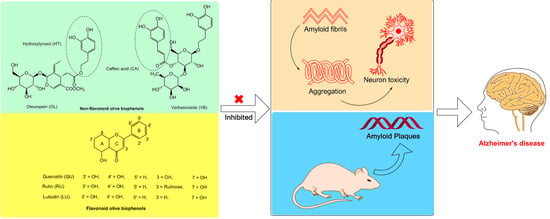
Olive Biophenols Reduces Alzheimer’s Pathology in SH-SY5Y Cells and APPswe Mice
Abstract
1. Introduction
2. Results
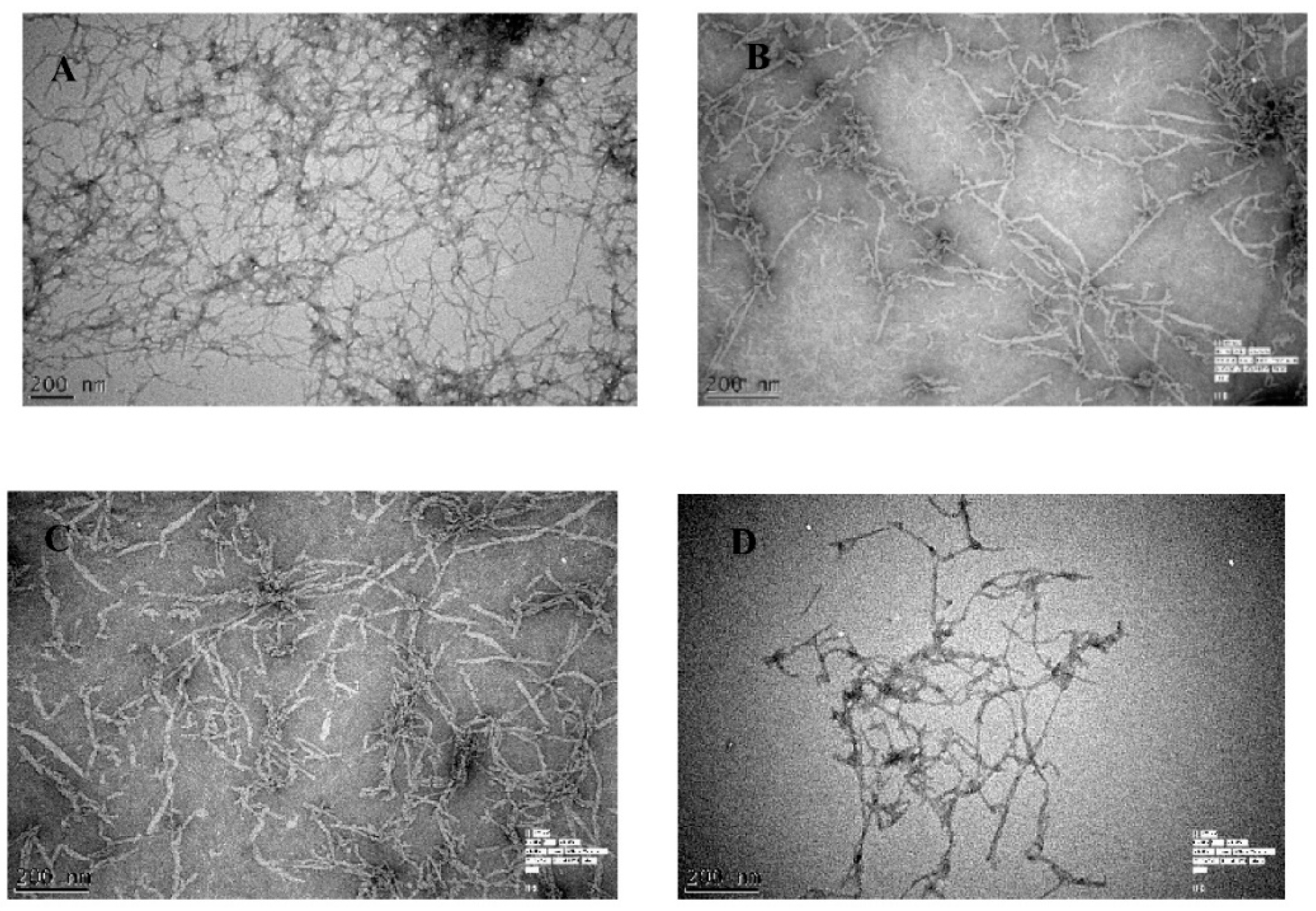
2.1. The Effect of Olive Biophenols on Aβ42 Aggregation (TEM)
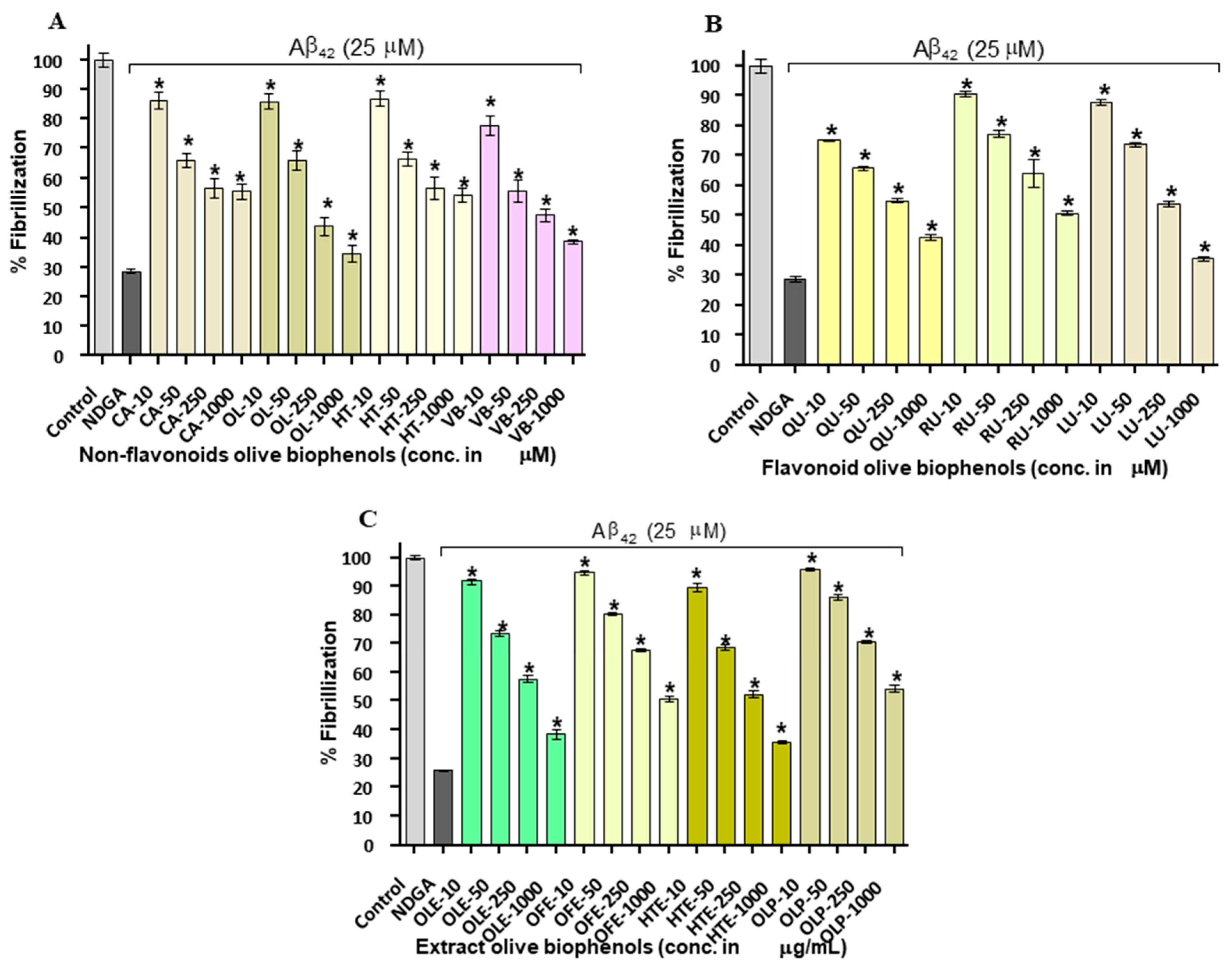
2.2. Aβ42 Fibril Inhibition by Olive Biophenols (ThT Fluorometric Assay)
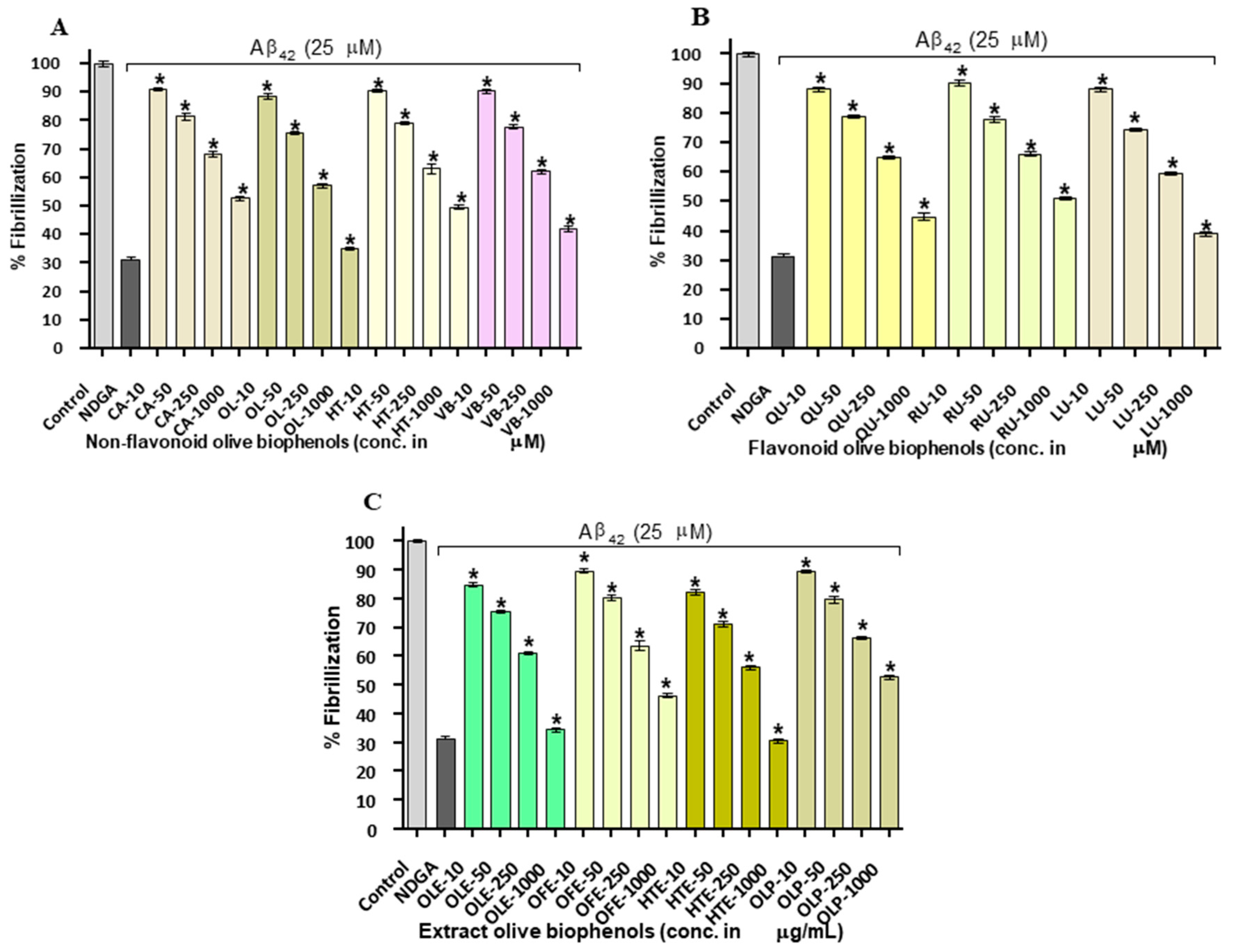
2.3. Congo Red Assay of Aβ42 Inhibition by Olive Biophenols
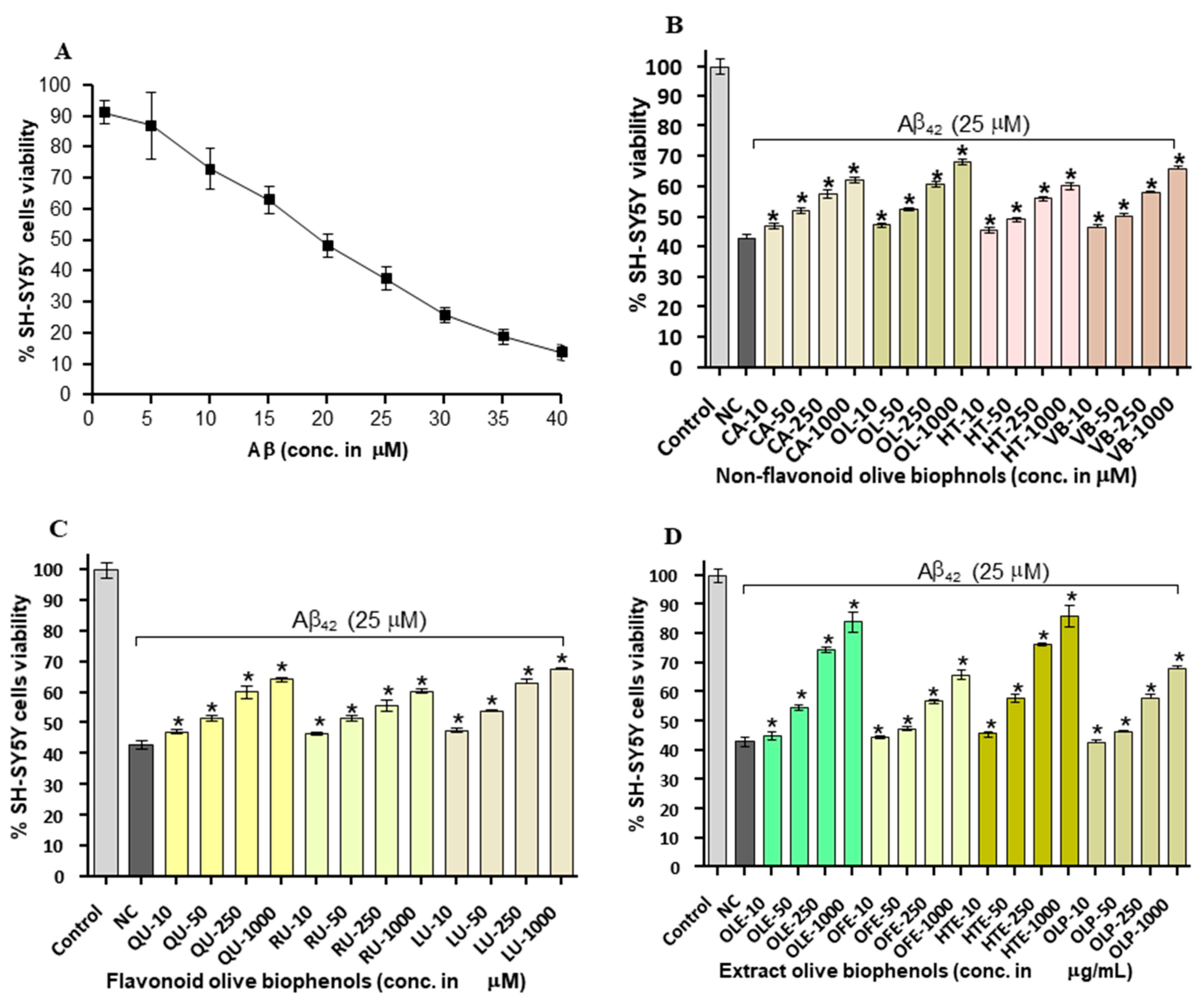
2.4. Neuroprotective Effects of Olive Biophenols against Aβ42 Induced Neurotoxicity in SH-SY5Y Cells
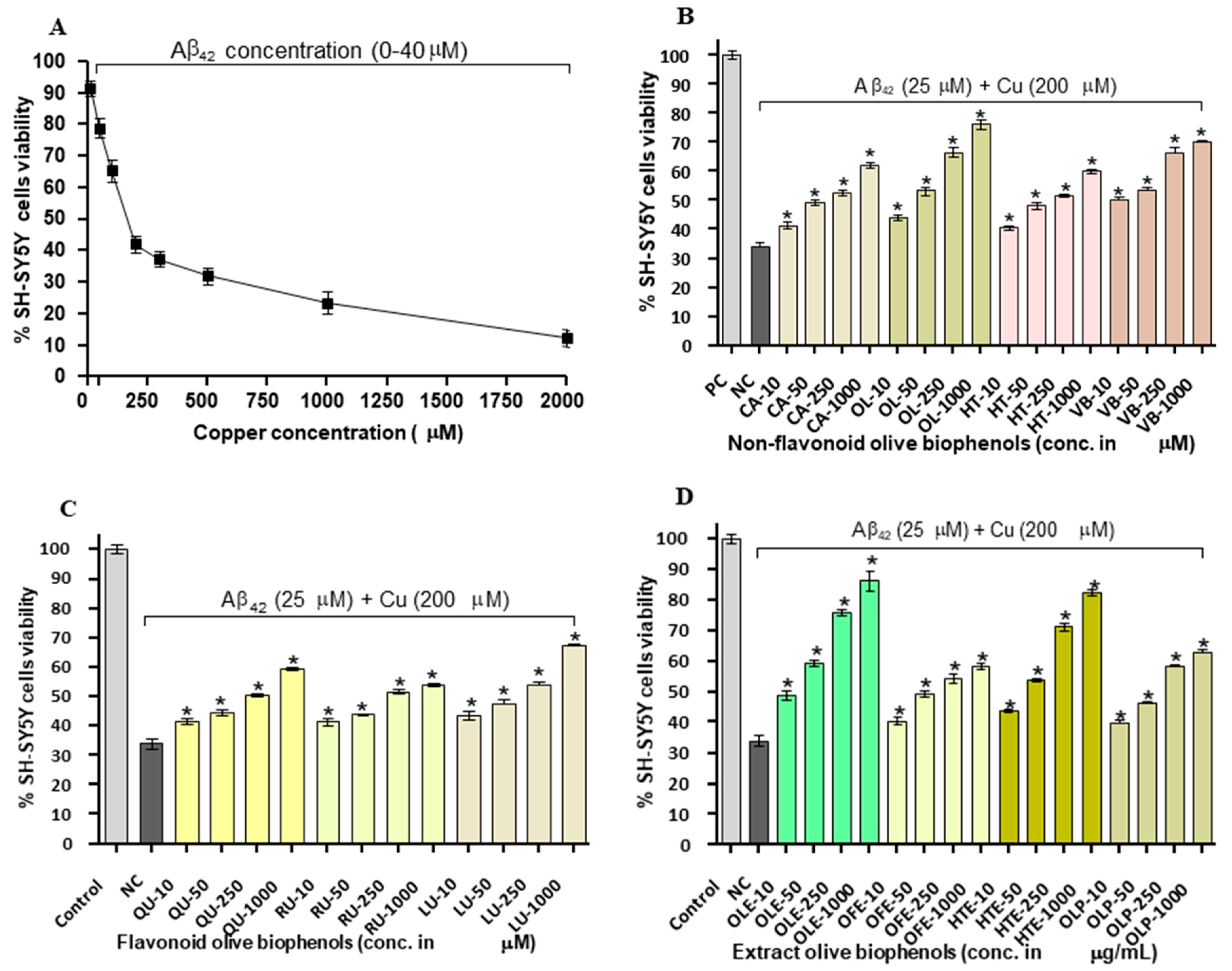
2.5. Neuroprotective Effect of Olive Biophenols against Copper-Amyloid Induced Neurotoxicity in SH-SY5Y Cells
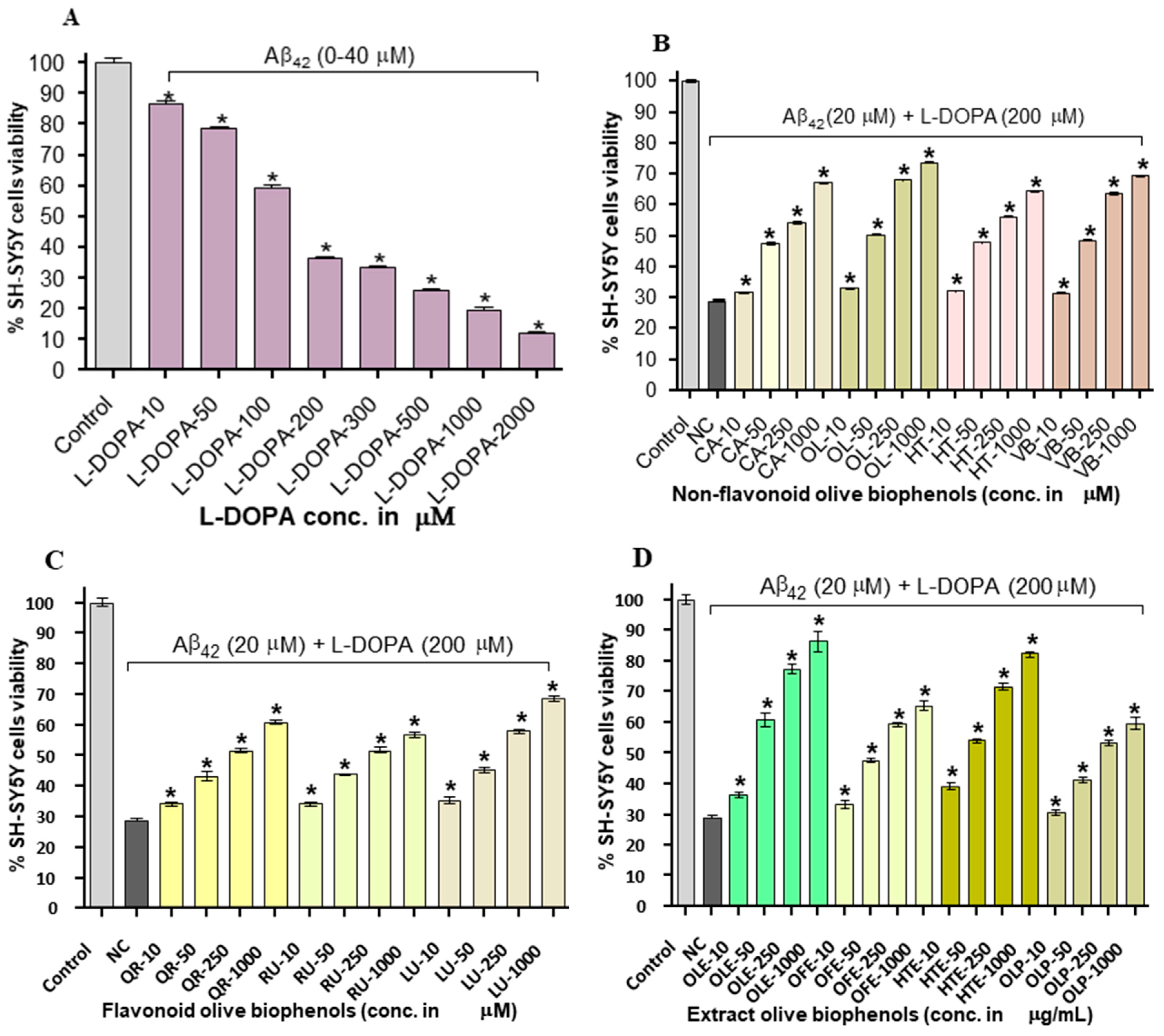
2.6. Neuroprotective Effect of Olive Biophenols against l-DOPA-Amyloid-Induced Neurotoxicity in SH-SY5Y Cells
2.7. Behavioural Analysis
2.7.1. Light and Dark Test
2.7.2. Novel Object Recognition
2.7.3. Barnes Maze Test
2.8. Amyloid Plaque Burden
2.9. Biochemical Analysis
2.9.1. Serum Cholesterol Level
2.9.2. Plasma Triglyceride Level
2.9.3. Plasma Glucose Level
3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Sample Preparation
4.3. Aβ42 Fibril Preparation and Aggregation Inhibitory Assay
4.3.1. Transmission Electron Microscope (TEM) Imaging
4.3.2. Thioflavin-T (ThT) Fluorometric Assay
4.3.3. Congo Red Binding Assay
4.4. Cell Culture
4.4.1. Aβ42 Induced SH-SY5Y Cells Toxicity and Olive Biophenols Treatment
4.4.2. Aβ42-Copper Induced SH-SY5Y Cell Toxicity and Olive Biophenols Treatment
4.4.3. Aβ42-l-DOPA Induced SH-SY5Y Cell Toxicity and Olive Biophenols Treatment
4.4.4. Cell Viability Assay
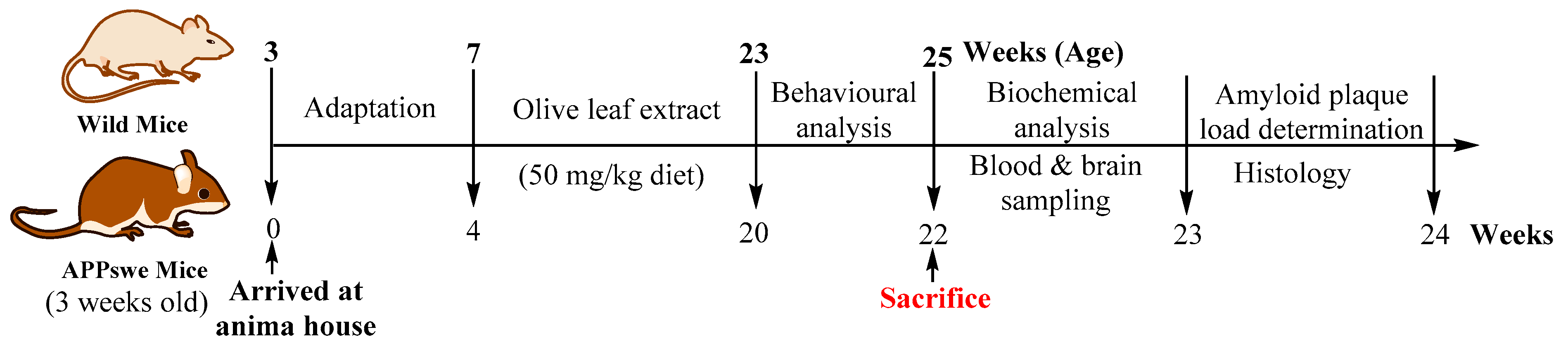
4.5. Animals and Ethical Considerations
4.6. Diet
4.7. Experimental Procedures
4.7.1. Light and Dark Test
4.7.2. Novel Object Recognition Test
4.7.3. Barnes Maze Test
4.7.4. Blood Biochemistry
4.7.5. Assessment of Amyloid Plaque Burden
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mawuenyega, K.G.; Sigurdson, W.; Ovod, V.; Munsell, L.; Kasten, T.; Morris, J.C.; Yarasheski, K.E.; Bateman, R.J. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science 2010, 330, 1774. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Kuperstein, I.; Broersen, K.; Benilova, I.; Rozenski, J.; Jonckheere, W.; Debulpaep, M.; Vandersteen, A.; Segers-Nolten, I.; Van Der Werf, K.; Subramaniam, V.; et al. Neurotoxicity of Alzheimer’s disease Abeta peptides is induced by small changes in the Abeta42 to Abeta40 ratio. EMBO J. 2010, 29, 3408–3420. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Takahashi, I.; Sato, H.; Kubota, Y.; Yoshida, S.; Muramatsu, Y. Age-related changes in the concentrations of major and trace elements in the brain of rats and mice. Biol. Trace Elem. Res. 2001, 80, 145–158. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Asada, M.; Kubota, M.; Maesako, M.; Watanabe, K.; Uemura, M.; Kihara, T.; Shimohama, S.; Takahashi, R.; Kinoshita, A.; et al. Copper enhances APP dimerization and promotes Abeta production. Neurosci. Lett. 2013, 547, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Sayre, L.M.; Moreira, P.I.; Smith, M.A.; Perry, G. Metal ions and oxidative protein modification in neurological disease. Ann. Ist. Super. Sanita 2005, 41, 143–164. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Eskici, G.; Axelsen, P.H. Copper and oxidative stress in the pathogenesis of Alzheimer’s disease. Biochemistry 2012, 51, 6289–6311. [Google Scholar] [CrossRef]
- Zoccolella, S.; dell’Aquila, C.; Abruzzese, G.; Antonini, A.; Bonuccelli, U.; Canesi, M.; Cristina, S.; Marchese, R.; Pacchetti, C.; Zagaglia, R.; et al. Hyperhomocysteinemia in levodopa-treated patients with Parkinson’s disease dementia. Mov. Disord. 2009, 24, 1028–1033. [Google Scholar] [CrossRef]
- Dorszewska, J.; Prendecki, M.; Lianeri, M.; Kozubski, W. Molecular Effects of l-DOPA Therapy in Parkinson’s Disease. Curr. Genom. 2014, 15, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, J.M.; Wang, H.; Pratico, D. Is hyperhomocysteinemia an Alzheimer’s disease (AD) risk factor, an AD marker, or neither? Trends Pharmacol. Sci. 2011, 32, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T.; Arning, E.; Wasek, B.; Nunbhakdi-Craig, V.; Sontag, J.M.; Sontag, E. Acute administration of l-DOPA induces changes in methylation metabolites, reduced protein phosphatase 2A methylation, and hyperphosphorylation of Tau protein in mouse brain. J. Neurosci. 2012, 32, 9173–9181. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.D.; Soto, C. Disrupting beta-amyloid aggregation for Alzheimer disease treatment. Curr. Top. Med. Chem. 2007, 7, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Chapter 4—Biophenols: Impacts and Prospects in Anti-Alzheimer Drug Discovery. In Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: New York, NY, USA, 2018; pp. 103–148. [Google Scholar]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. The protective role of plant biophenols in mechanisms of Alzheimer’s disease. J. Nutr. Biochem. 2017, 47, 1–20. [Google Scholar] [CrossRef]
- Omar, S.H. Biophenols pharmacology against the amyloidogenic activity in Alzheimer’s disease. Biomed. Pharmacother. 2017, 89, 396–413. [Google Scholar] [CrossRef] [PubMed]
- Segura-Carretero, A.; Menéndez-Menéndez, J.; Fernández-Gutiérrez, A. Chapter 19—Polyphenols in Olive Oil: The Importance of Phenolic Compounds in the Chemical Composition of Olive Oil. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 167–175. [Google Scholar]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Omar, S.H.; Kerr, P.G.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive (Olea europaea L.) Biophenols: A Nutriceutical against Oxidative Stress in SH-SY5Y Cells. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (β-secretase, Cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Churches, Q.I.; Caine, J.; Cavanagh, K.; Epa, V.C.; Waddington, L.; Tranberg, C.E.; Meyer, A.G.; Varghese, J.N.; Streltsov, V.; Duggan, P.J. Naturally occurring polyphenolic inhibitors of amyloid beta aggregation. Bioorg. Med. Chem. Lett. 2014, 24, 3108–3112. [Google Scholar] [CrossRef]
- Ono, K.; Li, L.; Takamura, Y.; Yoshiike, Y.; Zhu, L.; Han, F.; Mao, X.; Ikeda, T.; Takasaki, J.; Nishijo, H.; et al. Phenolic compounds prevent amyloid beta-protein oligomerization and synaptic dysfunction by site-specific binding. J. Biol. Chem. 2012, 287, 14631–14643. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef] [PubMed]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.P.; Yang, S.G.; Wang, Y.J.; Zhang, X.; Du, X.T.; Sun, X.X.; Zhao, M.; Huang, L.; Liu, R.T. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.A.; Ecroyd, H.; Kee, T.W.; Carver, J.A. The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J. 2009, 276, 5960–5972. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.W.; Wang, Y.J.; Su, Y.J.; Zhou, W.W.; Yang, S.G.; Zhang, R.; Zhao, M.; Li, Y.N.; Zhang, Z.P.; Zhan, D.W.; et al. Rutin inhibits beta-amyloid aggregation and cytotoxicity, attenuates oxidative stress, and decreases the production of nitric oxide and proinflammatory cytokines. Neurotoxicology 2012, 33, 482–490. [Google Scholar] [CrossRef]
- Shoval, H.; Lichtenberg, D.; Gazit, E. The molecular mechanisms of the anti-amyloid effects of phenols. Amyloid 2007, 14, 73–87. [Google Scholar] [CrossRef]
- Klunk, W.E.; Jacob, R.F.; Mason, R.P. Quantifying amyloid beta-peptide (Abeta) aggregation using the Congo red-Abeta (CR-abeta) spectrophotometric assay. Anal. Biochem. 1999, 266, 66–76. [Google Scholar] [CrossRef]
- Lorenzo, A.; Yankner, B.A. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc. Natl. Acad. Sci. USA 1994, 91, 12243–12247. [Google Scholar] [CrossRef]
- Chonpathompikunlert, P.; Han, J.; Toh, K.; Isoda, H.; Nagasaki, Y. TEMPOL protects human neuroblastoma SH-SY5Y cells against beta-amyloid-induced cell toxicity. Eur. J. Pharmacol. 2011, 650, 544–549. [Google Scholar] [CrossRef]
- Cassagnes, L.E.; Herve, V.; Nepveu, F.; Hureau, C.; Faller, P.; Collin, F. The catalytically active copper-amyloid-Beta state: Coordination site responsible for reactive oxygen species production. Angew. Chem. Int. Ed. Engl. 2013, 52, 11110–11113. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.; Li, X.; He, Y.; Chen, S.; Xiang, J. Amyloid-beta peptide (1–42) aggregation induced by copper ions under acidic conditions. Acta Biochim. Biophys. Sin. 2013, 45, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Bandaruk, Y.; Mukai, R.; Terao, J. Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-A in human neuroblastoma SH-SY5Y cells. Toxicol. Rep. 2014, 1, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Bell, R.D.; Sagare, A.; Zlokovic, B.V. Clearance of amyloid-beta peptide across the blood-brain barrier: Implication for therapies in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2009, 8, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.M.; Benghuzzi, H.; Tucci, M. The effect of conventional and sustained delivery of levodopa on SH-SY5Y neuroblastoma cells. Biomed. Sci. Instrum. 2005, 41, 382–387. [Google Scholar] [PubMed]
- Bourin, M.; Hascoet, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Ed Dami, T.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The polyphenol oleuropein aglycone protects TgCRND8 mice against Ass plaque pathology. PLoS ONE 2013, 8, e71702. [Google Scholar] [CrossRef]
- Burns, M.; Gaynor, K.; Olm, V.; Mercken, M.; LaFrancois, J.; Wang, L.; Mathews, P.M.; Noble, W.; Matsuoka, Y.; Duff, K. Presenilin redistribution associated with aberrant cholesterol transport enhances beta-amyloid production in vivo. J. Neurosci. 2003, 23, 5645–5649. [Google Scholar] [CrossRef]
- Refolo, L.M.; Malester, B.; LaFrancois, J.; Bryant-Thomas, T.; Wang, R.; Tint, G.S.; Sambamurti, K.; Duff, K.; Pappolla, M.A. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis. 2000, 7, 321–331. [Google Scholar] [CrossRef]
- Wahrle, S.; Das, P.; Nyborg, A.C.; McLendon, C.; Shoji, M.; Kawarabayashi, T.; Younkin, L.H.; Younkin, S.G.; Golde, T.E. Cholesterol-dependent gamma-secretase activity in buoyant cholesterol-rich membrane microdomains. Neurobiol. Dis. 2002, 9, 11–23. [Google Scholar] [CrossRef]
- Burgess, B.L.; McIsaac, S.A.; Naus, K.E.; Chan, J.Y.; Tansley, G.H.; Yang, J.; Miao, F.; Ross, C.J.; van Eck, M.; Hayden, M.R.; et al. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant A beta in plasma. Neurobiol. Dis. 2006, 24, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, M.; Zahiri, H.R.; Ceimo, J.; Cooper, K.; Gaul, W.; Connor, D.; Sparks, D.L. Is there a characteristic lipid profile in Alzheimer’s disease? J. Alzheimers Dis. 2004, 6, 585–589, discussion 673–581. [Google Scholar] [CrossRef] [PubMed]
- Suryadevara, V.; Storey, S.G.; Aronow, W.S.; Ahn, C. Association of abnormal serum lipids in elderly persons with atherosclerotic vascular disease and dementia, atherosclerotic vascular disease without dementia, dementia without atherosclerotic vascular disease, and no dementia or atherosclerotic vascular disease. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M859–M861. [Google Scholar] [PubMed]
- Olsson, M.; Ahlin, S.; Olsson, B.; Svensson, P.A.; Stahlman, M.; Boren, J.; Carlsson, L.M.; Sjoholm, K. Establishment of a transgenic mouse model specifically expressing human serum amyloid A in adipose tissue. PLoS ONE 2011, 6, e19609. [Google Scholar] [CrossRef] [PubMed]
- Hoppener, J.W.; Ahren, B.; Lips, C.J. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 2000, 343, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, K.; Akhtari, K.; Hassanzadeh, H.; Zarei, S.A.; Fakhraei, N.; Hassanzadeh, K. The role of structural C–H compared with phenolic OH sites on the antioxidant activity of oleuropein and its derivatives as a great non-flavonoid family of the olive components: A DFT study. Food Chem. 2014, 164, 251–258. [Google Scholar] [CrossRef]
- Bazoti, F.N.; Bergquist, J.; Markides, K.; Tsarbopoulos, A. Localization of the noncovalent binding site between amyloid-beta-peptide and oleuropein using electrospray ionization FT-ICR mass spectrometry. J. Am. Soc. Mass Spectrom. 2008, 19, 1078–1085. [Google Scholar] [CrossRef]
- Kurisu, M.; Miyamae, Y.; Murakami, K.; Han, J.; Isoda, H.; Irie, K.; Shigemori, H. Inhibition of amyloid beta aggregation by acteoside, a phenylethanoid glycoside. Biosci. Biotechnol. Biochem. 2013, 77, 1329–1332. [Google Scholar] [CrossRef]
- Liu, R.; Meng, F.; Zhang, L.; Liu, A.; Qin, H.; Lan, X.; Li, L.; Du, G. Luteolin isolated from the medicinal plant Elsholtzia rugulosa (Labiatae) prevents copper-mediated toxicity in beta-amyloid precursor protein Swedish mutation overexpressing SH-SY5Y cells. Molecules 2011, 16, 2084–2096. [Google Scholar] [CrossRef]
- Stefani, M.; Rigacci, S. Protein folding and aggregation into amyloid: The interference by natural phenolic compounds. Int. J. Mol. Sci. 2013, 14, 12411–12457. [Google Scholar] [CrossRef]
- Agholme, L.; Lindstrom, T.; Kagedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimers Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hureau, C.; Berthoumieu, O. Role of metal ions in the self-assembly of the Alzheimer’s amyloid-beta peptide. Inorg. Chem. 2013, 52, 12193–12206. [Google Scholar] [CrossRef] [PubMed]
- Syme, C.D.; Nadal, R.C.; Rigby, S.E.; Viles, J.H. Copper binding to the amyloid-beta (Abeta) peptide associated with Alzheimer’s disease: Folding, coordination geometry, pH dependence, stoichiometry, and affinity of Abeta-(1–28): Insights from a range of complementary spectroscopic techniques. J. Biol. Chem. 2004, 279, 18169–18177. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.W.; Dunlop, R.A.; Rowe, A.; Double, K.L.; Rodgers, K.J. l-DOPA is incorporated into brain proteins of patients treated for Parkinson’s disease, inducing toxicity in human neuroblastoma cells in vitro. Exp. Neurol. 2012, 238, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sagar, H.J. Clinical similarities and differences between Alzheimer’s disease and Parkinson’s disease. J. Neural. Transm. Suppl. 1987, 24, 87–99. [Google Scholar] [PubMed]
- Garcia-Alloza, M.; Robbins, E.M.; Zhang-Nunes, S.X.; Purcell, S.M.; Betensky, R.A.; Raju, S.; Prada, C.; Greenberg, S.M.; Bacskai, B.J.; Frosch, M.P. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 2006, 24, 516–524. [Google Scholar] [CrossRef]
- Malm, T.; Koistinaho, J.; Kanninen, K. Utilization of APPswe/PS1dE9 Transgenic Mice in Research of Alzheimer’s Disease: Focus on Gene Therapy and Cell-Based Therapy Applications. Int. J. Alzheimers Dis. 2011, 2011, 517160. [Google Scholar] [CrossRef] [PubMed]
- Savonenko, A.; Xu, G.M.; Melnikova, T.; Morton, J.L.; Gonzales, V.; Wong, M.P.; Price, D.L.; Tang, F.; Markowska, A.L.; Borchelt, D.R. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer’s disease: Relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol. Dis. 2005, 18, 602–617. [Google Scholar] [CrossRef]
- Bramanti, E.; Fulgentini, L.; Bizzarri, R.; Lenci, F.; Sgarbossa, A. beta-Amyloid amorphous aggregates induced by the small natural molecule ferulic acid. J. Phys. Chem. B 2013, 117, 13816–13821. [Google Scholar] [CrossRef]
- Kodali, R.; Williams, A.D.; Chemuru, S.; Wetzel, R. Abeta(1–40) forms five distinct amyloid structures whose beta-sheet contents and fibril stabilities are correlated. J. Mol. Biol. 2010, 401, 503–517. [Google Scholar] [CrossRef]
- Naiki, H.; Hasegawa, K.; Yamaguchi, I.; Nakamura, H.; Gejyo, F.; Nakakuki, K. Apolipoprotein E and antioxidants have different mechanisms of inhibiting Alzheimer’s beta-amyloid fibril formation in vitro. Biochemistry 1998, 37, 17882–17889. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.M.; Hartley, D.M.; Kusumoto, Y.; Fezoui, Y.; Condron, M.M.; Lomakin, A.; Benedek, G.B.; Selkoe, D.J.; Teplow, D.B. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 1999, 274, 25945–25952. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhao, L.; Zhu, B.; Li, Q.; Yew, D.T.; Yao, Z.; Xu, J. Protective effects of Ginkgo biloba extract (EGb761) and its constituents quercetin and ginkgolide B against beta-amyloid peptide-induced toxicity in SH-SY5Y cells. Chem.-Biol. Interact. 2009, 181, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Chetsawang, J.; Govitrapong, P.; Chetsawang, B. Hydrogen peroxide toxicity induces Ras signaling in human neuroblastoma SH-SY5Y cultured cells. J. Biomed. Biotechnol. 2010, 2010. [Google Scholar] [CrossRef]
- Borchelt, D.R.; Ratovitski, T.; van Lare, J.; Lee, M.K.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Price, D.L.; Sisodia, S.S. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 1997, 19, 939–945. [Google Scholar] [CrossRef]
- Mehraein, F.; Sarbishegi, M.; Aslani, A. Evaluation of effect of oleuropein on skin wound healing in aged male BALB/c mice. Cell J. 2014, 16, 25–30. [Google Scholar] [PubMed]
- Crawley, J.N.; Paylor, R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav. 1997, 31, 197–211. [Google Scholar] [CrossRef]
- Holmes, A.; Yang, R.J.; Crawley, J.N. Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J. Mol. Neurosci. 2002, 18, 151–165. [Google Scholar] [CrossRef]
- De Rosa, R.; Garcia, A.A.; Braschi, C.; Capsoni, S.; Maffei, L.; Berardi, N.; Cattaneo, A. Intranasal administration of nerve growth factor (NGF) rescues recognition memory deficits in AD11 anti-NGF transgenic mice. Proc. Natl. Acad. Sci. USA 2005, 102, 3811–3816. [Google Scholar] [CrossRef]
- Attar, A.; Liu, T.; Chan, W.T.; Hayes, J.; Nejad, M.; Lei, K.; Bitan, G. A shortened Barnes maze protocol reveals memory deficits at 4-months of age in the triple-transgenic mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e80355. [Google Scholar] [CrossRef]
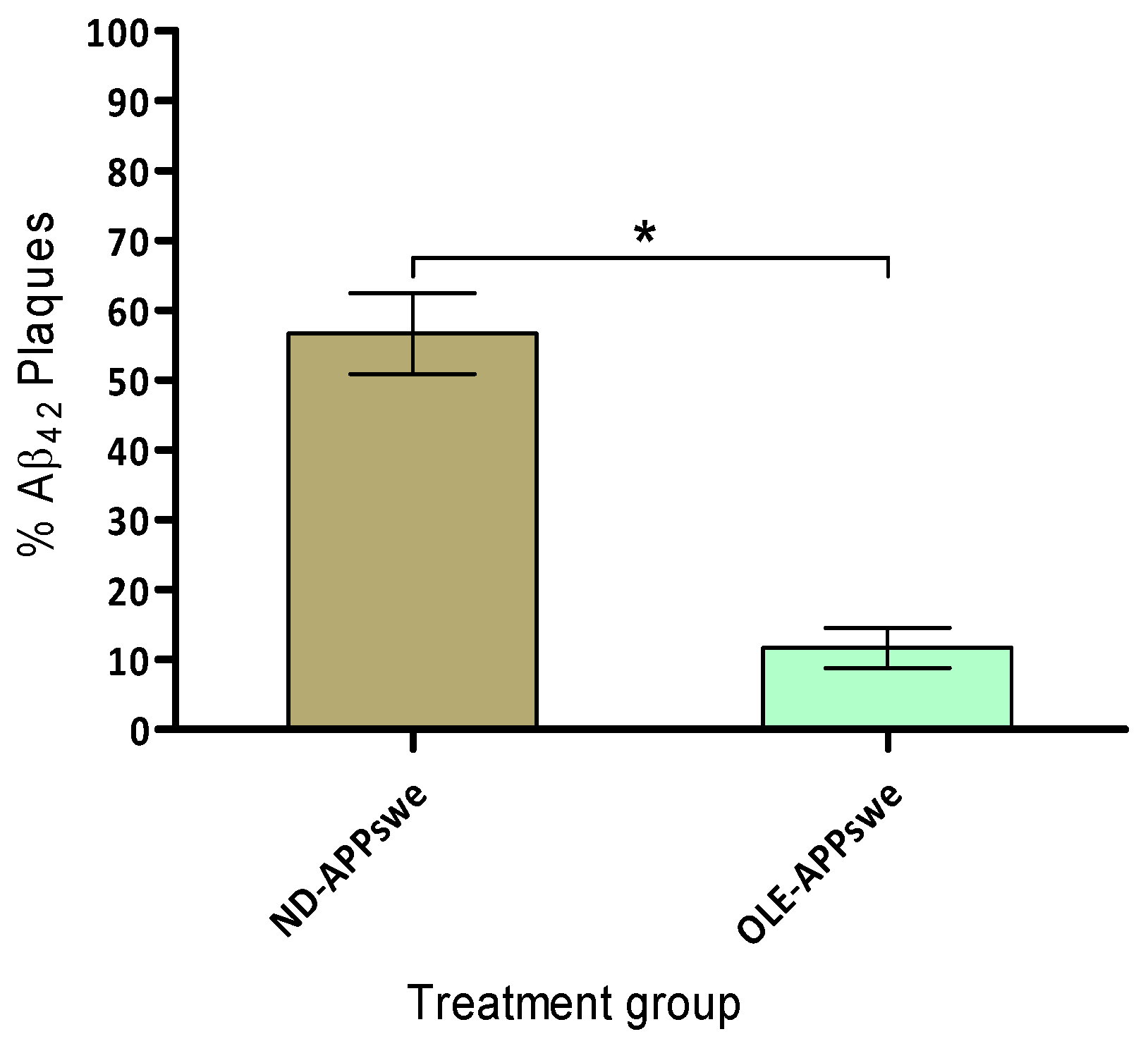
| Olive Biophenols | Thioflavin-T Assay | Congo-Red Assay | |||
|---|---|---|---|---|---|
| IC50 | % Inhibition | IC50 | % Inhibition | ||
| Non-flavonoids | Nordihroguaretic acid (NDGA) | 15.4 µM | 70 ± 0.5 | 14.4 µM | 69 ± 0.4 |
| Caffeic acid (CA) | ND | 46 ± 0.32 | ND | 47 ± 0.31 | |
| Hydroxytyrosol (HT) | ND | 45 ± 0.47 | 97.8 µM | 50 ± 0.4 | |
| Oleuropein (OL) | 22.9 µM | 61 ± 0.33 | 36.5 µM | 65 ± 0.3 | |
| Verbascoside (VB) | 22.6 µM | 61 ± 0.35 | 59.6 µM | 57 ± 0.51 | |
| Flavonoids | Luteolin (LU) | 36.9 µM | 64 ± 0.4 | 46.3 µM | 61 ± 0.33 |
| Quercetin (QU) | 45.9 µM | 57 ± 0.34 | 73.8 µM | 55 ± 0.71 | |
| Rutin (RU) | ND | 49 ± 0.25 | ND | 48 ± 0.33 | |
| Extracts | Olive leaf extract (OLE) | 45 µg/mL | 60 ± 0.36 | 41.1 µg/mL | 65 ± 0.4 |
| Olive fruit extract (OFE) | 95.9 µg/mL | 50 ± 0.43 | 80.9 µg/mL | 53 ± 0.51 | |
| Hydroxytyrosol extreme (HTE) | 30.4 µg/mL | 64 ± 0.34 | 28.4 µg/mL | 69 ± 0.42 | |
| Olivenol plus (OLP) | ND | ND | ND | ND | |
| Olive Biophenols | Aβ-SH-SY5Y Toxicity | Aβ-Cu-SH-SY5Y Toxicity | Aβ-l-DOPA-SH-SY5Y Toxicity | |
|---|---|---|---|---|
| % Viability | % Viability | % Viability | ||
| Non-flavonoids | Control (SH-SY5Y-media) | 100 ± 1.21 | 100 ± 1.13 | 100 ± 0.92 |
| Negative control | 37 ± 1.41 | 34 ± 1.53 | 12 ± 0.37 | |
| Caffeic acid (CA) | 62 ± 0.53 | 62 ± 0.93 | 67 ± 0.43 | |
| Hydroxytyrosol (HT) | 60 ± 1.00 | 60 ± 0.84 | 64 ± 1.02 | |
| Oleuropein (OL) | 68 ± 0.69 | 76 ± 1.61 | 74 ± 0.23 | |
| Verbascoside (VB) | 66 ± 1.11 | 70 ± 0.48 | 69 ± 0.66 | |
| Flavonoids | Luteolin (LU) | 65 ± 0.39 | 67 ± 0.52 | 69 ± 0.87 |
| Quercetin (QU) | 63 ± 0.29 | 60 ± 0.52 | 61 ± 0.73 | |
| Rutin (RU) | 59 ± 0.59 | 54 ± 0.71 | 57 ± 0.85 | |
| Extracts | Olive leaf extract (OLE) | 84 ± 3.17 | 87 ± 3.2 | 86 ± 3.2 |
| Olive fruit extract (OFE) | 68 ± 1.31 | 58 ± 0.69 | 65 ± 1.49 | |
| Hydroxytyrosol extreme (HTE) | 86 ± 3.6 | 82 ± 0.91 | 82 ± 0.96 | |
| Olivenol plus (OLP) | 68 ± 0.74 | 63 ± 0.96 | 60 ± 2.43 | |
| Animals | Normal Diet | OLE Diet |
|---|---|---|
| Wild mice (Control) | Yes | No |
| Transgenic Mice (Control) | Yes | No |
| Wild Mice (OLE) | No | Yes |
| Transgenic Mice (OLE) | No | Yes |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Olive Biophenols Reduces Alzheimer’s Pathology in SH-SY5Y Cells and APPswe Mice. Int. J. Mol. Sci. 2019, 20, 125. https://doi.org/10.3390/ijms20010125
Omar SH, Scott CJ, Hamlin AS, Obied HK. Olive Biophenols Reduces Alzheimer’s Pathology in SH-SY5Y Cells and APPswe Mice. International Journal of Molecular Sciences. 2019; 20(1):125. https://doi.org/10.3390/ijms20010125
Chicago/Turabian StyleOmar, Syed Haris, Christopher J. Scott, Adam S. Hamlin, and Hassan K. Obied. 2019. "Olive Biophenols Reduces Alzheimer’s Pathology in SH-SY5Y Cells and APPswe Mice" International Journal of Molecular Sciences 20, no. 1: 125. https://doi.org/10.3390/ijms20010125
APA StyleOmar, S. H., Scott, C. J., Hamlin, A. S., & Obied, H. K. (2019). Olive Biophenols Reduces Alzheimer’s Pathology in SH-SY5Y Cells and APPswe Mice. International Journal of Molecular Sciences, 20(1), 125. https://doi.org/10.3390/ijms20010125