Dme-Hsa Disease Database (DHDD): Conserved Human Disease-Related miRNA and Their Targeting Genes in Drosophila melanogaster
Abstract
1. Introduction
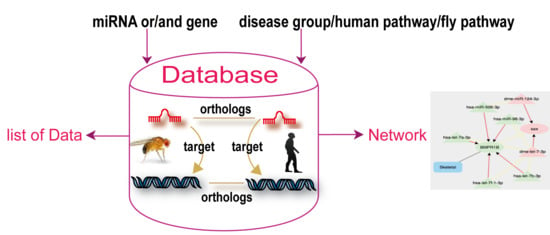
2. Database Description
2.1. Data Sources
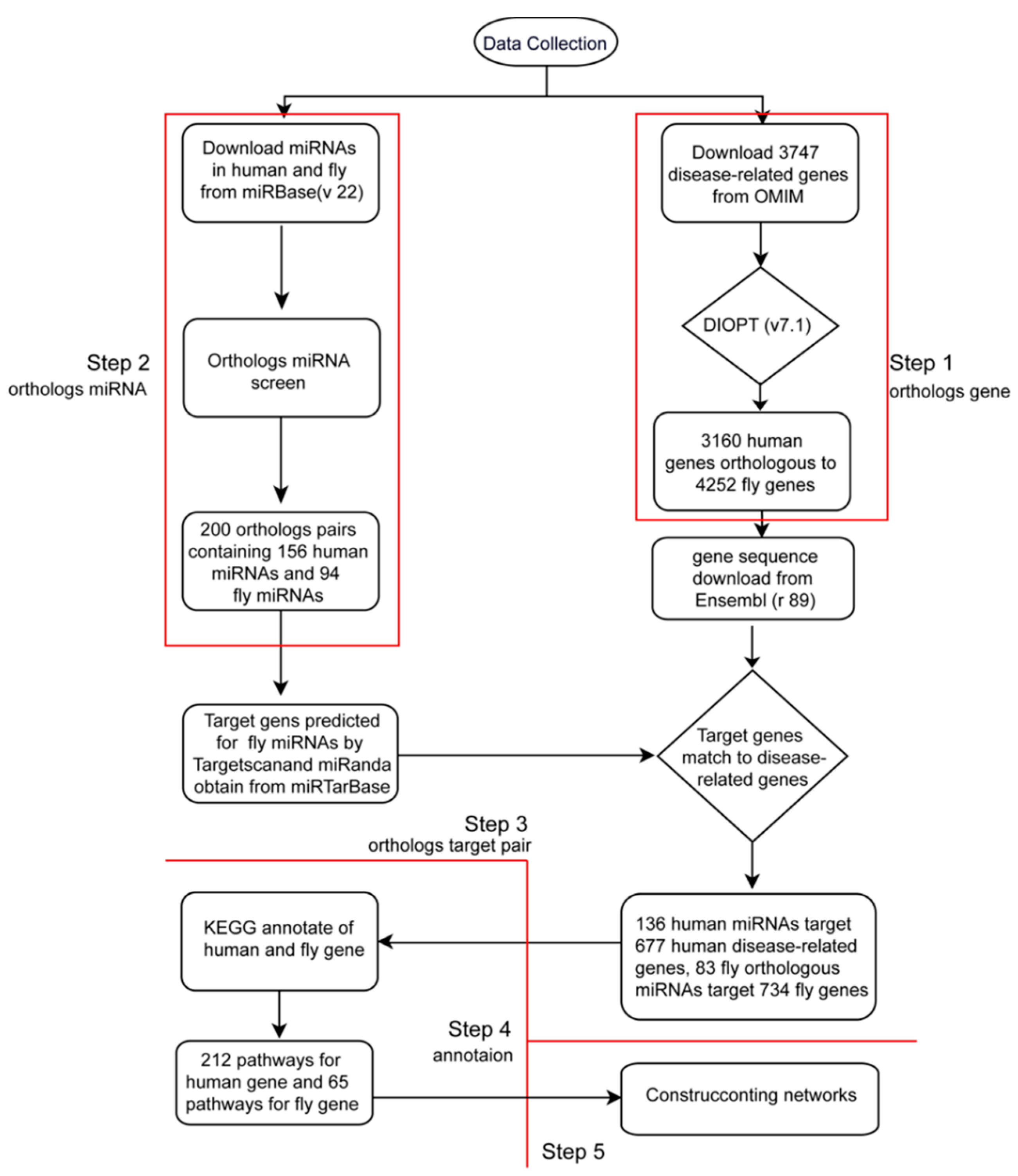
2.2. Database Construction
2.3. Database Content
2.3.1. Identification of Orthologs of Human Disease-Related Genes in D. melanogaster
2.3.2. Identification of Orthologs of Human Disease-Related miRNA in D. melanogaster
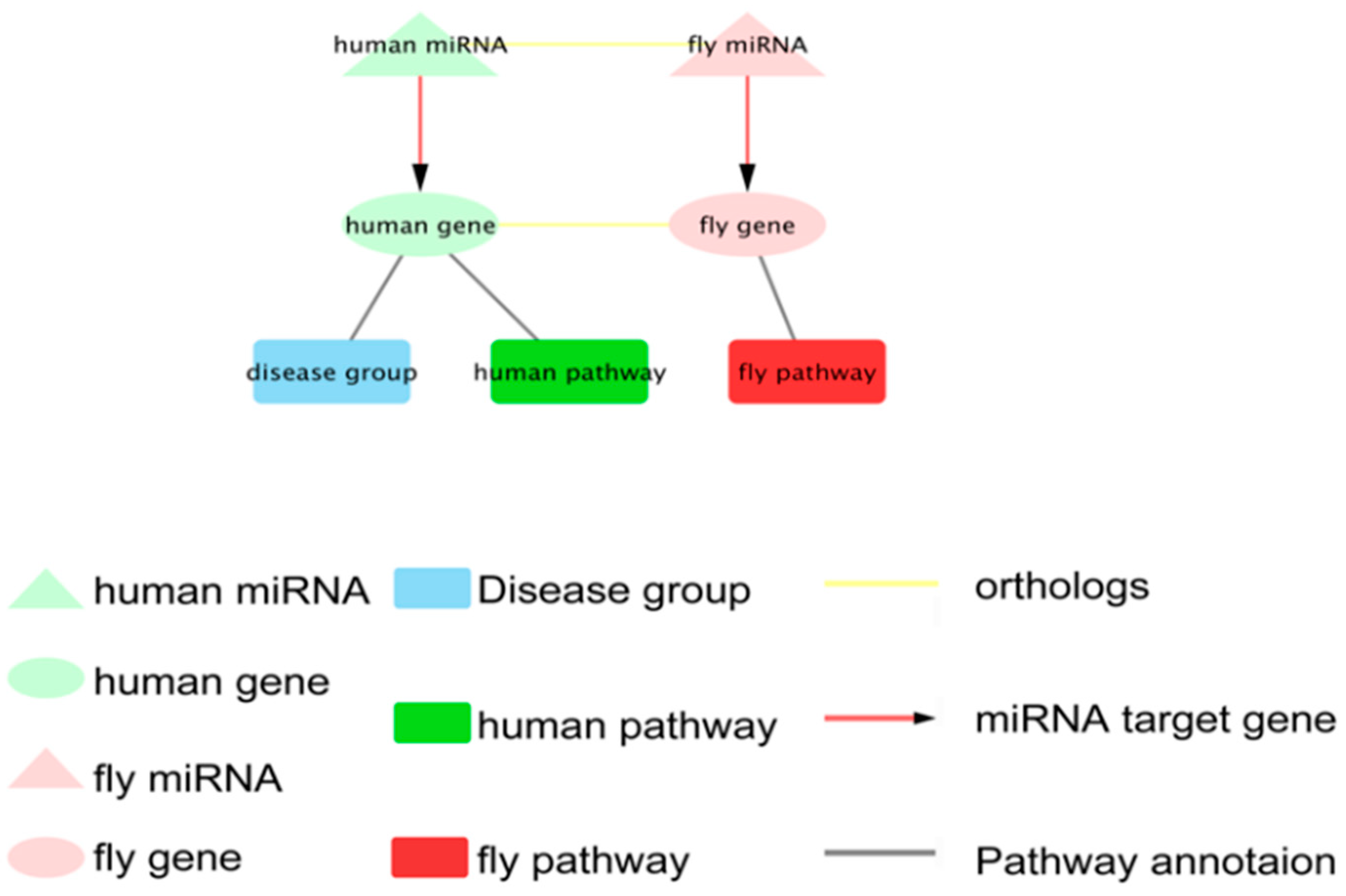
2.3.3. Identification the Orthologous Pairs of miRNA to Gene (OPMG) in Human and D. melanogaster
2.3.4. Pathway Annotation of Ortholog miRNA Target Genes between Human and D. melanogaster
2.3.5. Construction of Visual Networks
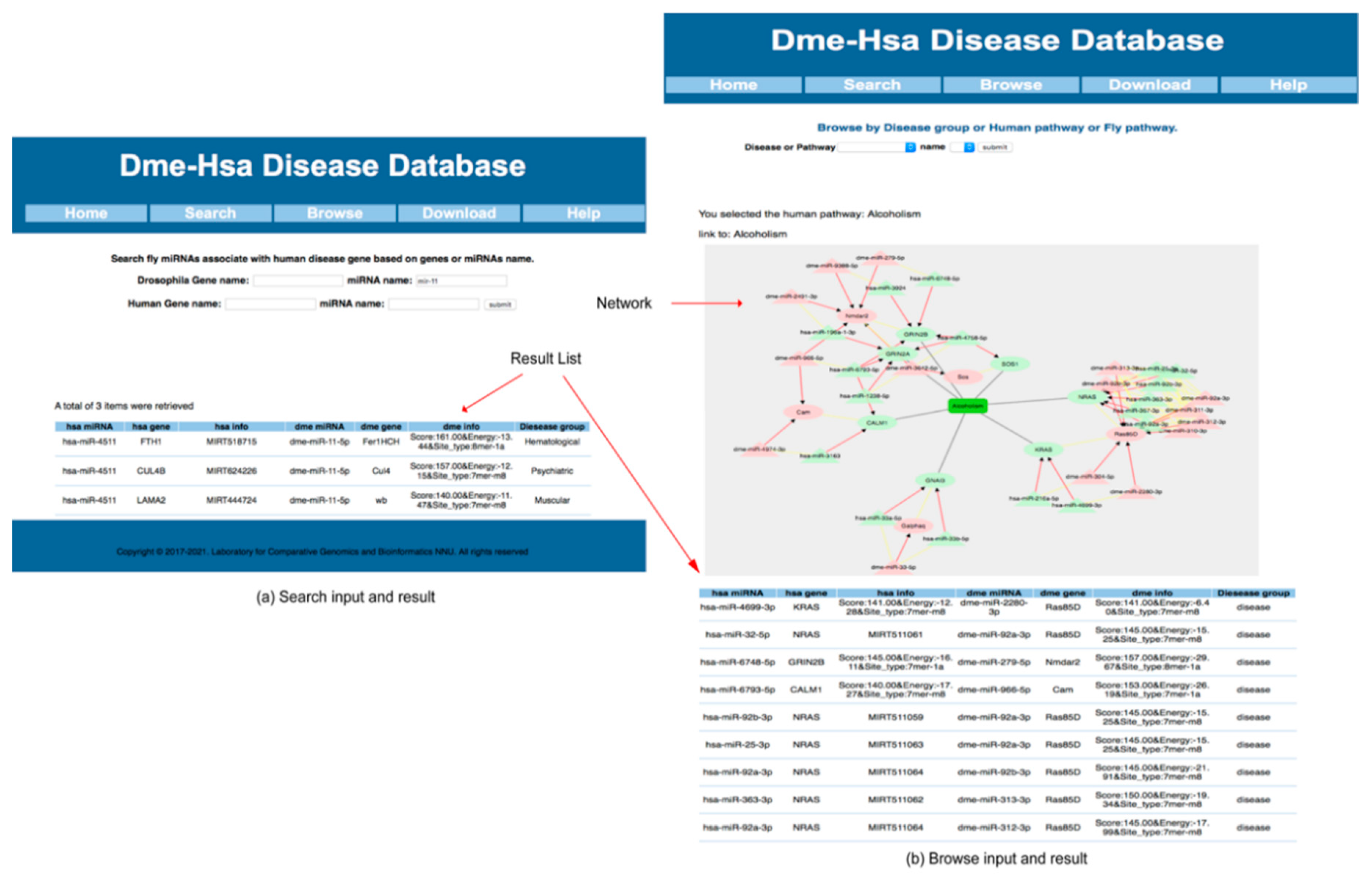
2.3.6. Web Server Introduction and Use
3. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Read, R.D.; Goodfellow, P.J.; Mardis, E.R.; Novak, N.; Armstrong, J.R.; Cagan, R.L. A Drosophila model of multiple endocrine neoplasia type 2. Genetics 2005, 171, 1057–1081. [Google Scholar] [CrossRef] [PubMed]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.A. Receptor tyrosine kinases: Specific outcomes from general signals. Cell 2000, 103, 13–15. [Google Scholar] [CrossRef]
- Lee, L.A.; Orr-Weaver, T.L. Regulation of cell cycles in Drosophila development: Intrinsic and extrinsic cues. Annu. Rev. Genet. 2003, 37, 545–578. [Google Scholar] [CrossRef] [PubMed]
- Karim, F.D.; Chang, H.C.; Therrien, M.; Wassarman, D.A.; Laverty, T.; Rubin, G.M. A screen for genes that function downstream of Ras1 during Drosophila eye development. Genetics 1996, 143, 315–329. [Google Scholar] [PubMed]
- Gao, X.; Neufeld, T.P.; Pan, D. Drosophila PTEN regulates cell growth and proliferation through PI3K-dependent and-independent pathways. Dev. Biol. 2000, 221, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D. Using Drosophila as a model insect. Nat. Rev. Genet. 2000, 1, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Read, R.D. Drosophila melanogaster as a model system for human brain cancers. Glia 2011, 59, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Hirth, F. Drosophila melanogaster in the study of human neurodegeneration. CNS Neurol. Disord. Drug Targets 2010, 9, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.J.; Amrein, H.; Izatt, J.A.; Choma, M.A.; Reedy, M.C.; Rockman, H.A. Drosophila as a model for the identification of genes causing adult human heart disease. Proc. Natl. Acad. Sci. USA 2006, 103, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Tipping, M.; Perrimon, N. Drosophila as a model for context-dependent tumorigenesis. J. Cell. Physiol. 2014, 229, 27–33. [Google Scholar] [PubMed]
- Herz, H.M.; Bergmann, A. Genetic analysis of ESCRT function in Drosophila: A tumour model for human Tsg101. Biochem. Soc. Trans. 2009, 37, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Vogel, H. Drosophila Models of Neurodegenerative Diseases. Annu. Rev. Pathol. 2009, 4, 315–342. [Google Scholar] [CrossRef] [PubMed]
- Alfa, R.W.; Kim, S.K. Using Drosophila to discover mechanisms underlying type 2 diabetes. Dis. Models Mech. 2016, 9, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kounatidis, I.; Ligoxygakis, P. Drosophila as a model to study the role of blood cells in inflammation, innate immunity and cancer. Front. Cell. Infect. Microbiol. 2014, 3, 113. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.D.; Thummel, C.S. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007, 6, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; Reiter, L.T.; Bier, E.; Gribskov, M. Homophila: Human disease gene cognates in Drosophila. Nucleic Acids Res. 2002, 30, 149–151. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.P.; Westerlund, I.; Sonnhammer, E.L. OrthoDisease: A database of human disease orthologs. Hum. Mutat. 2004, 24, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Flockhart, I.; Vinayagam, A.; Bergwitz, C.; Berger, B.; Perrimon, N.; Mohr, S.E. An integrative approach to ortholog prediction for disease-focused and other functional studies. BMC Bioinform. 2011, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar] [PubMed]
- Naeini, M.M.; Ardekani, A.M. Noncoding RNAs and Cancer. Avicenna J. Med. Biotechnol. 2009, 1, 55–70. [Google Scholar] [PubMed]
- Zhao, W.G.; Yu, S.N.; Lu, Z.H.; Ma, Y.H.; Gu, Y.M.; Chen, J. The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting KRAS. Carcinogenesis 2010, 31, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, S.P.; Zhao, Y.H. MicroRNA-143/-145 in Cardiovascular Diseases. BioMed Res. Int. 2015, 2015, 531740. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Landreh, M.; Cao, K.; Abe, M.; Hendriks, G.J.; Kennerdell, J.R.; Zhu, Y.; Wang, L.S.; Bonini, N.M. The microRNA miR-34 modulates ageing and neurodegeneration in Drosophila. Nature 2012, 482, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, Y. A survey on database resources for microRNA-disease relationships. Brief. Funct. Genom. 2017, 16, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, Y.; Hao, Y.; Juan, L.; Teng, M.; Zhang, X.; Li, M.; Wang, G.; Liu, Y. miR2Disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009, 37, D98–D104. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiu, C.; Tu, J.; Geng, B.; Yang, J.; Jiang, T.; Cui, Q. HMDD v2.0: A database for experimentally supported human microRNA and disease associations. Nucleic Acids Res. 2014, 42, D1070–D1074. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Z.R.; Cheng, F.X.; Li, W.H.; Liu, G.X.; Tang, Y. Computational prediction of microRNA networks incorporating environmental toxicity and disease etiology. Sci. Rep. 2014, 4, 5576. [Google Scholar] [CrossRef] [PubMed]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.; Akanni, W.; Amode, M.R.; Barrell, D.; Billis, K.; Carvalho-Silva, D.; Cummins, C.; Clapham, P.; Fitzgerald, S.; Gil, L.; et al. Ensembl 2016. Nucleic Acids Res. 2016, 44, D710–D716. [Google Scholar] [CrossRef] [PubMed]
- Marygold, S.J.; Crosby, M.A.; Goodman, J.L.; FlyBase Consortium. Using FlyBase, a Database of Drosophila Genes and Genomes. Methods Mol. Biol. 2016, 1478, 1–31. [Google Scholar] [PubMed]
- Amberger, J.S.; Bocchini, C.A.; Schiettecatte, F.; Scott, A.F.; Hamosh, A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015, 43, D789–D798. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.I.; Cusick, M.E.; Valle, D.; Childs, B.; Vidal, M.; Barabasi, A.L. The human disease network. Proc. Natl. Acad. Sci. USA 2007, 104, 8685–8690. [Google Scholar] [CrossRef] [PubMed]
- Ruby, J.G.; Stark, A.; Johnston, W.K.; Kellis, M.; Bartel, D.P.; Lai, E.C. Evolution, biogenesis, expression, and target predictions of a substantially expanded set of Drosophila microRNAs. Genome Res. 2007, 17, 1850–1864. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Nam, J.W.; Farh, K.K.; Chiang, H.R.; Shkumatava, A.; Bartel, D.P. Expanding the microRNA targeting code: Functional sites with centered pairing. Mol. Cell 2010, 38, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.org resource: Targets and expression. Nucleic Acids Res. 2008, 36, D149–D153. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-H.; Chang, N.-W.; Shrestha, S.; Hsu, S.-D.; Lin, Y.-L.; Lee, W.-H.; Yang, C.-D.; Hong, H.-C.; Wei, T.-Y.; Tu, S.-J. miRTarBase 2016: Updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res. 2015, 44, D239–D247. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.D.; Cagan, R.L. Drosophila Lung Cancer Models Identify Trametinib plus Statin as Candidate Therapeutic. Cell Rep. 2016, 14, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.K.; Lee, S.; Park, S.H.; Lee, J.H.; Han, S.Y.; Kim, S.T.; Kim, Y.K.; Jeon, S.; Koo, B.S.; Cho, K.S. Inhibition of JNK/dFOXO pathway and caspases rescues neurological impairments in Drosophila Alzheimer’s disease model. Biochem. Biophys. Res. Commun. 2012, 419, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Siddique, Y.H.; Naz, F.; Jyoti, S.; Ali, F.; Fatima, A.; Khanam, S. Protective effect of Geraniol on the transgenic Drosophila model of Parkinson’s disease. Environ. Toxicol. Pharmacol. 2016, 43, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Fortini, M.E.; Skupski, M.P.; Boguski, M.S.; Hariharan, I.K. A survey of human disease gene counterparts in the Drosophila genome. J. Cell Biol. 2000, 150, F23–F30. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Crowther, D.C. Functional genomics in Drosophila models of human disease. Brief. Funct. Genom. 2012, 11, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Wen, S.; Chen, D.; Jin, P. Small regulatory RNAs in neurodevelopmental disorders. Hum. Mol. Genet. 2009, 18, R18–R26. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wu, J.; Yuan, L. MicroRNA expression analysis of adult-onset Drosophila Alzheimer’s disease model. Curr. Alzheimer Res. 2014, 11, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wu, J.; Zhang, D.; Wan, C.; Yuan, L. The Role of miR-124 in Drosophila Alzheimer's Disease Model by Targeting Delta in Notch Signaling Pathway. Curr. Mol. Med. 2015, 15, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, I.; Alaniz, M.E.; Renwick, N.; Cela, C.; Fulga, T.A.; Van Vactor, D.; Tuschl, T.; Clark, L.N.; Shelanski, M.L.; McCabe, B.D.; et al. Dysregulation of microRNA-219 promotes neurodegeneration through post-transcriptional regulation of tau. J. Clin. Investig. 2015, 125, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, A.; Hong, S.M.; Hruban, R.H.; Goggins, M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin. Cancer Res. 2012, 18, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Rachagani, S.; Macha, M.A.; Menning, M.S.; Dey, P.; Pai, P.; Smith, L.M.; Mo, Y.Y.; Batra, S.K. Changes in microRNA (miRNA) expression during pancreatic cancer development and progression in a genetically engineered KrasG12D; Pdx1-Cre mouse (KC) model. Oncotarget 2015, 6, 40295–40309. [Google Scholar] [CrossRef] [PubMed]
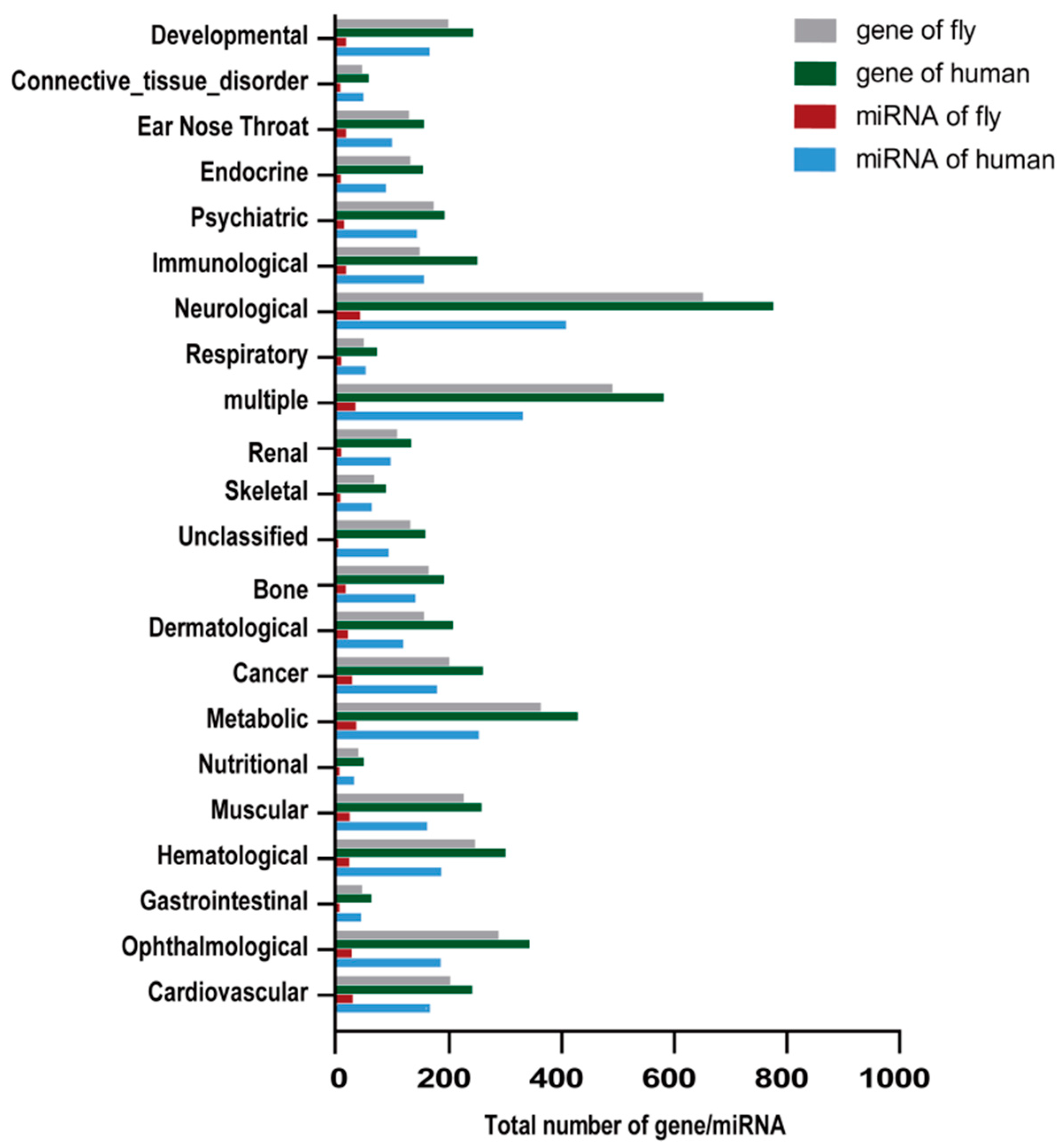
| Disease | Human | Fly | ||
|---|---|---|---|---|
| gene | miRNA | gene | miRNA | |
| Bone | 10 | 35 | 11 | 13 |
| Cancer | 15 | 54 | 16 | 31 |
| Cardiovascular | 6 | 10 | 5 | 7 |
| Connective tissue disorder | 5 | 20 | 7 | 13 |
| Dermatological | 3 | 10 | 3 | 10 |
| Developmental | 2 | 3 | 3 | 3 |
| Ear Nose Throat | 6 | 25 | 7 | 11 |
| Endocrine | 5 | 14 | 7 | 7 |
| Gastrointestinal | 2 | 4 | 4 | 4 |
| Hematological | 2 | 3 | 2 | 2 |
| Immunological | 9 | 31 | 9 | 12 |
| Metabolic | 8 | 33 | 7 | 11 |
| Muscular | 11 | 37 | 14 | 17 |
| Neurological | 25 | 55 | 27 | 36 |
| Nutritional | 1 | 1 | 1 | 1 |
| Ophthalmological | 9 | 25 | 11 | 21 |
| Psychiatric | 8 | 19 | 8 | 11 |
| Renal | 6 | 7 | 6 | 6 |
| Skeletal | 1 | 5 | 1 | 2 |
| Unclassified | 4 | 19 | 4 | 15 |
| multiple | 28 | 53 | 30 | 31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, G.; Sun, L.; Qin, S.; Li, R.; Chen, L.; Jin, P.; Ma, F. Dme-Hsa Disease Database (DHDD): Conserved Human Disease-Related miRNA and Their Targeting Genes in Drosophila melanogaster. Int. J. Mol. Sci. 2018, 19, 2642. https://doi.org/10.3390/ijms19092642
Wei G, Sun L, Qin S, Li R, Chen L, Jin P, Ma F. Dme-Hsa Disease Database (DHDD): Conserved Human Disease-Related miRNA and Their Targeting Genes in Drosophila melanogaster. International Journal of Molecular Sciences. 2018; 19(9):2642. https://doi.org/10.3390/ijms19092642
Chicago/Turabian StyleWei, Guanyun, Lianjie Sun, Shijie Qin, Ruimin Li, Liming Chen, Ping Jin, and Fei Ma. 2018. "Dme-Hsa Disease Database (DHDD): Conserved Human Disease-Related miRNA and Their Targeting Genes in Drosophila melanogaster" International Journal of Molecular Sciences 19, no. 9: 2642. https://doi.org/10.3390/ijms19092642
APA StyleWei, G., Sun, L., Qin, S., Li, R., Chen, L., Jin, P., & Ma, F. (2018). Dme-Hsa Disease Database (DHDD): Conserved Human Disease-Related miRNA and Their Targeting Genes in Drosophila melanogaster. International Journal of Molecular Sciences, 19(9), 2642. https://doi.org/10.3390/ijms19092642