 2018, 67, 815–824. [Google Scholar] [CrossRef]
- Yasuda, S.; Sho, M.; Yamato, I.; Yoshiji, H.; Wakatsuki, K.; Nishiwada, S.; Yagita, H.; Nakajima, Y. Simultaneous Blockade of Programmed Death 1 and Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) Induces Synergistic Anti-Tumour Effectin Vivo. Clin. Exp. Immunol. 2013, 172, 500–506. [Google Scholar] [CrossRef]
- Balachandran, V.; Cavnar, M.; Zeng, S.; Bamboat, Z.; Ocuin, L.; Obaid, H.; Sorenson, E.; Popow, R.; Ariyan, C.; Rossi, F.; et al. Imatinib Potentiates Antitumor T Cell Responses in Gastrointestinal Stromal Tumor Through the Inhibition of Ido. Nat. Med. 2011, 17, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Soo, R.; Lim, S.; Syn, N.; Teng, R.; Soong, R.; Mok, T.; Cho, B. Immune Checkpoint Inhibitors in Epidermal Growth Factor Receptor Mutant Non-Small Cell Lung Cancer: Current Controversies and Future Directions. Lung Cancer 2018, 115, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Loi, S.; Divisekera, U.; Ngiow, S.; Duret, H.; Yagita, H.; Teng, M.; Smyth, M. Anti-Erbb-2 Mab Therapy Requires Type I and II Interferons and Synergizes with Anti-PD-1 or Anti-CD137 Mab Therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 7142–7147. [Google Scholar] [CrossRef]
- Smith, J.; Wang, S.; Nadella, S.; Jablonski, S.; Weiner, L. Cholecystokinin Receptor Antagonist Alters Pancreatic Cancer Microenvironment and Increases Efficacy of Immune Checkpoint Antibody Therapy in Mice. Cancer Immunol. Immunother. 2017, 67, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Khajeh Alizadeh Attar, M.; Anwar, M.; Eskian, M.; Keshavarz-Fathi, M.; Choi, S.; Rezaei, N. Basic Understanding and Therapeutic Approaches to Target Toll-Like Receptors in Cancerous Microenvironment and Metastasis. Med. Res. Rev. 2017, 38, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Pan, P.; Wang, G.; Yin, B.; Ozao, J.; Ku, T.; Divino, C.; Chen, S. Reversion of Immune Tolerance in Advanced Malignancy: Modulation of Myeloid-Derived Suppressor Cell Development by Blockade of Stem-Cell Factor Function. Blood 2007, 111, 219–228. [Google Scholar] [CrossRef]
- Garton, A.; Seibel, S.; Lopresti-Morrow, L.; Crew, L.; Janson, N.; Mandiyan, S.; Trombetta, E.; Pankratz, S.; LaVallee, T.; Gedrich, R. Anti-KIT Monoclonal Antibody Treatment Enhances the Antitumor Activity of Immune Checkpoint Inhibitors by Reversing Tumor-Induced Immunosuppression. Mol. Cancer Ther. 2017, 16, 671–680. [Google Scholar] [CrossRef]
- Akalu, Y.; Rothlin, C.; Ghosh, S. TAM Receptor Tyrosine Kinases as Emerging Targets of Innate Immune Checkpoint Blockade for Cancer Therapy. Immunol. Rev. 2017, 276, 165–177. [Google Scholar] [CrossRef]
- Matlung, H.; Szilagyi, K.; Barclay, N.; van den Berg, T. The CD47-Sirpα Signaling Axis as an Innate Immune Checkpoint in Cancer. Immunol. Rev. 2017, 276, 145–164. [Google Scholar] [CrossRef]
- Wu, L.; Yu, G.; Deng, W.; Mao, L.; Yang, L.; Ma, S.; Bu, L.; Kulkarni, A.; Zhang, W.; Zhang, L.; et al. Anti-CD47 Treatment Enhances Anti-Tumor T-Cell Immunity and Improves Immunosuppressive Environment in Head and Neck Squamous Cell Carcinoma. OncoImmunology 2018, 7, e1397248. [Google Scholar] [CrossRef] [PubMed]
- Ring, N.; Herndler-Brandstetter, D.; Weiskopf, K.; Shan, L.; Volkmer, J.; George, B.; Lietzenmayer, M.; McKenna, K.; Naik, T.; McCarty, A.; et al. Anti-Sirpα Antibody Immunotherapy Enhances Neutrophil and Macrophage Antitumor Activity. Proc. Natl. Acad. Sci. USA 2017, 114, E10578–E10585. [Google Scholar] [CrossRef] [PubMed]
- Fucà, G.; de Braud, F.; Di Nicola, M. Immunotherapy-Based Combinations. Curr. Opin. Oncol. 2018, 30, 345–351. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahavi, D.J.; Weiner, L.M. Targeting Multiple Receptors to Increase Checkpoint Blockade Efficacy. Int. J. Mol. Sci. 2019, 20, 158. https://doi.org/10.3390/ijms20010158
Zahavi DJ, Weiner LM. Targeting Multiple Receptors to Increase Checkpoint Blockade Efficacy. International Journal of Molecular Sciences. 2019; 20(1):158. https://doi.org/10.3390/ijms20010158
Chicago/Turabian StyleZahavi, David J., and Louis M. Weiner. 2019. "Targeting Multiple Receptors to Increase Checkpoint Blockade Efficacy" International Journal of Molecular Sciences 20, no. 1: 158. https://doi.org/10.3390/ijms20010158
APA StyleZahavi, D. J., & Weiner, L. M. (2019). Targeting Multiple Receptors to Increase Checkpoint Blockade Efficacy. International Journal of Molecular Sciences, 20(1), 158. https://doi.org/10.3390/ijms20010158