Genome-Wide Analysis Elucidates the Role of CONSTANS-like Genes in Stress Responses of Cotton
Abstract
1. Introduction
2. Results
2.1. Gene Identification and Conserved Domain Retrieval
2.2. Multiple Sequence Alignment of Conserved Domains
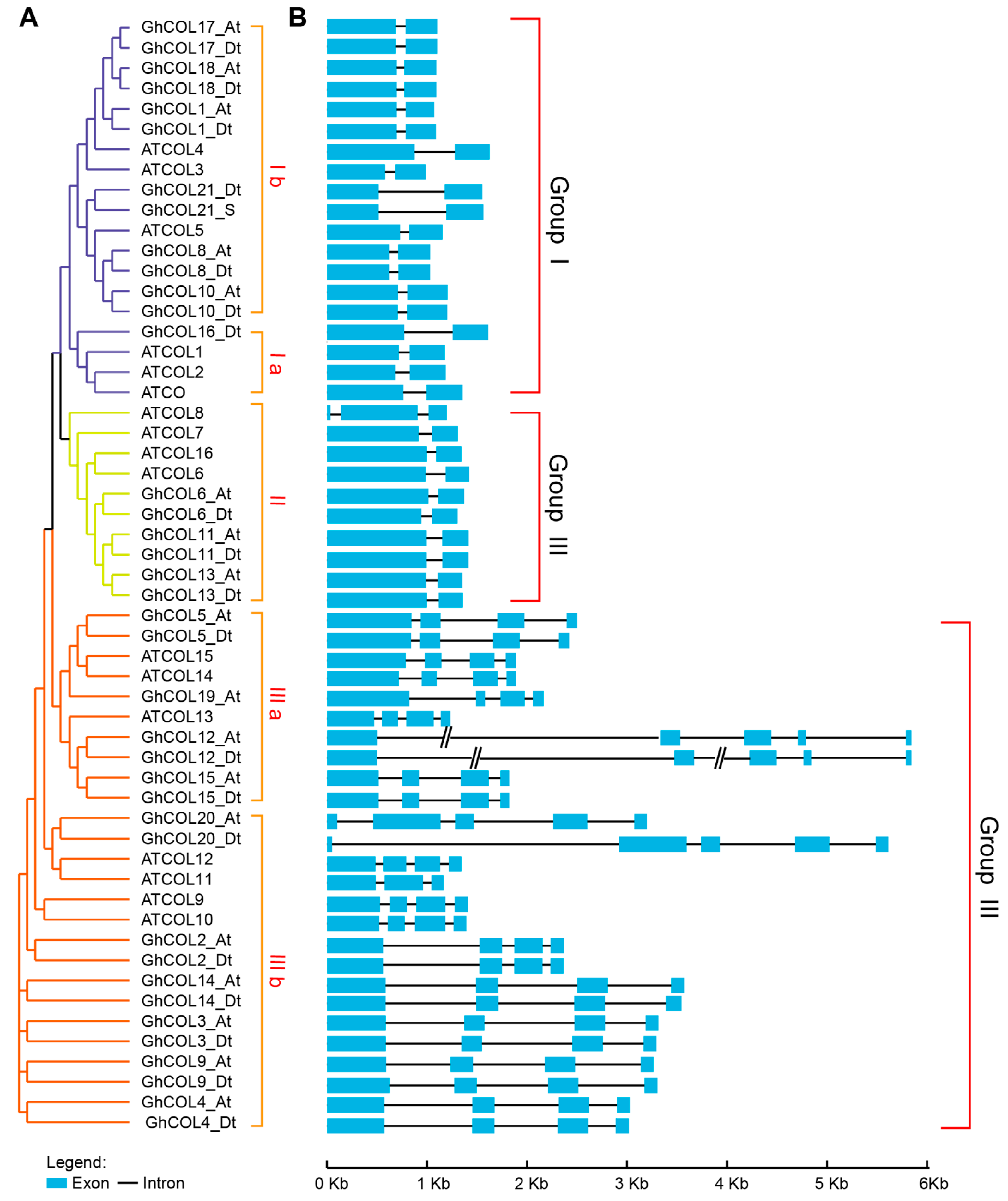
2.3. Phylogenetic Analysis of CO-like Genes among Typical Dicots and Monocots
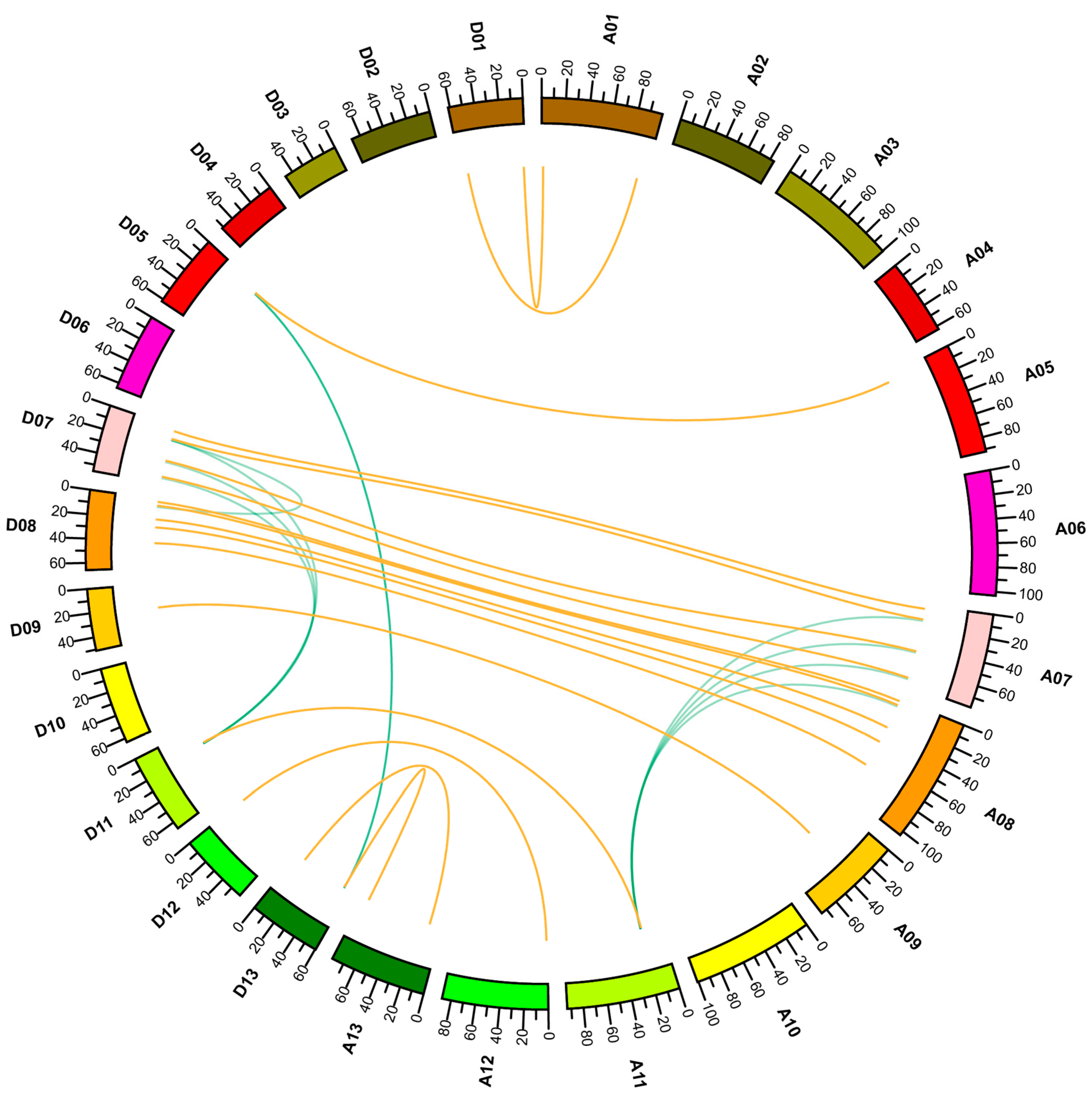
2.4. Gene Expansion and Synteny Analysis
2.5. The Effect of Domestication on CO-like Genes
2.6. Gene Structure Analysis
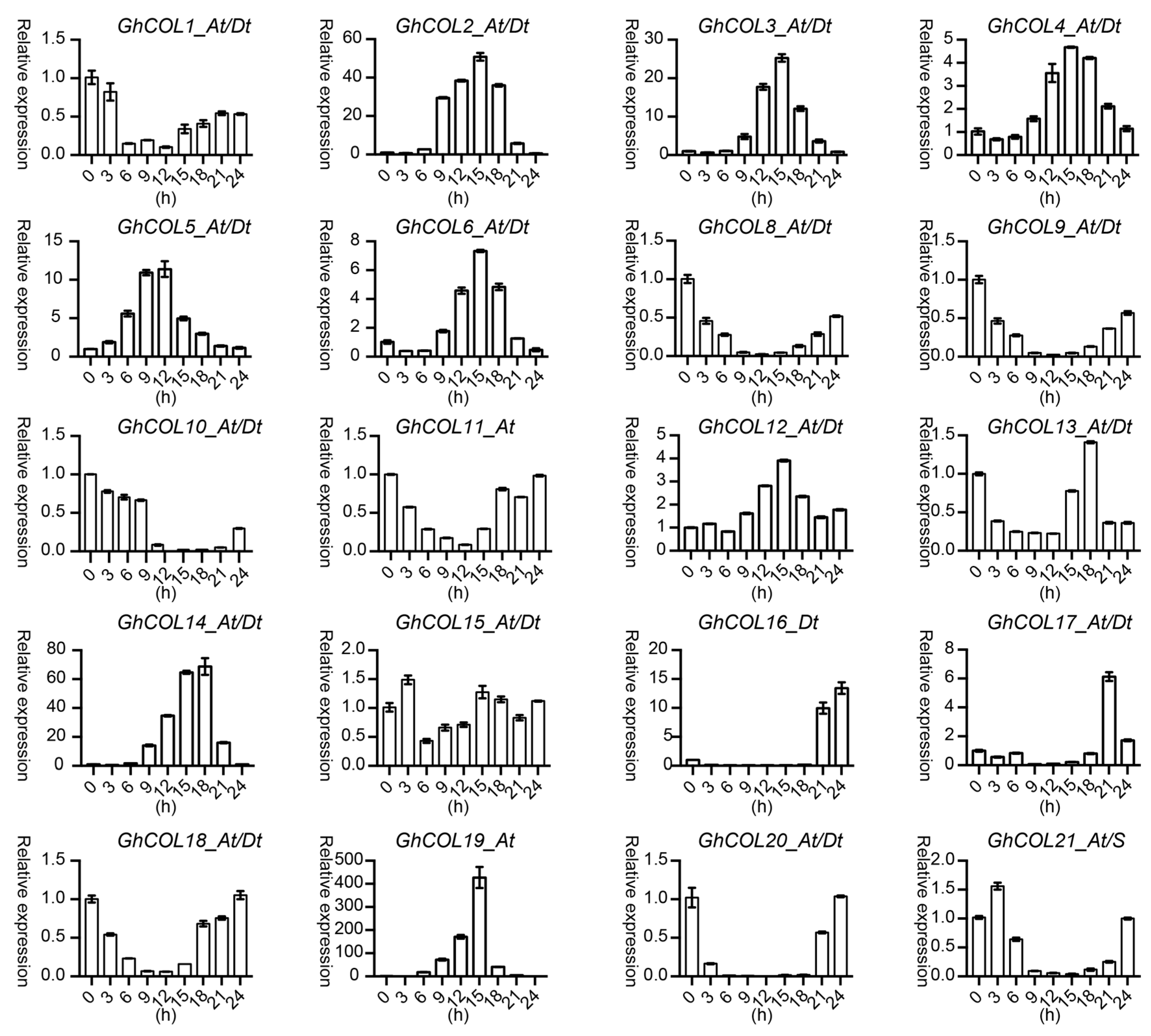
2.7. Gene-Expression Patterns in Different Tissues
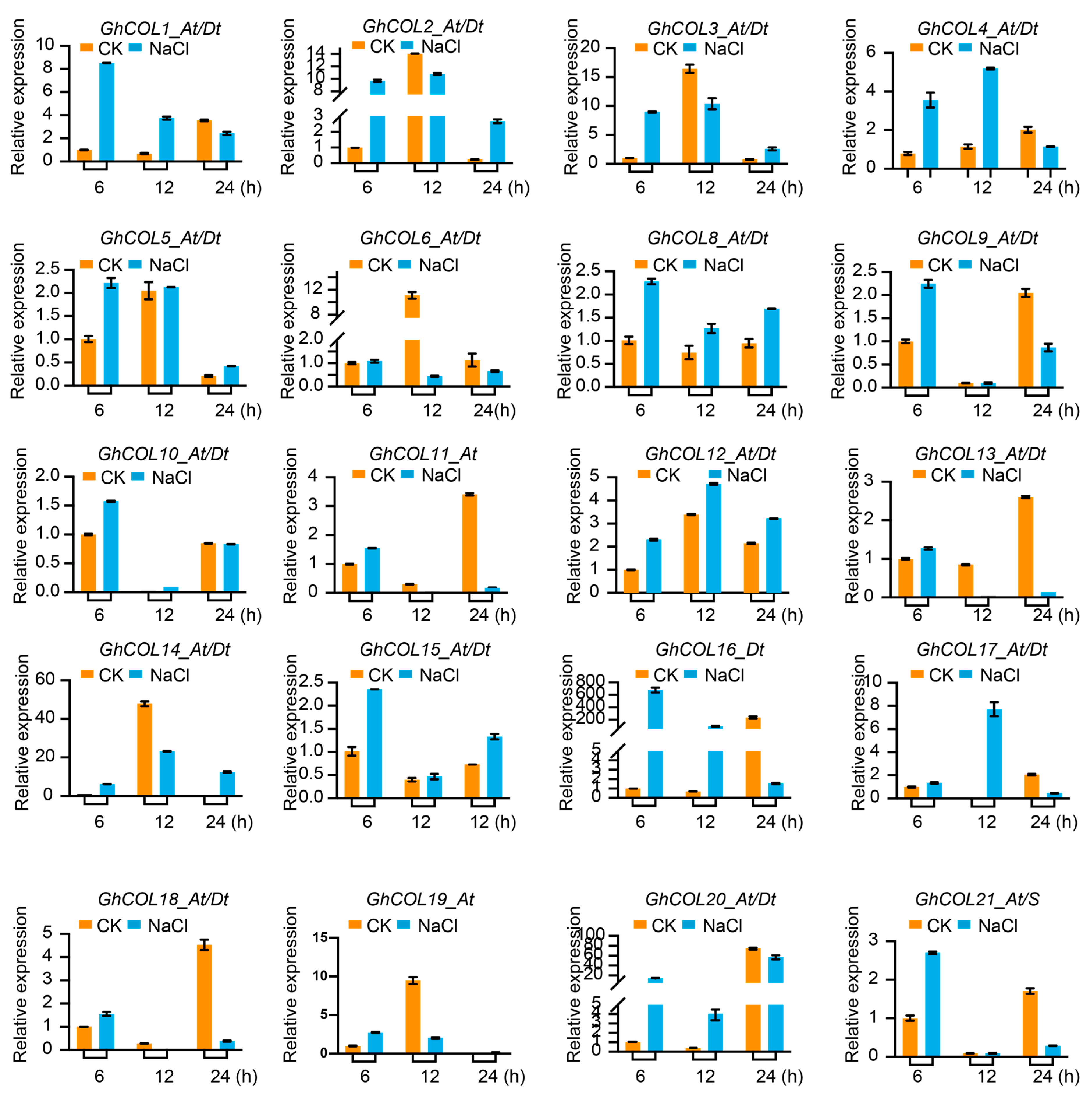
2.8. Response of CO-like Genes to Photoperiod and Stress
3. Discussion
3.1. CO-like Genes Have Experienced Expansion
3.2. CO-like Genes Were Highly Conserved during the Evolution
3.3. CO-like Genes Play Diverse Roles under Stress
4. Materials and Methods
4.1. Gene Identification and Sequence Retrieval
4.2. Conserved Sequence and Phylogenetic Analysis
4.3. Chromosomal Location and Collinearity Analysis
4.4. Calculating Synonymous and Non-Synonymous Substitution Rates
4.5. Annotation and Analysis of Transposable Elements
4.6. Gene Structure Analysis
4.7. Transcriptome Data Analysis and Gene-Expression Heatmap
4.8. Real-Time PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Griffiths, S.; Dunford, R.P.; Coupland, G.; Laurie, D.A. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol. 2003, 131, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Robson, F.; Costa, M.M.R.; Hepworth, S.R.; Vizir, I.; Pin, M.; Reeves, P.H.; Putterill, J.; Coupland, G. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2002, 28, 619–631. [Google Scholar] [CrossRef]
- Torok, M.; Etkin, L.D. Two B or not two B? Overview of the rapidly expanding B-box family of proteins. Differentiation 2001, 67, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. Plant TFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef]
- Putterill, J.J.; Plummer, K.M.; Newcomb, R.D.; Marshall, S.D.G. The Carboxylesterase Gene Family from Arabidopsis thaliana. J. Mol. Evol. 2003, 57, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Yano, M.; Katayose, Y.; Ashikari, M.; Yamanouchi, U.; Monna, L.; Fuse, T.; Baba, T.; Yamamoto, K.; Umehara, Y.; Nagamura, Y.; et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 2000, 12, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Tian, W.; Wei, M.; Yan, W.; He, H.; Zhou, D.; Huang, X.; Li, S.; Ouyang, X. The DTH8-Hd1 module mediates day-length-dependent regulation of rice flowering. Mol. Plant 2017, 10, 948–961. [Google Scholar] [CrossRef] [PubMed]
- Hassidim, M.; Harir, Y.; Yakir, E.; Kron, I.; Green, R.M. Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis. Planta 2009, 230, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-F.; Wang, Z.-Y. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005, 43, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez-Herrera, N.; Trimborn, L.; Menje, M.; Henschel, M.; Robers, L.; Kaufholdt, D.; Hänsch, R.; Adrian, J.; Ponnu, J.; Hoecker, U. The transcription factor COL12 is a substrate of the COP1/SPA E3 ligase and regulates flowering time and plant architecture. Plant Physiol. 2018, 176, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, F.; Dong, S.; Liu, W.; Wang, H.; Chen, Z.; Wang, J. CONSTANS-like 9 (COL9) delays the flowering time in Oryza sativa by repressing the Ehd1 pathway. Biochem. Biophys. Res. Commun. 2016, 479, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wu, F.; Wan, J. Flowering time regulation by the CONSTANS-Like gene OsCOL10. Plant Signal. Behav. 2017, 12, e1267893. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Jin, M.; Wang, J.; Wu, F.; Sheng, P.; Cheng, Z.; Wang, J.; Zheng, X.; Chen, L.; Wang, M.; et al. OsCOL10, a CONSTANS-Like Gene, functions as a flowering time repressor downstream of Ghd7 in rice. Plant Cell. Physiol. 2016, 57, 798–812. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhang, Y.; Zhang, M.; Zhan, X.; Shen, X.; Yu, P.; Chen, D.; Liu, Q.; Sinumporn, S.; Hussain, K.; et al. The rice CONSTANS-like protein OsCOL15 suppresses flowering by promoting Ghd7 and repressing RID1. Biochem. Biophys. Res. Commun. 2018, 495, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zheng, X.M.; Chen, D.; Zhang, Y.; Ma, W.; Zhang, H.; Sun, L.; Yang, Z.; Zhao, C.; Zhan, X.; et al. OsCOL16, encoding a CONSTANS-like protein, represses flowering by up-regulating Ghd7 expression in rice. Plant Sci. 2017, 260, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Hettiarachchi, G.H.C.M.; Deng, X.-W.; Holm, M. Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 2006, 18, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shen, J.; Xu, Y.; Li, X.; Xiao, J.; Xiong, L. Ghd2, a CONSTANS-like gene, confers drought sensitivity through regulation of senescence in rice. J. Exp. Bot. 2016, 67, 5785–5798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, R.; Li, H.; Zhao, T.; Liu, J.; Lin, C.; Liu, B. CONSTANS-LIKE 7 (COL7) is involved in phytochrome B (phyB)-mediated light-quality regulation of auxin homeostasis. Mol. Plant 2014, 7, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Z.; Li, H.; Zhao, X.; Liu, X.; Ortiz, M.; Lin, C.; Liu, B. CONSTANS-LIKE 7 regulates branching and shade avoidance response in Arabidopsis. J. Exp. Bot. 2013, 64, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, S.; Sun, D.; Liu, W.; Gu, F.; Liu, Y.; Guo, T.; Wang, H.; Wang, J.; Chen, Z. CONSTANS-Like 9 (OsCOL9) interacts with receptor for activated C-Kinase 1(OsRACK1) to regulate blast resistance through salicylic acid and ethylene signaling pathways. PLoS ONE 2016, 11, e0166249. [Google Scholar] [CrossRef]
- Yang, Z.; Gong, Q.; Qin, W.; Yang, Z.; Cheng, Y.; Lu, L.; Ge, X.; Zhang, C.; Wu, Z.; Li, F. Genome-wide analysis of WOX genes in upland cotton and their expression pattern under different stresses. BMC Plant Biol. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gong, Q.; Wang, L.; Jin, Y.; Xi, J.; Li, Z.; Qin, W.; Yang, Z.; Lu, L.; Chen, Q.; et al. Genome-wide study of YABBY genes in upland cotton and their expression patterns under different stresses. Front. Genet. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Yang, Z.; Chen, E.; Sun, G.; He, S.; Butt, H.I.; Zhang, C.; Zhang, X.; Yang, Z.; Du, X.; et al. A phi-class glutathione S-transferase gene for Verticillium wilt resistance in Gossypium arboreum identified in a genome-wide association study. Plant Cell Physiol. 2018, 59, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.-H.; Feng, L.; Zheng, Y.; Li, D.-D.; Li, X.-B. Genome-wide functional analysis of cotton (Gossypium hirsutum) in response to drought. PLoS ONE 2013, 8, e80879. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Li, F.; Ye, W.; Wang, J.; Song, G.; Yue, Z.; Cong, L.; Shang, H.; Zhu, S.; et al. The draft genome of a diploid cotton Gossypium raimondii. Nat. Genet. 2012, 44, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.H.; Wendel, J.F.; Gundlach, H.; Guo, H.; Jenkins, J.; Jin, D.; Llewellyn, D.; Showmaker, K.C.; Shu, S.; Udall, J.; et al. Repeated polyploidization of Gossypium genomes and the evolution of spinnable cotton fibres. Nature 2012, 492, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fan, G.; Wang, K.; Sun, F.; Yuan, Y.; Song, G.; Li, Q.; Ma, Z.; Lu, C.; Zou, C.; et al. Genome sequence of the cultivated cotton Gossypium arboreum. Nat. Genet. 2014, 46, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Fan, G.; Lu, C.; Xiao, G.; Zou, C.; Kohel, R.J.; Ma, Z.; Shang, H.; Ma, X.; Wu, J.; et al. Genome sequence of cultivated upland cotton (Gossypium hirsutum TM-1) provides insights into genome evolution. Nat. Biotechnol. 2015, 33, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 33, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Vandepoele, K.; Simillion, C.; Van de Peer, Y. Evidence that rice and other cereals are ancient aneuploids. Plant Cell 2003, 15, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Bennetzen, J.L.; Wang, H. The contributions of transposable elements to the structure, function, and evolution of plant genomes. Annu. Rev. Plant Biol. 2014, 65, 505–530. [Google Scholar] [CrossRef] [PubMed]
- Renny-Byfield, S.; Page, J.T.; Udall, JA.; Sanders, W.S.; Peterson, D.G.; Arick, M.A.; Grover, C.E.; Wendel, J.F. Independent domestication of two old world cotton species. Genome. Biol. Evol. 2016, 8, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Shimamoto, K. Heading date 1 (Hd1), an ortholog of Arabidopsis CONSTANS, is a possible target of human selection during domestication to diversify flowering times of cultivated rice. Genes Genet. Syst. 2011, 86, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tu, L.; Lin, M.; Lin, Z.; Wang, P.; Yang, Q.; Ye, Z.; Shen, C.; Li, J.; Zhang, L.; et al. Asymmetric subgenome selection and cis-regulatory divergence during cotton domestication. Nat. Genet. 2017, 49, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, I.R. Using bioinformatic and phylogenetic approaches to classify transposable elements and understand their complex evolutionary histories. Mob. DNA 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jung, S.; Cheng, C.-H.; Ficklin, S.P.; Lee, T.; Zheng, P.; Jones, D.; Percy, R.G.; Main, D. CottonGen: A genomics, genetics and breeding database for cotton research. Nucleic Acids Res. 2014, 42, D1229–D1236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed]



| Paralogous Pairs | Ka | Ks | Ka/Ks |
|---|---|---|---|
| GhCOL1_At-GhCOL1_Dt | 0.03 | 0.07 | 0.526 |
| GhCOL2_At-GhCOL2_Dt | 0.00 | 0.02 | 0.149 |
| GhCOL3_At-GhCOL3_Dt | 0.01 | 0.04 | 0.199 |
| GhCOL4_At-GhCOL4_Dt | 0.01 | 0.05 | 0.275 |
| GhCOL5_At-GhCOL5_Dt | 0.01 | 0.04 | 0.365 |
| GhCOL6_At_GhCOL6_Dt | 0.01 | 0.03 | 0.550 |
| GhCOL8_At_GhCOL8_Dt | 0.01 | 0.04 | 0.298 |
| GhCOL9_At_GhCOL9_Dt | 0.00 | 0.06 | 0.054 |
| GhCOL10_At-GhCOL10_Dt | 0.01 | 0.06 | 0.159 |
| GhCOL11_At-GhCOL11_Dt | 0.03 | 0.05 | 0.561 |
| GhCOL12_At-GhCOL12_Dt | 0.01 | 0.02 | 0.552 |
| GhCOL13_At-GhCOL13_Dt | 0.01 | 0.03 | 0.365 |
| GhCOL14_At-GhCOL14_Dt | 0.01 | 0.06 | 0.131 |
| GhCOL15_At-GhCOL15_Dt | 0.01 | 0.04 | 0.276 |
| GhCOL17_At-GhCOL17_Dt | 0.01 | 0.03 | 0.205 |
| GhCOL18_At-GhCOL18_Dt | 0.01 | 0.05 | 0.274 |
| GhCOL20_At-GhCOL20_Dt | 0.04 | 0.05 | 0.993 |
| GhCOL21_S-GhCOL21_Dt | 0.04 | 0.07 | 0.515 |
| GhCOL3_Dt-GhCOL14_Dt | 0.09 | 0.41 | 0.213 |
| GhCOL4_Dt-GhCOL14_Dt | 0.09 | 0.40 | 0.223 |
| GhCOL9_Dt-GhCOL2_Dt | 0.11 | 0.53 | 0.211 |
| GhC0L18_Dt-GhCOL17_Dt | 0.09 | 0.73 | 0.119 |
| GhCOL2_Dt-GhCOL14_Dt | 0.09 | 0.53 | 0.175 |
| GhCOL3_At-GhCOL14_At | 0.08 | 0.41 | 0.204 |
| GhCOL2_At-GhCOL14_At | 0.09 | 0.45 | 0.210 |
| GhCOL9_At-GhCOL14_At | 0.09 | 0.45 | 0.210 |
| GhCOL4_At-GhCOL14_At | 0.09 | 0.39 | 0.230 |
| Type | Number of Elements | Length Occupied (bp) | Percentage of Sequence (%) | Number of Elements | Length Occupied (bp) | Percentage of Sequence (%) |
|---|---|---|---|---|---|---|
| 2000 bp region | 10,000 bp region | |||||
| DNA transponsons | 1 | 283 | 0.1 | 6 | 109 | 0.04 |
| CMC-EnSpm | 0 | 0 | 0 | 4 | 1172 | 0.42 |
| MULE-MuDR | 0 | 0 | 0 | 0 | 0 | 0 |
| PIF-Harbinger | 1 | 283 | 0.1 | 1 | 283 | 0.1 |
| TcMar-Pogo | 0 | 0 | 0 | 0 | 0 | 0 |
| hAT | 0 | 0 | 0 | 0 | 0 | 0 |
| hAT-Ac | 0 | 0 | 0 | 0 | 0 | 0 |
| hAT-Tag1 | 0 | 0 | 0 | 0 | 0 | 0 |
| hAT-Tip100 | 0 | 0 | 0 | 0 | 0 | 0 |
| hAT-Charlie | 0 | 0 | 0 | 1 | 32 | 0.01 |
| Retroelements | 20 | 6199 | 2.23 | 51 | 25,761 | 9.27 |
| LINE: | 0 | 0 | 0 | 0 | 0 | 0 |
| L1 | 4 | 1085 | 0.39 | 4 | 1085 | 0.39 |
| LTR: | 8 | 2557 | 0.92 | 0 | 0 | 0 |
| Caulimovirus | 0 | 0 | 0 | 0 | 0 | 0 |
| Copia | 8 | 2557 | 0.92 | 36 | 19,874 | 7.15 |
| Gypsy | 0 | 0 | 0 | 11 | 4802 | 1.73 |
| RC: | 0 | 0 | 0 | 0 | 0 | 0 |
| Helitron | 0 | 0 | 0 | 0 | 0 | 0 |
| Low_complexity | 36 | 1880 | 0.68 | 117 | 6419 | 2.31 |
| Simple_repeat | 144 | 6167 | 2.22 | 481 | 20,564 | 7.4 |
| Unspecified | 24 | 3581 | 1.29 | 113 | 38,583 | 13.88 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, W.; Yu, Y.; Jin, Y.; Wang, X.; Liu, J.; Xi, J.; Li, Z.; Li, H.; Zhao, G.; Hu, W.; et al. Genome-Wide Analysis Elucidates the Role of CONSTANS-like Genes in Stress Responses of Cotton. Int. J. Mol. Sci. 2018, 19, 2658. https://doi.org/10.3390/ijms19092658
Qin W, Yu Y, Jin Y, Wang X, Liu J, Xi J, Li Z, Li H, Zhao G, Hu W, et al. Genome-Wide Analysis Elucidates the Role of CONSTANS-like Genes in Stress Responses of Cotton. International Journal of Molecular Sciences. 2018; 19(9):2658. https://doi.org/10.3390/ijms19092658
Chicago/Turabian StyleQin, Wenqiang, Ya Yu, Yuying Jin, Xindong Wang, Ji Liu, Jianping Xi, Zhi Li, Huiqin Li, Ge Zhao, Wei Hu, and et al. 2018. "Genome-Wide Analysis Elucidates the Role of CONSTANS-like Genes in Stress Responses of Cotton" International Journal of Molecular Sciences 19, no. 9: 2658. https://doi.org/10.3390/ijms19092658
APA StyleQin, W., Yu, Y., Jin, Y., Wang, X., Liu, J., Xi, J., Li, Z., Li, H., Zhao, G., Hu, W., Chen, C., Li, F., & Yang, Z. (2018). Genome-Wide Analysis Elucidates the Role of CONSTANS-like Genes in Stress Responses of Cotton. International Journal of Molecular Sciences, 19(9), 2658. https://doi.org/10.3390/ijms19092658





