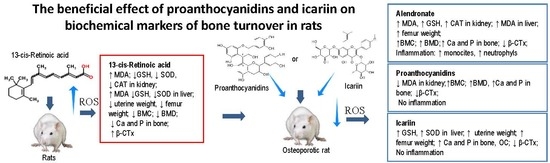
The Beneficial Effect of Proanthocyanidins and Icariin on Biochemical Markers of Bone Turnover in Rats
Abstract
1. Introduction
2. Results
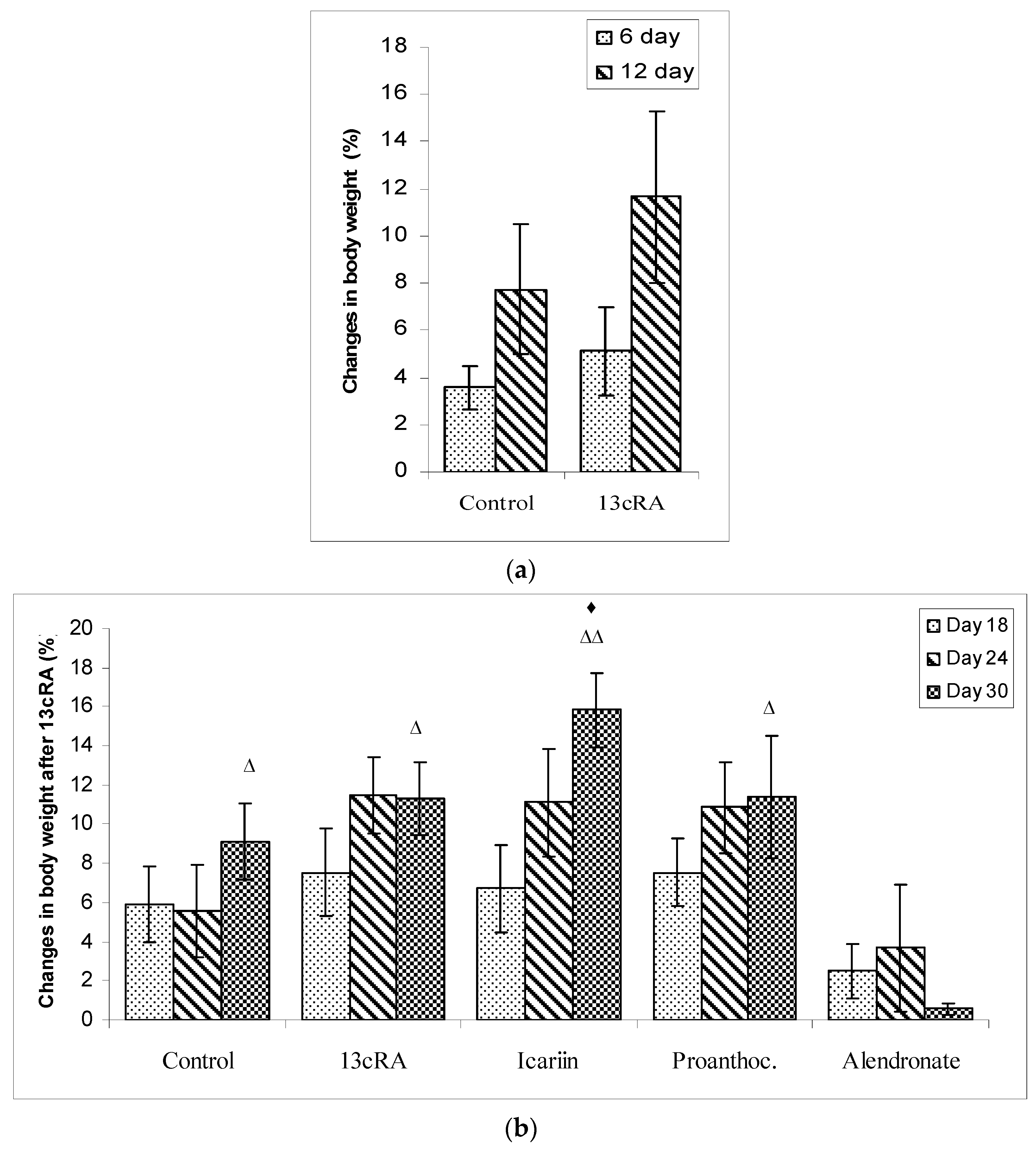
2.1. Body Weights
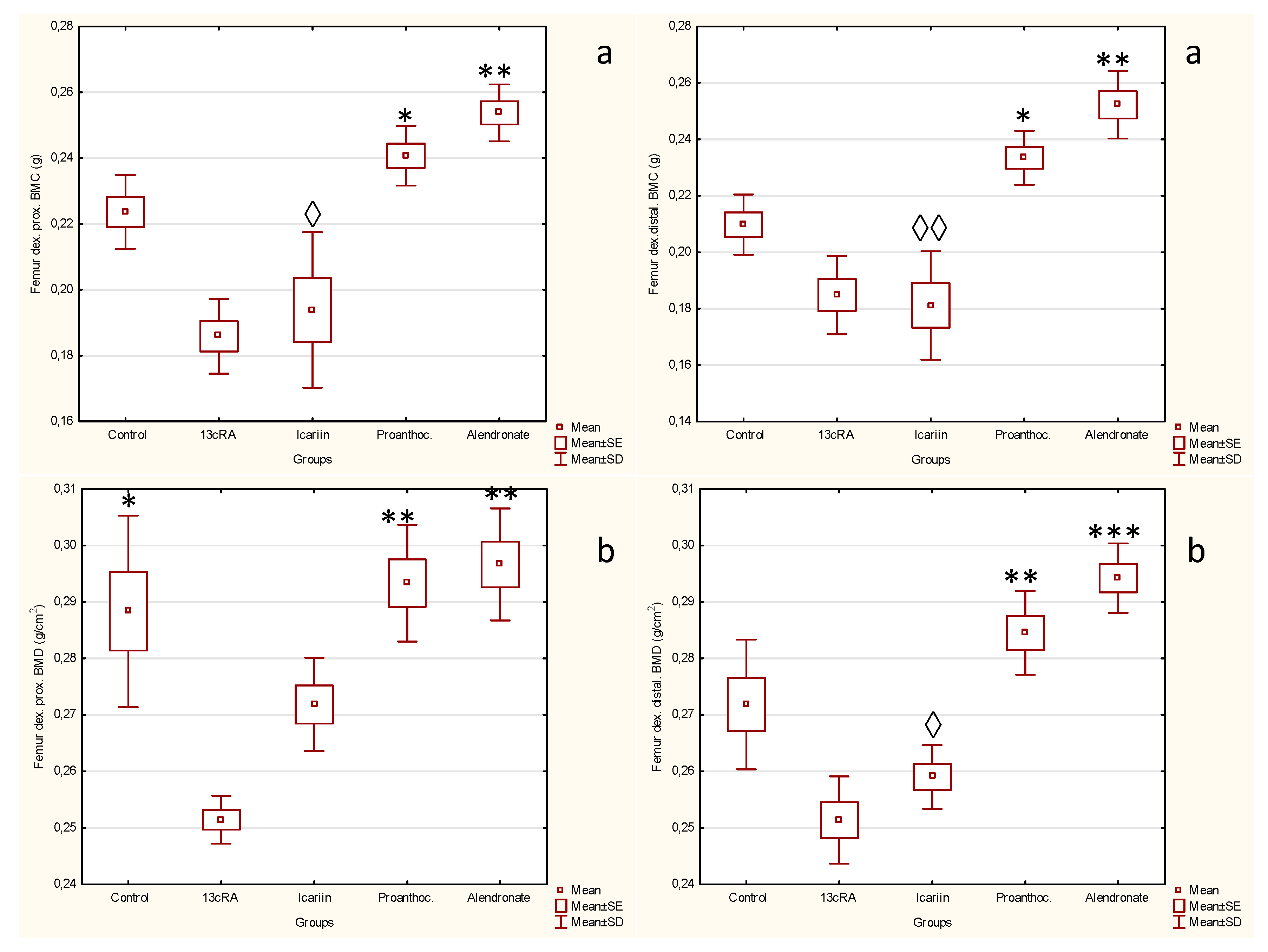
2.2. Effect of Icariin, Proanthocyanidins and Alendronate on Bone Health and Metabolism in Rats with Retinoic Acid-Induced Bone Loss
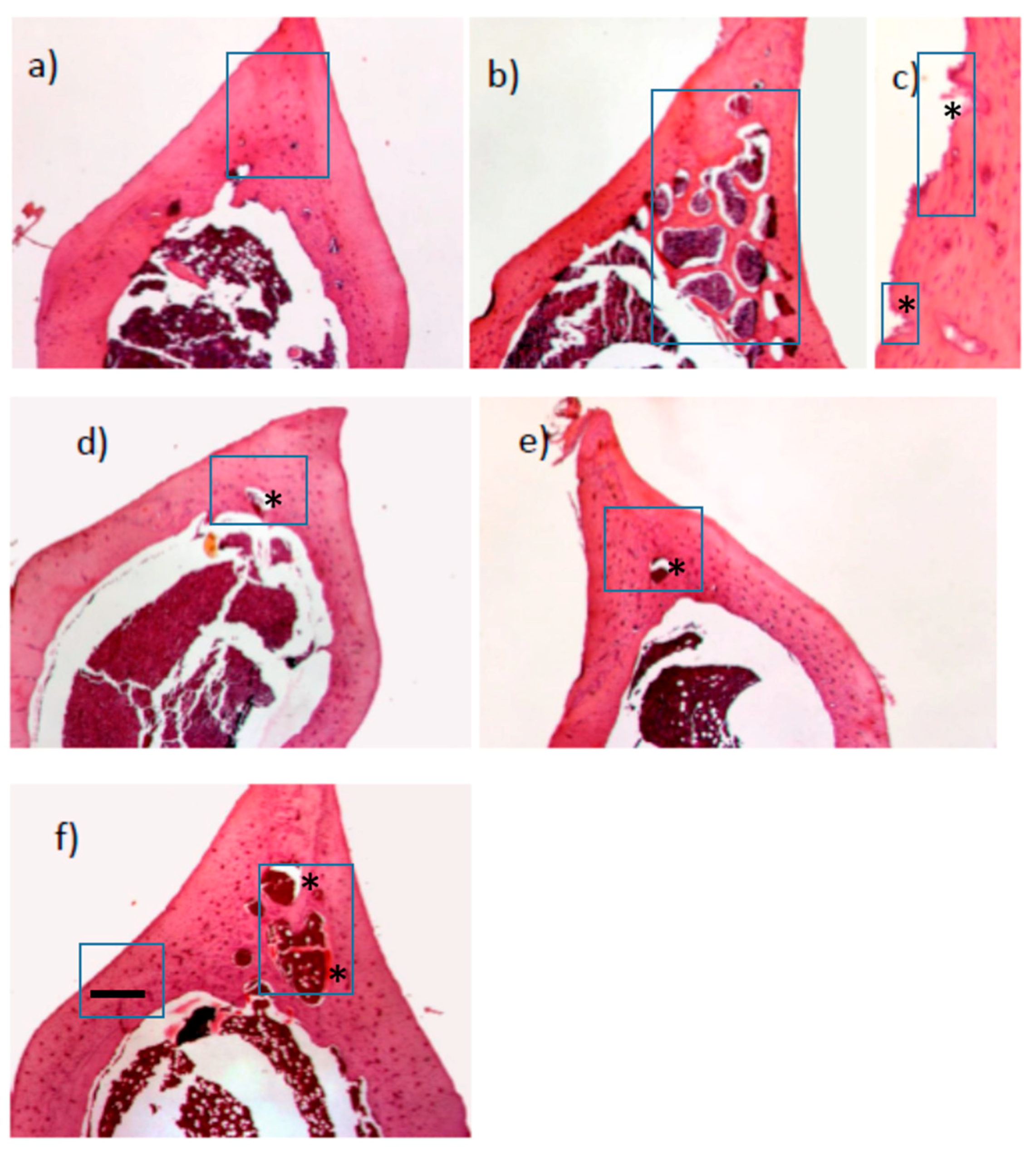
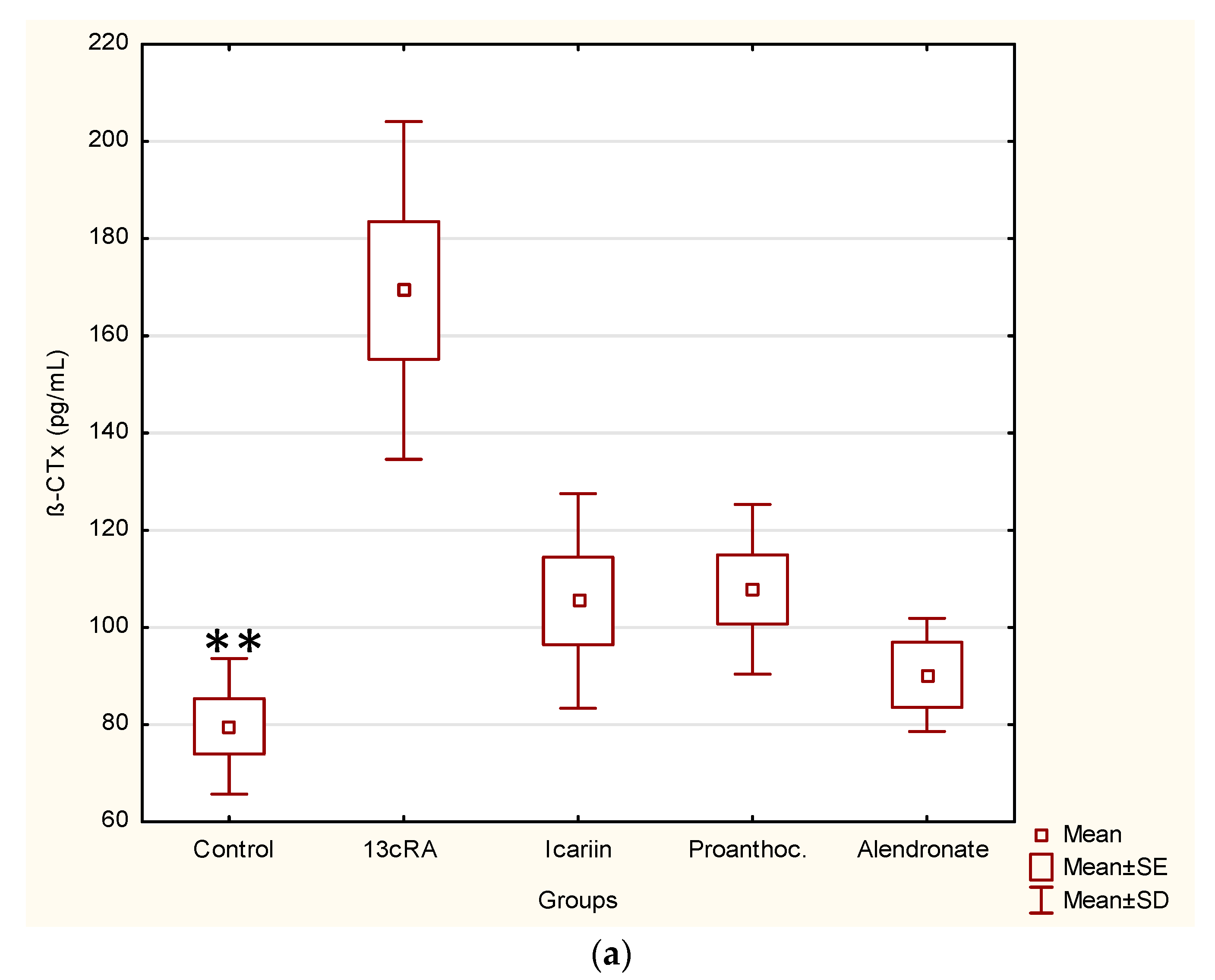
2.3. Effect of Icariin, Proanthocyanidins and Alendronate on Osteoclast and Osteoblast Activities in Rats with Retinoic Acid-Induced Bone Loss
2.4. Effect of Icariin, Proanthocyanidins and Alendronate on Antioxidant and Anti-Inflammatory Markers in Rats with Retinoic Acid-Induced Bone Loss
2.5. Oestrogenic-Like Activity of Icariin, Proanthocyanidins and Alendronate in Rats with Retinoic Acid-Induced Bone Loss
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Proanthocyanidins and Icariin
4.3. Animals and Experimental Design
4.4. Body Weight, Blood and Serum Biochemistry
4.5. Bone Analysis
4.6. Bone Ash Contents, and Ca and P Analysis
4.7. Vitamin D Level and Biochemical Markers of Bone Turnover
4.8. Histological Examination of Rats’ Bone
4.9. Oxidative/Antioxidative Stress Markers Analysis in Liver and Kidney
4.10. Inflammatory Cytokines Analysis
4.11. Uterine Weight
4.12. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMD | bone mineral density |
| BMC | bone mineral content |
| VDR | vitamin D receptor |
| WHI | Women’s Health Initiative |
| MWS | Million Women Study |
| 13cRA | 13 cis-retinoic acid |
| HRT | hormone replacement therapy |
| OS | oxidative stress |
| SOD | superoxide dismutase |
| CAT | catalase |
| OC | osteocalcin |
| CTx | C-terminal telopeptide |
| β-CTx | β-CrossLaps marker of bone resorption |
| HE | hematoxylin and eosin |
| MDA | malondialdehyde |
| SDS | sodium dodecyl sulphate |
| DTNB | 5,5′-Dithiobis(2-nitrobenzoic acid) |
| RDO | rapid decalcified |
| GSH | glutathione |
| AP | anterior-posterior length |
| ML | medio-lateral length |
| ROS | reactive oxygen species |
References
- Oršolić, N.; Goluža, E.; Đikić, D.; Lisičić, D.; Sašilo, K.; Rođak, E.; Jeleč, Z.; Lazarus, M.V.; Orct, T. Role of flavonoids on oxidative stress and mineral contents in the retinoic acid-induced bone loss model of rat. Eur. J. Nutr. 2014, 53, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jin, Y.; Lee, S.H.; Khadka, D.B.; Cho, W.J.; Lee, K.Y. Berberine bioisostere Q8 compound stimulates osteoblast differentiation and function in vitro. Pharmacol. Res. 2017, 119, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Yeh, J.K.; Cao, J.J.; Chyu, M.C.; Wang, J.S. Green tea and bone health: Evidence from laboratory studies. Pharmacol. Res. 2011, 64, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Emblica officinalis (Amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms. Pharmacol. Res. 2016, 111, 180–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Choi, H.S.; Choi, H.S.; Chung, K.Y.; Lee, B.J.; Maeng, H.J.; Seo, M.D. Quercetin Directly Interacts with Vitamin D Receptor (VDR): Structural Implication of VDR Activation by Quercetin. Biomol. Ther. 2016, 24, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Barboza, G.; Guizzardi, S.; Moine, L.; Tolosa de Talamoni, N. Oxidative stress, antioxidants and intestinal calcium absorption. World J. Gastroenterol. 2017, 23, 2841–2853. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. A review of propolis antitumor action in vivo and in vitro. J. ApiProd. ApiMed. Sci. 2010, 2, 1–20. [Google Scholar] [CrossRef]
- Lephart, E.D. Modulation of Aromatase by Phytoestrogens. Enzyme Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Lv, S.; Li, C.; Tao, J.; Jia, C.; Jiang, M.; Hou, Y.; Hou, X.; Deng, J.; Bai, G. Bioactivity-based antioxidative components screening and evaluation in grape seed proanthocyanidin extract. J. Food Sci. Technol. 2017, 54, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhang, W.; Yuan, W.; Du, H.; Ming, K.; Yao, F.; Bai, J.; Chen, Y.; Liu, J.; Wang, D.; et al. Phosphorylation of Icariin Can Alleviate the Oxidative Stress Caused by the Duck Hepatitis Virus A through Mitogen-Activated Protein Kinases Signaling Pathways. Front Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef] [PubMed]
- Diel, P. Tissue-specific estrogenic response and molecular mechanisms. Toxicol. Lett. 2002, 127, 217–224. [Google Scholar] [CrossRef]
- Chiang, C.H.; Huang, C.C.; Chan, W.L.; Huang, P.H.; Chen, T.J.; Chung, C.M.; Lin, S.J.; Chen, J.W.; Leu, H.B. Oral alendronate use and risk of cancer in postmenopausal women with osteoporosis: A. nationwide study. J. Bone Miner. Res. 2012, 27, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Kuller, L.H.; Prentice, R.L.; Stefanick, M.L.; Manson, J.E.; Gass, M.; Aragaki, A.K.; Ockene, J.K.; Lane, D.S.; Sarto, G.E.; et al. WHI Investigators. Breast cancer after use of estrogen plus progestin in postmenopausal women. N. Engl. J. Med. 2009, 360, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; La Croix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Writing Group for the Women’s Health Initiative Investigators. Writing group for the Women’s Health Initiative investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [PubMed]
- Conte, P.; Guarneri, V. Safety of intravenous and oral bisphosphonates and compliance with dosing regimens. Oncologist 2004, 9, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Fonseca, F.; Santos-Silva, A.R.; Della-Coletta, R.; Vargas, P.A.; Lopes, A. Alendronate-associated osteonecrosis of the jaws: A review of the main topic. Med. Oral Patol. Oral. Cir. Bucal 2014, 19, 106–111. [Google Scholar] [CrossRef]
- Pitts, C.J.; Kearns, A.E. Update on medications with adverse skeletal effects. Mayo Clin. Proc. 2011, 86, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.; Melhus, H. Vitamin A antagonizes calcium response to vitamin D in man. J. Bone Miner. Res. 2001, 16, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Jeleč, Ž.; Nemrava, J.; Balta, V.; Gregorović, G.; Jeleč, D. The effect of quercetin on bone mineral status and markers of bone turnover in retinoic acid-induced osteoporosis. Pol. J. Food Nutr. Sci. 2018, 68, 149–162. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Martin-Millan, M.; Plotkin, L.I.; Stewart, S.A.; Roberson, P.K.; Kousteni, S.; O’Brien, C.A.; Bellido, T.; Parfitt, A.M.; et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 2007, 282, 27285–27297. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bergamini, C.M. Oxidative damage and the pathogenesis of menopause related disturbances and diseases. Clin. Chem. Lab. Med. 2016, 54, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Conte da Frota, M.L., Jr.; Gomes da Silva, E.; Behr, G.A.; Roberto de Oliveira, M.; Dal-Pizzol, F.; Klamt, F.; Moreira, J.C. All-trans retinoic acid induces free radical generation and modulate antioxidant enzyme activities in rat sertoli cells. Mol. Cell Biochem. 2006, 285, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.R.; Soliman, A.M. Oxidative Stress as a Risk Factor of Osteoporotic Model Induced by Vitamin A in Rats. Aust. J. Basic Appl. Sci. 2009, 3, 1559–1568. [Google Scholar]
- Frankel, T.L.; Seshadri, M.S.; McDowall, D.B.; Cornish, C.J. Hypervitaminosis A and calcium-regulating.hormones in the rat. J. Nutr. 1986, 116, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, A.; Zaragozá, R.; Vivó-Sese, I.; Viña, J.R.; Miralles, V.J. Retinol, at concentrations greater than the physiological limit, induces oxidative stress and apoptosis in human dermal fibroblasts. Exp. Dermatol. 2004, 13, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hough, S.; Avioli, L.V.; Muir, H.; Gelderblom, D.; Jenkins, G.; Kurasi, H.; Slatopolsky, E.; Bergfeld, M.A.; Teitelbaum, S.L. Effects of hypervitaminosis A on the bone and mineral metabolism of the rat. Endocrinology 1988, 122, 2933–2939. [Google Scholar] [CrossRef] [PubMed]
- Kindmark, A.; Melhus, H.; Ljunghall, S.; Ljunggren, O. Inhibitory effects of 9-cis and all trans retinoic acid on 1, 25 (OH) vitamin D-induced bone resorption. Calcif. Tissue Int. 1995, 57, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Kneissel, M.; Studer, A.; Cortesi, R.; Susa, M. Retinoid-induced bone thinning is caused by subperiosteal osteoclast activity in adult rodents. Bone 2005, 36, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the Skeleton. Trends Endocrinol. MeTab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Liao, E.Y.; Luo, X.H.; Wang, W.B.; Wu, X.P.; Zhou, H.D.; Dai, R.C.; Liao, H.J.; Yang, C. Effects of different nylestriol/levonorgestrel dosages on bone metabolism in female Sprague-Dawley rats with retinoic acid-induced osteoporosis. Endocr. Res. 2003, 29, 23–42. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.N.; Ritter, C.; Brown, A.J.; Slatoplosky, E. Retinoic acid suppresses parathyroid hormone (PTH) secretion and PreproPTH mRNA levels in bovine parathyroid cell culture. J. Clin. Investig. 1994, 93, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Muthusami, S.; Ramachandran, I.; Muthusamy, B.; Vasudevan, G.; Prabhu, V.; Subramaniam, V.; Jagadeesan, A.; Narasimhan, S. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin. Chim. Acta 2005, 360, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Scheven, B.A.; Hamilton, N.J. Retinoic acid and 1, 25-dihydroxy vitamin D stimulate osteoclast formation by different mechanisms. Bone 1990, 11, 53–59. [Google Scholar] [CrossRef]
- Iolascon, G.; Frizzi, L.; Di Pietro, G.; Capaldo, A.; Luciano, F.; Gimigliano, F. Bone quality and bone strength: Benefits of the bone-forming approach. Clin. Cases Miner. Bone Metab. 2014, 11, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and Grape Seed Byproducts as a Source of Antioxidant and Anti-Inflammatory Proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.L.; Huang, H.B.; Fang, L.; Hu, J.N.; Jin, Z.M.; Wang, R.W. Protective effect of ginkgo proanthocyanidins against cerebral ischemia/reperfusion injury associated with its antioxidant effects. Neural Regen. Res. 2016, 11, 1779–1783. [Google Scholar] [PubMed]
- Hozayen, W.G.; El-Desouky, M.A.; Soliman, H.A.; Ahmed, R.R.; Khaliefa, A.K. Antiosteoporotic effect of Petroselinum crispum, Ocimum basilicum and Cichorium intybus L. in glucocorticoid-induced osteoporosis in rats. BMC Complement. Altern. Med. 2016, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Zafar, T.A.; Weaver, C.M.; Zhao, Y.; Martin, B.R.; Wastney, M.E. Nondigestible oligosaccharides increase calcium absorption and suppress bone resorption in ovariectomized rats. Int. J. Nutr. 2004, 134, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Hoenderop, J.G.; Nilius, B.; Bindels, R.J. Estrogen effects on calcitropic hormones and calcium homestasis. Endocr. Rev. 1994, 15, 301–309. [Google Scholar]
- Bartelt, A.; Widenmaier, S.B.; Schlein, C.; Johann, K.; Goncalves, R.L.S.; Eguchi, K.; Fischer, A.W.; Parlakgül, G.; Snyder, N.A.; Nguyen, T.B.; et al. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 2018, 24, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, B.; Dong, H.; Shi, J.; Fang, D. Icariin induces synoviolin expression through NFE2L1 to protect neurons from ER stress-induced apoptosis. PLoS ONE 2015, 10, e0119955. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dai, X.; Jiang, Y.; Zhang, Z.; Bao, L.; Li, Y.; Zhang, F.; Ma, X.; Cai, X.; Jing, L.; et al. Grape seed proanthocyanidin extracts alleviate oxidative stress and ER stress in skeletal muscle of low dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Mol. Nutr. Food Res. 2013, 57, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Benghuzzi, H.; Mohamed, A.; Aneja, A.; Cameron, J.A.; Tucci, M. The effects of sustained delivery of alendronate on the kidney in ovariectomized female rats. Biomed. Sci. Instrum. 2012, 48, 35–42. [Google Scholar] [PubMed]
- Conwell, L.S.; Chang, A.B. Bisphosphonates for osteoporosis in people with cystic fibrosis. Cochrane Database Syst. Rev. 2012, 18, CD002010. [Google Scholar] [CrossRef]
- Perazzella, M.A.; Markowitz, G.S. Bisphosphonate Nephrotoxicity. Kidney Int. 2008, 74, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Kapucu, C.; Cetinel, S.; Cikler, E.; Ayanoğlu-Dülger, G. Gastroprotective effect of leukotriene receptor blocker montelukast in alendronat-induced lesions of the rat gastric mucosa. Prostag. Leukot. Essent. Fatty Acids 2005, 72, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shikama, Y.; Nagai, Y.; Okada, S.; Oizumi, T.; Shimauchi, H.; Sugawara, S.; Endo, Y. Pro-IL-1β accumulation in macrophages by alendronate and its prevention by clodronate. Toxicol. Lett. 2010, 199, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Park-Wyllie, L.; Mamdani, M.; Juurlink, D.; Hawker, G.A.; Gunraj, N.; Austin, P.C.; Whelan, D.B.; Weiler, P.J.; Laupacis, A. Bisphosphonate Use and the Risk of Subtrochanteric or Femoral Shaft Fractures in Older Women. JAMA 2011, 305, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yu, Z.; Funayama, H.; Yamaguchi, K.; Sasano, T.; Sugawara, S.; Endo, Y. Histidine decarboxylase-stimulating and inflammatory effects of alendronate in mice: Involvement of mevalonate pathway, TNFalpha, macrophages, and T-cells. Int. Immunopharmacol. 2007, 7, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.J.; Joo, Y.B.; Jung, Y.O.; Ju, J.H.; Cho, M.L.; Oh, H.J.; Jhun, J.Y.; Park, M.K.; Park, J.S.; Kang, C.M.; et al. Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp. Mol. Med. 2011, 43, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Kunštić, M.; Kukolj, M.; Gračan, R.; Nemrava, J. Oxidative stress, polarization of macrophages and tumour angiogenesis: Efficacy of caffeic acid. Chem. Biol. Interact. 2016, 256, 111–124. [Google Scholar] [CrossRef] [PubMed]

| Groups a | Biochemical Parameters-Enzymes (X ± SD) | |||||
|---|---|---|---|---|---|---|
| AST (U L−1) | ALT (U L−1) | ALP (U L−1) | GGT (U L−1) | LDH (U L−1) | Amylase (U L−1) | |
| Control | 78.33 ± 7.73 | 46.40 ± 9.52 | 170.13 ± 24.54 | 0.00 ± 0.00 | 464.50 ± 130.97 | 1751.00 ± 609.96 |
| 13cRA | 96.16 ± 13.34 | 43.16 ± 4.66 | 224.00 ± 15.01 ♦ | 0.66 ± 0.51 | 725.66 ± 157.59 | 2046.16 ± 213.69 |
| Icariin | 83.00 ± 7.01 | 51.16 ± 5.19 | 164.00 ± 20.36 | 0.33 ± 0.51 | 385.83 ± 65.98 * | 1549.66 ± 397.01 |
| Proanthoc | 85.16 ± 8.51 | 42.40 ± 6.94 | 186.66 ± 26.01 | 0.33 ± 0.51 | 286.50 ± 45.85 ** | 1831.66 ± 247.39 |
| Alendronate | 87.66 ± 7.50 | 42.00 ± 2.64 | 115.00 ± 12.00 ** | 0.33 ± 0.57 | 402.00 ± 122.74 | 2184.00 ± 419.06 |
| Groups a | Leucocytes (109 L−1) | Differential Blood Count (X ± SD) | ||||
|---|---|---|---|---|---|---|
| Lymphocytes (%) | Monocytes (%) | Neutrophils (%) | Basophils (%) | Eosinophils (%) | ||
| Control | 2.70 ± 1.17 | 79.06 ± 7.99 | 0.42 ± 0.21 | 13.46 ± 2.97 | 0.38 ± 0.21 | 0.61 ± 0.27 |
| 13cRA | 3.88 ± 1.12 | 83.96 ± 2.74 | 0.45 ± 0.16 | 15.18 ± 2.28 | 0.55 ± 0.17 | 0.73 ± 0.36 |
| Icariin | 4.01 ± 1.01 | 78.01 ± 3.57 | 0.40 ± 0.18 | 20.15 ± 2.91 | 0.40 ± 0.16 | 0.70 ± 0.34 |
| Proanthoc | 4.70 ± 0.75 | 73.90 ± 4.81 | 0.35 ± 0.12 | 21.90 ± 3.13 | 0.66 ± 0.33 | 0.71 ± 0.24 |
| Alendronate | 5.35 ± 1.36 ♦ | 70.16 ± 3.29 | 0.86 ± 0.20 ♦ | 28.00 ± 4.33 ♦ | 1.23 ± 0.50 | 0.73 ± 0.41 |
| Groups a | Relative Bone Weight (g/100 g) | |||||
|---|---|---|---|---|---|---|
| Right Femur | Left Femur | |||||
| X ± SD | Min Value | Max Value | X ± SD | Min Value | Max Value | |
| Control | 0.363 ± 0.014 ** | 0.350 | 0.386 | 0.347 ± 0.009 * | 0.335 | 0.362 |
| 13cRA | 0.314 ± 0.009 | 0.299 | 0.321 | 0.302 ± 0.012 | 0.285 | 0.316 |
| Icariin | 0.346 ± 0.004 * | 0.341 | 0.355 | 0.333 ± 0.005 | 0.323 | 0.342 |
| Proanthoc | 0.333 ± 0.005 | 0.326 | 0.341 | 0.323 ± 0.017 | 0.303 | 0.342 |
| Alendronate | 0.373 ± 0.015 ** | 0.356 | 0.385 | 0.374 ± 0.036 * | 0.351 | 0.416 |
| Groups a | Femur Geometric Characteristic (cm /100 g) (X ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| AP φ Proximal Epiphysis | ML φ Proximal Epiphysis | AP φ Mid- Diaphysis | ML φ Mid- Diaphysis | AP φ Distal Epiphysis | ML Distal Epiphysis | Femur Length V. Trochanter-Condyle | |
| Control | 0.153 ± 0.004 | 0.223 ± 0.010 | 0.129 ± 0.004 | 0.172 ± 0.021 * | 0.154 ± 0.009 | 0.207 ± 0.015 | 1.405 ± 0.050 |
| 13cRA | 0.137 ± 0.009 | 0.214 ± 0.011 | 0.118 ± 0.005 | 0.141 ± 0.010 | 0.150 ± 0.008 | 0.213 ± 0.009 | 1.325 ± 0.044 |
| Icariin | 0.168 ± 0.010 | 0.247 ± 0.016 | 0.141 ± 0.006 ** | 0.149 ± 0.011 | 0.194 ± 0.010 ** | 0.236 ± 0.012 | 1.546 ± 0.043 *** |
| Proanthoc. | 0.153 ± 0.008 | 0.236 ± 0.014 | 0.131 ± 0.007 | 0.145 ± 0.008 | 0.173 ± 0.014 | 0.235 ± 0.017 | 1.427 ± 0.096 |
| Alendronate | 0.146 ± 0.004 | 0.222 ± 0.005 | 0.128 ± 0.005 | 0.149 ±0.004 | 0.169 ± 0.015 | 0.228 ± 0.001 | 1.390 ± 0.036 |
| Groups a | Content of Calcium and Phosphorus in Femur (X ± SD) | Content of Calcium and Phosphorus in Serum (X ± SD) | Serum 25-Hydroxy Vitamin D (nmol/L) | ||
|---|---|---|---|---|---|
| Calcium (mg Ca/g) | Phosphorus (mg P/g) | Calcium (mmol/L) | Phosphorus (mmol/L) | ||
| Control | 134.50 ± 3.59 * | 57.25 ± 1.25 * | 2.31 ± 0.01 | 1.96 ± 0.15 | 28.1 ± 5.16 |
| 13cRA | 121.00 ± 3.25 | 49.29 ± 2.15 | 2.39 ± 0.04 | 1.93 ± 0.26 | 20.60 ± 3.46 |
| Icariin | 138.80 ± 3.39 * | 57.50 ± 1.94 * | 2.32 ± 0.08 | 2.38 ± 0.18 | 25.08 ± 2.22 |
| Proanthoc. | 147.20 ± 2.15 * | 58.80 ± 0.86 * | 2.43 ± 0.11 | 2.32 ± 0.20 | 23.2 ± 2.11 |
| Alendronate | 136.00 ± 5.17 * | 61.00 ± 2.41 * | 2.46 ± 0.37 | 2.05 ± 0.15 | 22.71 ± 2.32 |
| Groups a | Kidney (X ± SD) | |||
|---|---|---|---|---|
| MDA (nmol/mg of Kidney Proteins) | GSH (µg/mg of Kidney Proteins) | SOD (U/mg of Kidney Proteins) | CAT (U/mg of Kidney Proteins) | |
| Control | 4.09 ± 1.45 * | 8.46 ± 0.22 | 4.07 ± 1.17 | 21.99 ± 3.85 * |
| 13cRA | 11.49 ± 3.09 ♦ | 3.60 ± 0.64 ♦ | 3.94 ± 1.28 | 8.93 ± 1.78 ♦ |
| Icariin | 5.75 ± 1.60 | 6.38 ± 2.06 | 3.51 ± 1.67 | 18.40 ± 4.27 * |
| Proanthoc. | 4.63 ± 0.78 * | 7.48 ± 2.11 | 3.04 ± 2.02 | 38.38 ± 5.94 * |
| Alendronate | 9.84 ± 2.39 | 10.63 ± 1.05 ** | 7.74 ± 3.02 * | 52.39 ± 6.92 **◊ |
| Groups a | Liver (X±SD) | |||
|---|---|---|---|---|
| MDA (nmol/mg of Liver Proteins) | GSH (µg/mg of Liver Proteins) | SOD (U/mg of Liver Proteins) | CAT (U/mg of Liver Proteins) | |
| Control | 10.22 ± 1.37 * | 8.89 ± 1.41 | 3.67 ± 1.68 | 4.98 ± 1.02 |
| 13cRA | 14.05 ± 1.53 ♦ | 5.64 ± 1.09 | 1.66 ± 0.32 | 4.66 ± 1.49 |
| Icariin | 13.02 ± 1.89 | 9.83 ± 2.36 * | 4.24 ± 1.03 * | 4.67 ± 1.35 |
| Proanthoc. | 11.47 ± 2.15 | 5.96 ± 0.75 | 1.63 ± 0.16 ◊ | 4.32 ± 0.20 |
| Alendronate | 18.05 ± 5.17 ♦ | 5.08 ± 2.08 | 1.76 ± 0.35 | 4.07 ± 0.15 |
| Groups a | Phytoestrogenic Activity-Relative Weight of the Uterus (X ± SD) | |||
|---|---|---|---|---|
| Uterine Weight (g) | Increase (%) in Relation to 13cRA | Min. Value | Max. Value | |
| Control | 0.239 ± 0.029 | 19.49 | 0.205 | 0.263 |
| 13cRA | 0.200 ± 0.011 | - | 0.182 | 0.216 |
| Icariin | 0.287 ± 0.035 ** | 43.42 | 0.237 | 0.336 |
| Proanthoc | 0.241 ± 0.031 | 20.91 | 0.213 | 0.283 |
| Alendronate | 0.235 ± 0.018 | 17.43 | 0.223 | 0.250 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oršolić, N.; Nemrava, J.; Jeleč, Ž.; Kukolj, M.; Odeh, D.; Terzić, S.; Fureš, R.; Bagatin, T.; Bagatin, D. The Beneficial Effect of Proanthocyanidins and Icariin on Biochemical Markers of Bone Turnover in Rats. Int. J. Mol. Sci. 2018, 19, 2746. https://doi.org/10.3390/ijms19092746
Oršolić N, Nemrava J, Jeleč Ž, Kukolj M, Odeh D, Terzić S, Fureš R, Bagatin T, Bagatin D. The Beneficial Effect of Proanthocyanidins and Icariin on Biochemical Markers of Bone Turnover in Rats. International Journal of Molecular Sciences. 2018; 19(9):2746. https://doi.org/10.3390/ijms19092746
Chicago/Turabian StyleOršolić, Nada, Johann Nemrava, Željko Jeleč, Marina Kukolj, Dyana Odeh, Svjetlana Terzić, Rajko Fureš, Tomica Bagatin, and Dinko Bagatin. 2018. "The Beneficial Effect of Proanthocyanidins and Icariin on Biochemical Markers of Bone Turnover in Rats" International Journal of Molecular Sciences 19, no. 9: 2746. https://doi.org/10.3390/ijms19092746
APA StyleOršolić, N., Nemrava, J., Jeleč, Ž., Kukolj, M., Odeh, D., Terzić, S., Fureš, R., Bagatin, T., & Bagatin, D. (2018). The Beneficial Effect of Proanthocyanidins and Icariin on Biochemical Markers of Bone Turnover in Rats. International Journal of Molecular Sciences, 19(9), 2746. https://doi.org/10.3390/ijms19092746