Nitric Oxide Enhancing Resistance to PEG-Induced Water Deficiency is Associated with the Primary Photosynthesis Reaction in Triticum aestivum L.
Abstract
1. Introduction
2. Results
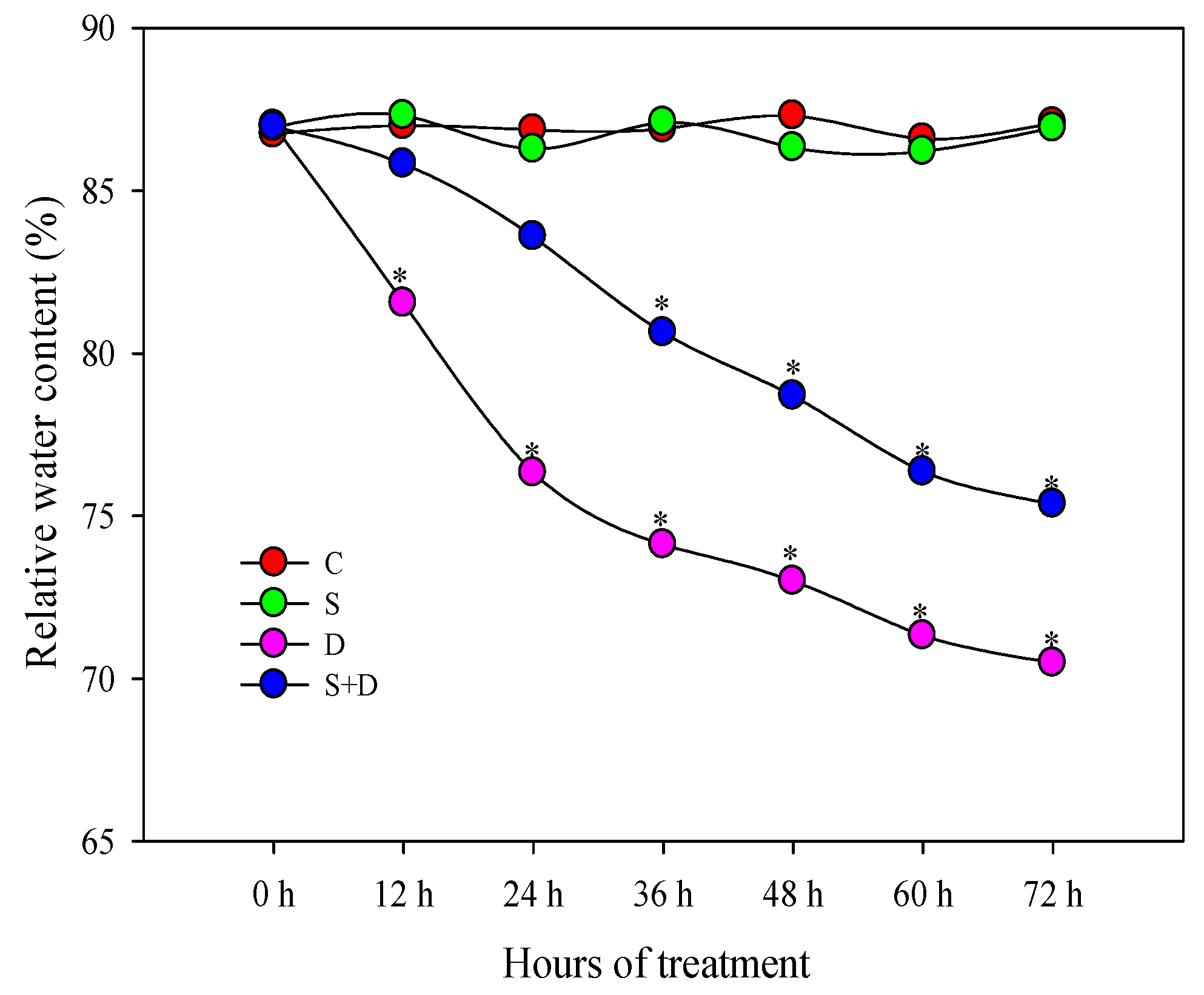
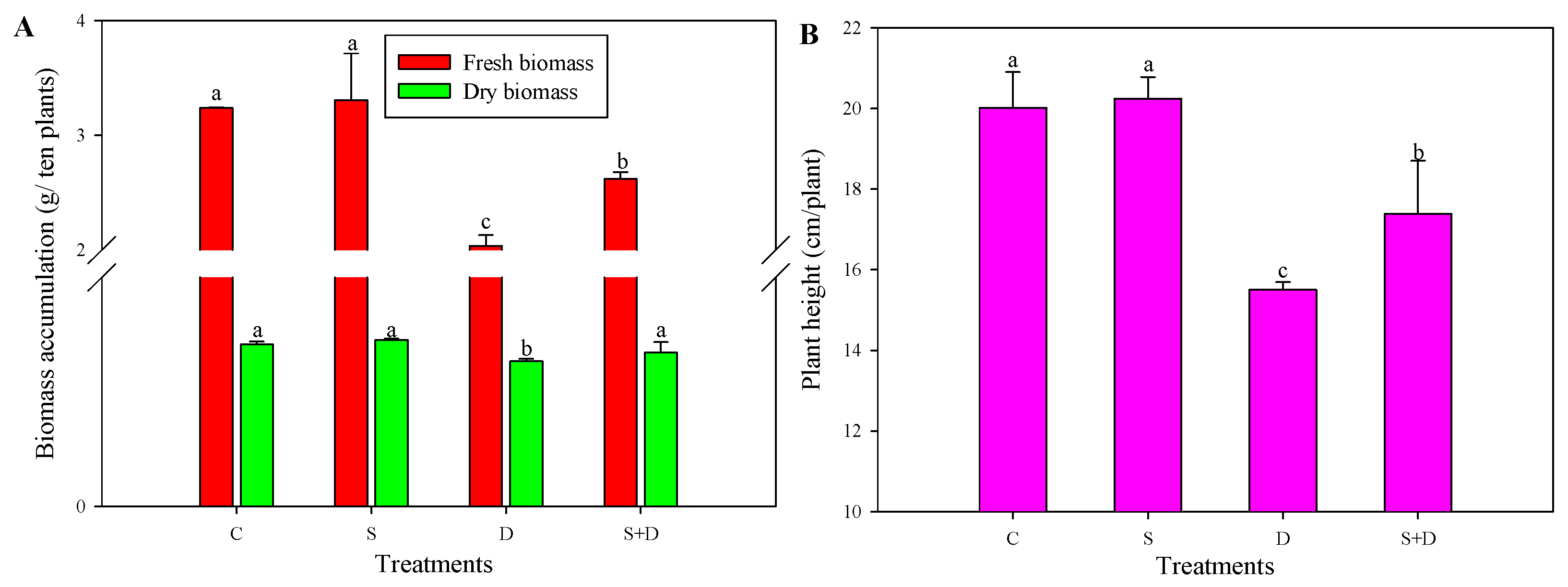
2.1. Morphological Change in Response to NO and D Stress
2.2. Quantitative Identification of Phosphoproteins Using iTRAQ
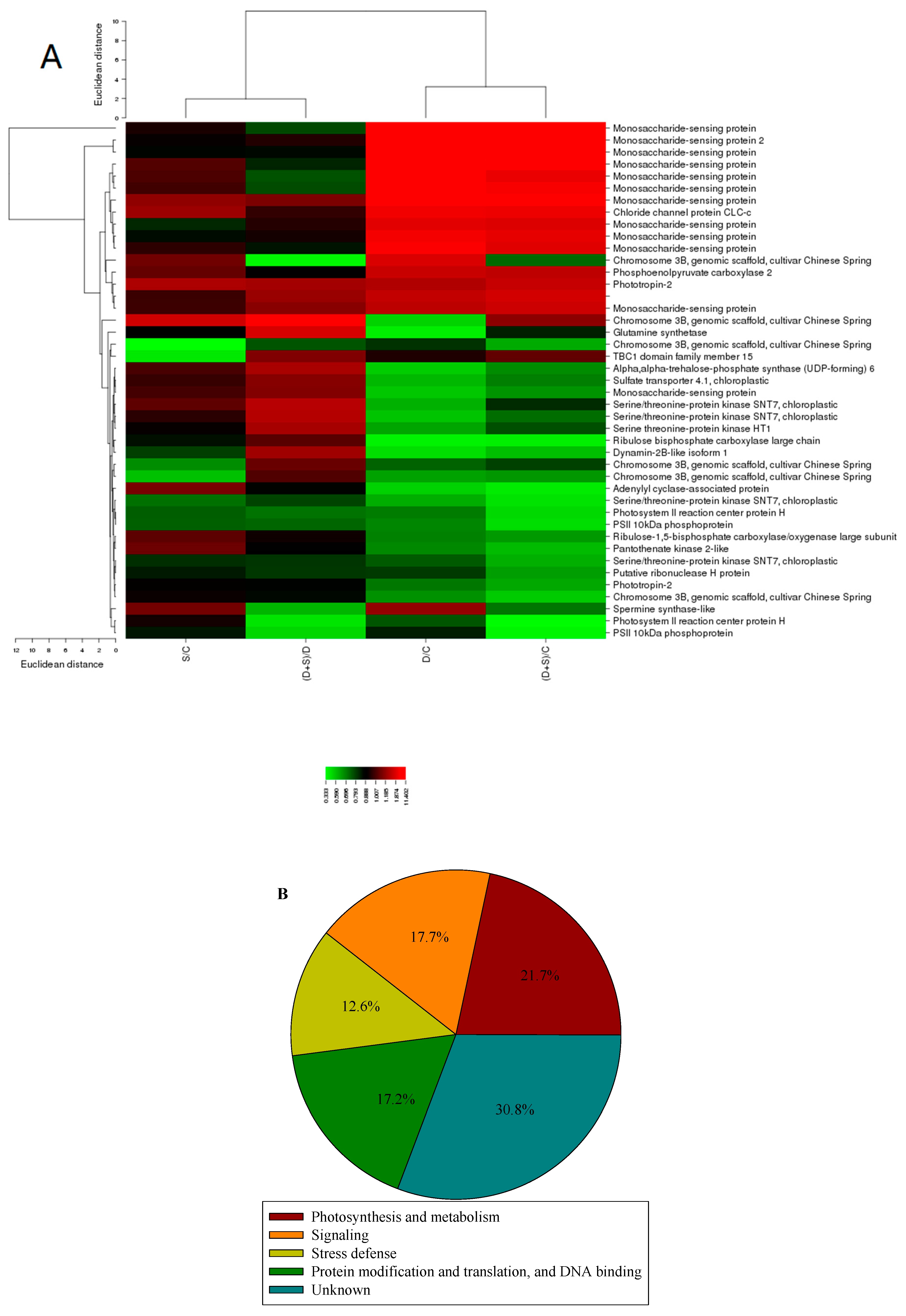
2.3. Gene ontology and Principle Component Analysis (PCA) Analysis of Differentially Expressed Phosphorylated Protein Species in Response to D Stress and NO
2.4. Metabolism-Related Differentially Expressed Phosphorylated Protein Species Accumulation in Response to NO and D Stress
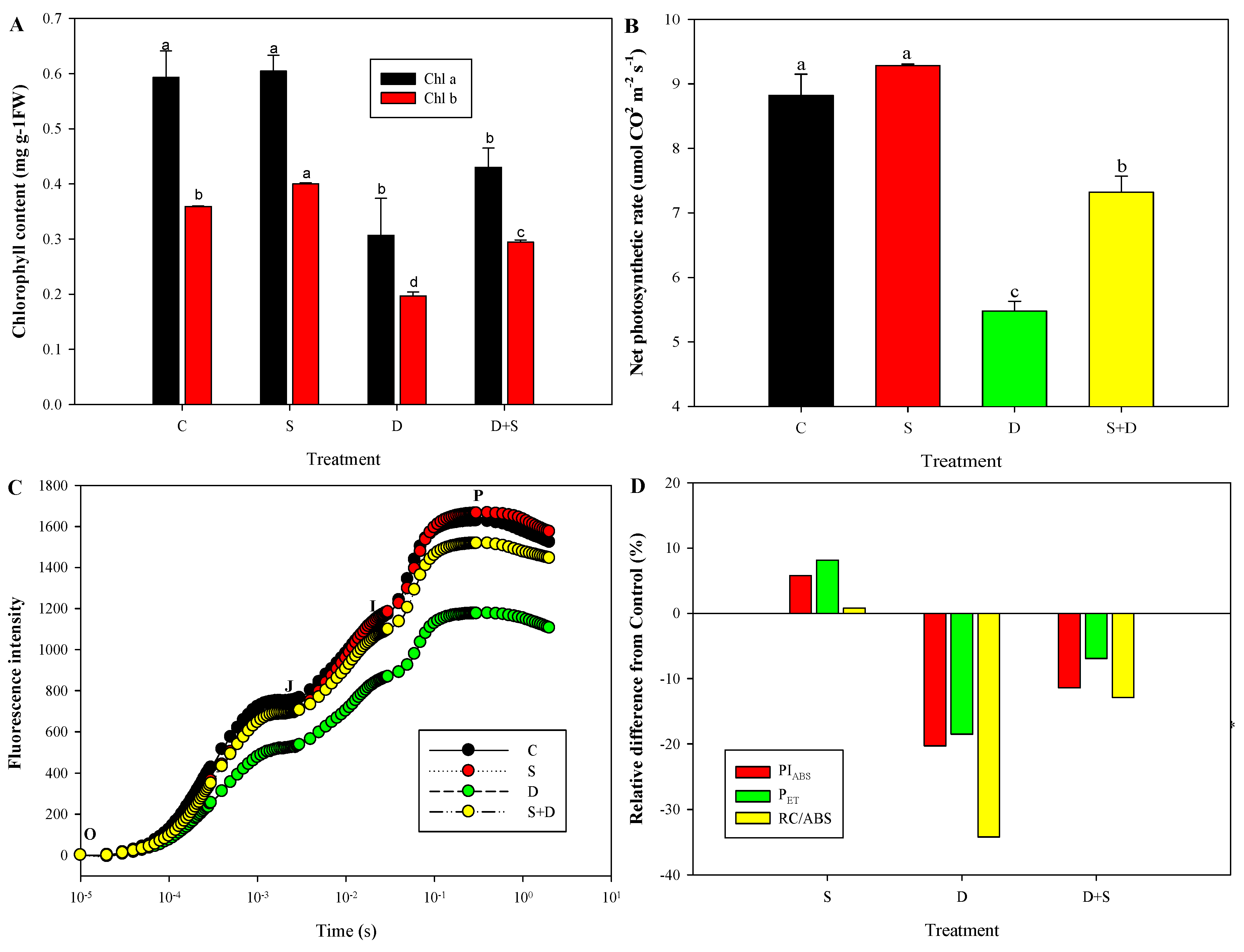
2.5. Physiological Changes of Photosynthesis-Related Parameters in Response to NO and D Stress
3. Discussion
3.1. Phosphoprotomics Elucidates Molecular Mechanism of NO-Induced D Tolerance in Wheat Seedlings
3.2. Comparison between Present Phosphoproteomic Data and Previous Studies
3.3. Phosphorylation is Involved in the Photosynthesis Metabolism against D
3.4. NO-Enhanced D Tolerance Correlated with Primary Reaction of Photosynthesis
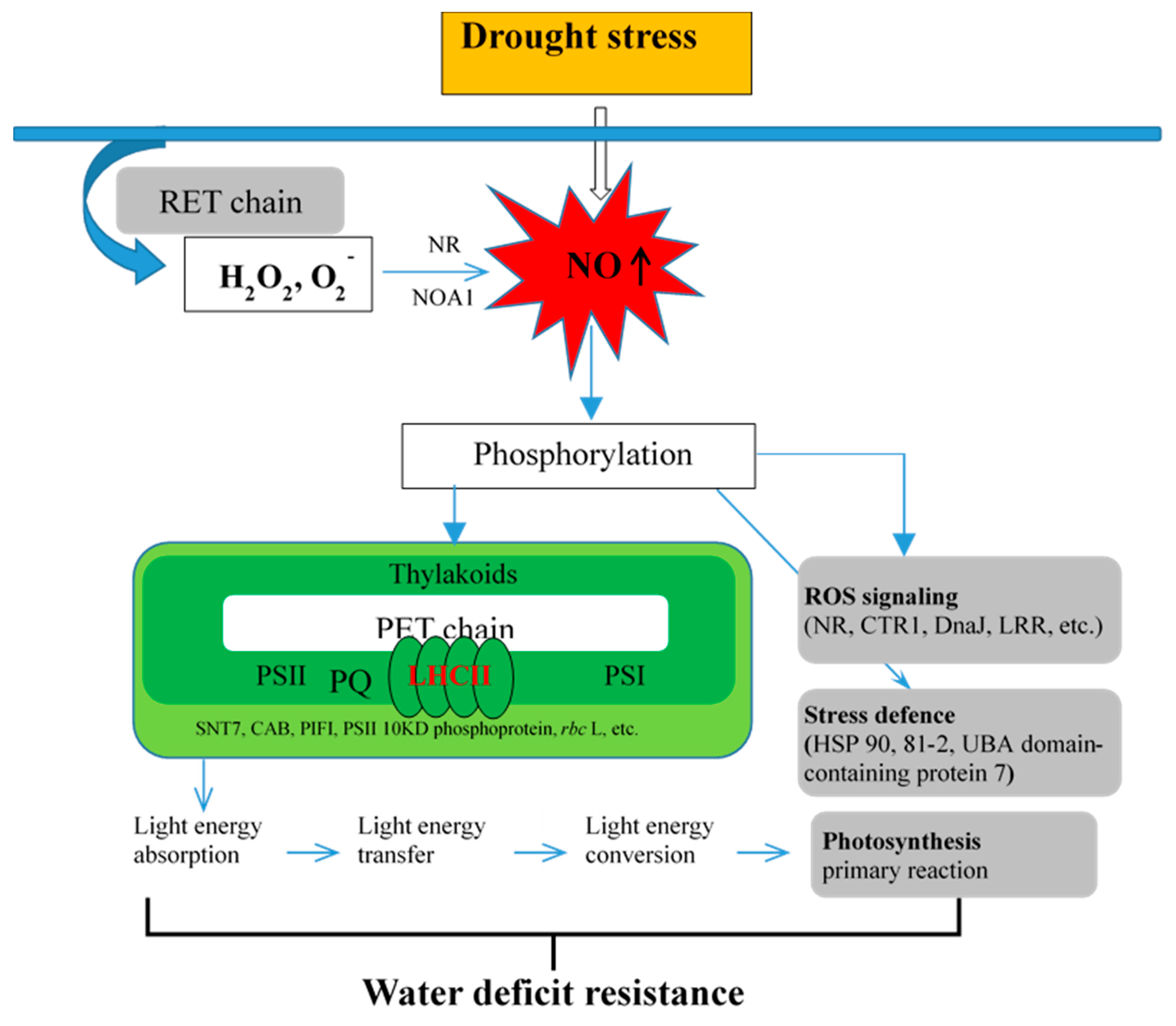
3.5. Putative Mechanism of NO Regulates Primary Reaction of Photosynthesis in Response to D Stress
4. Materials and Methods
4.1. Plant Materials and D and NO Treatments
4.2. Phenotypic Analysis
4.3. Physiological Analysis
4.4. Protein Extraction
4.5. Protein Digestion and iTRAQ Labeling
4.6. Enrichment of Phosphorylated Peptides Using TiO2 Beads
4.7. Liquid Chromatograph -Mass Spectrometry Analysis
4.8. Data Workflow
4.9. Identification of Differentially Phosphorylated Peptides
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Khan, M.N.; Mobin, M.; Mohammad, F.; Corpas, F.J. Nitric Oxide Action in Abiotic Stress Responses in Plants; Springer International Publishing: Basel, Switzerland, 2015; pp. 51–52. [Google Scholar]
- Shao, R.X.; Wang, K.B.; Shangguan, Z.P. Cytokinin-induced photosynthetic adaptability of Zea mays L. to drought stress associated with nitric oxide signal: Probed by ESR spectroscopy and fast OJIP fluorescence rise. J. Plant Physiol. 2010, 167, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Samouilov, A.; Kuppusamy, P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim. Biophys. Acta Bioenerg. 1999, 250–262. [Google Scholar] [CrossRef]
- Sahay, S.; Gupta, M. An update on nitric oxide and its benign role in plant responses under metal stress. Nitric Oxide-Biol. Chem. 2017, 67, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.F.; Zhao, H.J.; Hong, J.P.; Han, Y.L.; Li, H.; Zhao, W.C. Effects of exogenous nitric oxide on photosynthesis, antioxidant capacity and proline accumulation in wheat seedlings subjected to osmotic stress. World J. Agric. Sci. 2008, 4, 307–313. [Google Scholar]
- Liao, W.B.; Huang, G.B.; Yu, J.H.; Zhang, M.L. Nitric oxide and hydrogen peroxide alleviate drought stress in marigold explants and promote its adventitious root development. Plant Physiol. Biochem. 2012, 58, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Cantrel, C.; Vazquez, T.; Puyaubert, J.; Rezé, N.; Lesch, M.; Kaiser, W.M.; Dutilleul, C.; Guillas, I.; Zachowski, A.; Baudouin, E. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011, 189, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.; Shabbir, N.; Shahbaz, M.; Majeed, S.; Raheel, M.; Hassan, W.; Amir Sohail, M. Cross talk between nitric oxide and phytohormones regulate plant development during abiotic stresses. In Phytohormones-Signaling Mechanisms and Crosstalk in Plant Development and Stress Responses; InTech: Kowloon, Hong Kong, 2017. [Google Scholar] [CrossRef]
- Bian, Y.; Deng, X.; Yan, X.; Zhou, J.; Yuan, L.; Yan, Y. Integrated proteomic analysis of Brachypodium distachyon roots and leaves reveals a synergistic network in the response to drought stress and recovery. Sci. Rep. 2017, 7, 46183. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.J. A multimodel assessment of the climate change effect on the drought severity-duration-frequency relationship. Hydrol. Process. 2013, 27, 2800–2813. [Google Scholar] [CrossRef]
- Shao, R.X.; Yang, Q.H.; Xin, L.F.; Shangguan, Z.P. Effects of exogenous nitric oxide and cytokinin on the growth and photosynthesis of wheat seedlings under water deficiency. J. Food Agric. Environ. 2012, 10, 1451–1456. [Google Scholar]
- Procházková, D.; Haisel, D.; Wilhelmová, N.; Pavlíková, D.; Száková, J. Effects of exogenous nitric oxide on photosynthesis. Photosynthetica 2013, 51, 483–489. [Google Scholar] [CrossRef]
- Shao, R.X.; Li, L.L.; Zheng, H.F.; Zhang, J.Y.; Yang, S.J.; Ma, Y.; Xin, L.F.; Su, X.Y.; Ran, W.L.; Mao, J.; et al. Effects of exogenous nitric oxide on photosynthesis of maize seedlings under drought stress. Sci. Agric. Sin. 2016, 2, 251–259. [Google Scholar]
- Shi, H.T.; Ye, T.T.; Zhu, J.K.; Chan, Z.L. Constitutive production of nitric oxide leads to enhanced drought stress resistance and extensive transcriptional reprogramming in Arabidopsis. J. Exp. Bot. 2014, 65, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.M.; Tian, D.G.; Todd, C.D.; Luo, Y.; Hu, X. Comparative proteome analyses reveal that nitric oxide is an important signal molecule in the response of rice to aluminum toxicity. J. Proteome Res. 2013, 12, 1316–1330. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Y.; Liu, F.; Pang, C.Y.; Fan, S.L.; Song, M.Z.; Wang, D.; Li, W.H.; Yu, S.X. Label-free quantitative proteomics analysis of cotton leaf response to nitric oxide. J. Proteome Res. 2011, 10, 5416–5432. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, L.; Valot, B.; Tardieu, F.; Zivy, M. Phosphoproteome dynamics upon changes in plant water status reveal early events associated with rapid growth adjustment in maize leaves. Mol. Cell. Proteom. 2012, 11, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lv, D.W.; Ge, P.; Bian, Y.W.; Chen, G.X.; Zhu, G.R.; Li, X.H.; Yan, Y.M. Phosphoproteome analysis reveals new drought response and defense mechanisms of seedling leaves in bread wheat (Triticum aestivum L.). J. Proteome 2014, 109, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.L.; Meng, Y.Y.; Song, M.Z.; Pang, C.Y.; Wei, H.L.; Liu, J.; Zhan, X.J.; Lan, J.Y.; Feng, C.H.; Zhang, S.X.; et al. Quantitative phosphoproteomics analysis of nitric oxide-responsive phosphoproteins in cotton leaf. PLoS ONE 2014, 9, e94261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ma, C.Y.; Lv, D.W.; Zhen, S.M.; Li, X.H.; Yan, Y.M. Comparative phosphoproteome analysis of the developing grains in bread wheat (Triticum aestivum L.) under well-watered and water-deficiency conditions. J. Proteome Res. 2014, 13, 4281–4297. [Google Scholar] [CrossRef] [PubMed]
- Parankusam, S.; Adimulam, S.S.; Bhatnagarmathur, P.; Sharma, K.K. Nitric oxide (NO) in plant heat stress tolerance: Current knowledge and perspectives. Front. Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Grieco, M.; Kangasjärvi, S.; Aro, E.M. Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol. 2010, 152, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Polverari, A.; Molesini, B.; Pezzotti, M.; Buonaurio, R.; Marte, M.; Delledonne, M. Nitric oxide-mediated transcriptional changes in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2003, 16, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Pesaresi, P.; Hertle, A.; Pribil, M.; Schneider, A.; Kleine, T.; Leister, D. Optimizing photosynthesis under fluctuating light: The role of the Arabidopsis SNT7 kinase. Plant Signal. Behav. 2010, 5, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Portis, A.R. A novel nucleus-encoded chloroplast protein, PIFI, is involved in NAD(P)H dehydrogenase complex-mediated chlororespiratory electron transport in Arabidopsis. Plant Physiol. 2007, 144, 1742–1752. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, R.; Dietz, K.J. Reduction–oxidation network for flexible adjustment of cellular metabolism in photoautotrophic cells. Plant Cell Environ. 2012, 35, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Reichheld, J.P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Wientjes, E.; van Amerongen, H.; Croce, R. LHCII is an antenna of both photosystems after long-term acclimation. Biochim. Biophys. Acta Bioenerg. 1827, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Bos, I.; Bland, K.M.; Tian, L.J.; Croce, R.; Frankel, L.K.; van Amerongen, H.; Bricker, T.M.; Wientjes, E. Multiple LHCII antennae can transfer energy efficiently to a single Photosystem I. Biochim. Biophys. Acta Bioenerg. 1858, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Koziol, A.G.; Borza, T.; Ishida, K.I.; Keeling, P.; Lee, R.W.; Durnford, D.G. Tracing the evolution of the light-harvesting antennae in chlorophyll a/b-containing organisms. Plant Physiol. 2007, 143, 1802–1816. [Google Scholar] [CrossRef] [PubMed]
- Bolaños, J.P.; HerreroMendez, A.; FernandezFernandez, S.; Almeida, A. Linking glycolysis with oxidative stress in neural cells: A regulatory role for nitric oxide. Biochem. Soc. Trans. 2007, 35, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.Z.; Li, G.Z.; Xu, W.; Peng, X.Q.; Han, Q.X.; Zhu, Y.J.; Guo, T.C. Proteomics reveals the effects of salicylic acid on growth and tolerance to subsequent drought stress in wheat. J. Proteome Res. 2012, 11, 6066–6079. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Xia, R.X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006, 163, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Hiscox, J.D.; Israelstam, G.F. Erratum: A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1980, 58, 403. [Google Scholar] [CrossRef]
- Arnon, D.I. Copperenzymesin isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [PubMed]
- Pi, E.; Qu, L.; Hu, J.; Huang, Y.; Qiu, L.; Lu, H.; Jiang, B.; Liu, C.; Peng, T.; Zhao, Y.; et al. Mechanisms of soybean roots’ tolerances to salinity revealed by proteomic and phosphoproteomic comparisons between two cultivars. Mol. Cell. Proteom. 2016, 15, 266–288. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, J.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.R.; Thingholm, T.E.; Jensen, O.N.; Roepstorff, P.; Jørgensen, T.J. Highly selective enrichment of phosphorylated peptides from peptide mixturesusing titanium dioxide microcolumns. Mol. Cell. Proteom. 2005, 4, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, S.A.; Villén, J.; Gerber, S.A.; Rush, J.; Gygi, S.P. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 2006, 24, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.L.; Stacey, G.; Hendrick, J.P.; Hemmings, H.C. General anesthetics selectively modulate glutamatergic and dopaminergic signaling site-specific phosphorylation in vivo. Neuropharmacology 2007, 53, 619–630. [Google Scholar] [CrossRef] [PubMed]
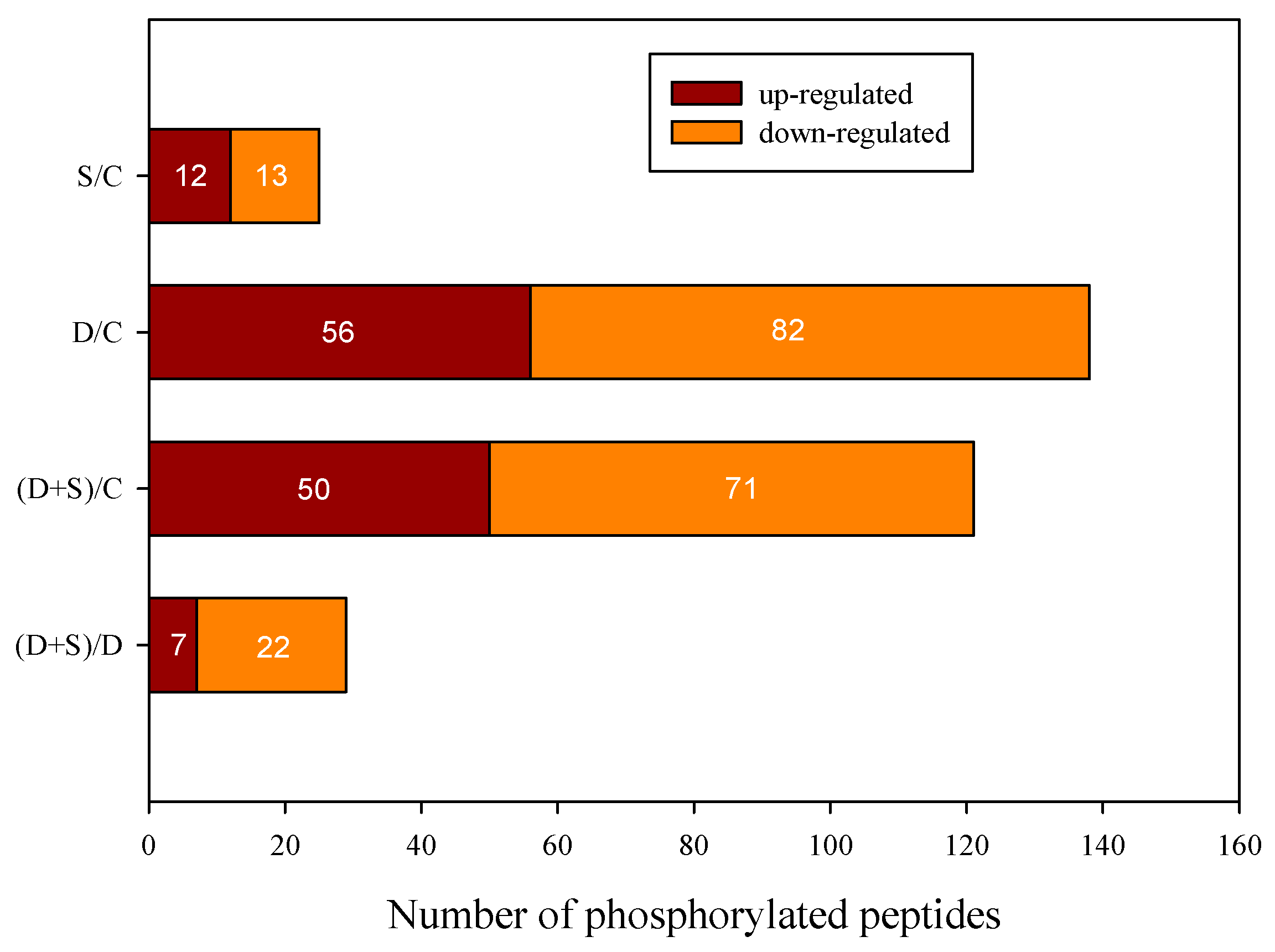
| Groups | Increase | Decrease | Each Total | Total Phosphorylated Proteins |
|---|---|---|---|---|
| S/C | 12 | 13 | 25 | 148 |
| D/C | 37 | 61 | 96 | |
| (S + D)/C | 33 | 57 | 88 | |
| (S + D)/D | 7 | 17 | 24 |
| Description | Phosphosite a | Accession | Coverage b (%) | Proteins c | Unique Peptide d | MW [kDa] e | Calc. pI f | Fold Ratio g |
|---|---|---|---|---|---|---|---|---|
| (S/C, D/C, (S + D)/C, (S + D)/D) | ||||||||
| Lhcb | S(11): 94.0 | F2CRC1 | 12.24 | 4 | 2 | 26.72 | 6.11 | 0.73/0.22/0.27/1.23 ↑ |
| SNT7 | T(1): 100.0; S(5): 100.0 | M8CIW2 | 2.59 | 1 | 2 | 68.90 | 9.01 | 1.04/0.62/0.82/1.32 ↑ |
| T(4): 100.0; S(8): 100.0 | 0.95/0.59/0.75/1.26 ↑ | |||||||
| S(5): 100.0 | 0.82/0.76/0.62/0.82 | |||||||
| S(5): 100.0 | 0.74/0.63/0.49/0.78 | |||||||
| PIFI | Class II | W5I170 | 14.98 | 3 | 1 | 24.69 | 4.77 | 0.79/0.60/0.72/1.20 ↑ |
| T(2): 100.0 | B3TN78 | 19.18 | 1 | 1 | 7.83 | 8.53 | 0.75/0.69/0.52/0.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, R.; Zheng, H.; Jia, S.; Jiang, Y.; Yang, Q.; Kang, G. Nitric Oxide Enhancing Resistance to PEG-Induced Water Deficiency is Associated with the Primary Photosynthesis Reaction in Triticum aestivum L. Int. J. Mol. Sci. 2018, 19, 2819. https://doi.org/10.3390/ijms19092819
Shao R, Zheng H, Jia S, Jiang Y, Yang Q, Kang G. Nitric Oxide Enhancing Resistance to PEG-Induced Water Deficiency is Associated with the Primary Photosynthesis Reaction in Triticum aestivum L. International Journal of Molecular Sciences. 2018; 19(9):2819. https://doi.org/10.3390/ijms19092819
Chicago/Turabian StyleShao, Ruixin, Huifang Zheng, Shuangjie Jia, Yanping Jiang, Qinghua Yang, and Guozhang Kang. 2018. "Nitric Oxide Enhancing Resistance to PEG-Induced Water Deficiency is Associated with the Primary Photosynthesis Reaction in Triticum aestivum L." International Journal of Molecular Sciences 19, no. 9: 2819. https://doi.org/10.3390/ijms19092819
APA StyleShao, R., Zheng, H., Jia, S., Jiang, Y., Yang, Q., & Kang, G. (2018). Nitric Oxide Enhancing Resistance to PEG-Induced Water Deficiency is Associated with the Primary Photosynthesis Reaction in Triticum aestivum L. International Journal of Molecular Sciences, 19(9), 2819. https://doi.org/10.3390/ijms19092819




