Two Different Strategies to Enhance Osseointegration in Porous Titanium: Inorganic Thermo-Chemical Treatment Versus Organic Coating by Peptide Adsorption
Abstract
:1. Introduction
- (1)
- (2)
- grafting of an arginine-glycine-aspartic acid (RGD) cell adhesive peptide, derived from a bone extracellular matrix (ECM) [43,44,45,46] on the surface of the scaffold. ECM-derived molecules, such as RGD peptides, are capable of interacting with cell-expressed receptors like integrins, which trigger the biological processes required for an optimal osseointegration [33].
2. Results
2.1. Structure of Porous System
2.2. Mechanical Properties
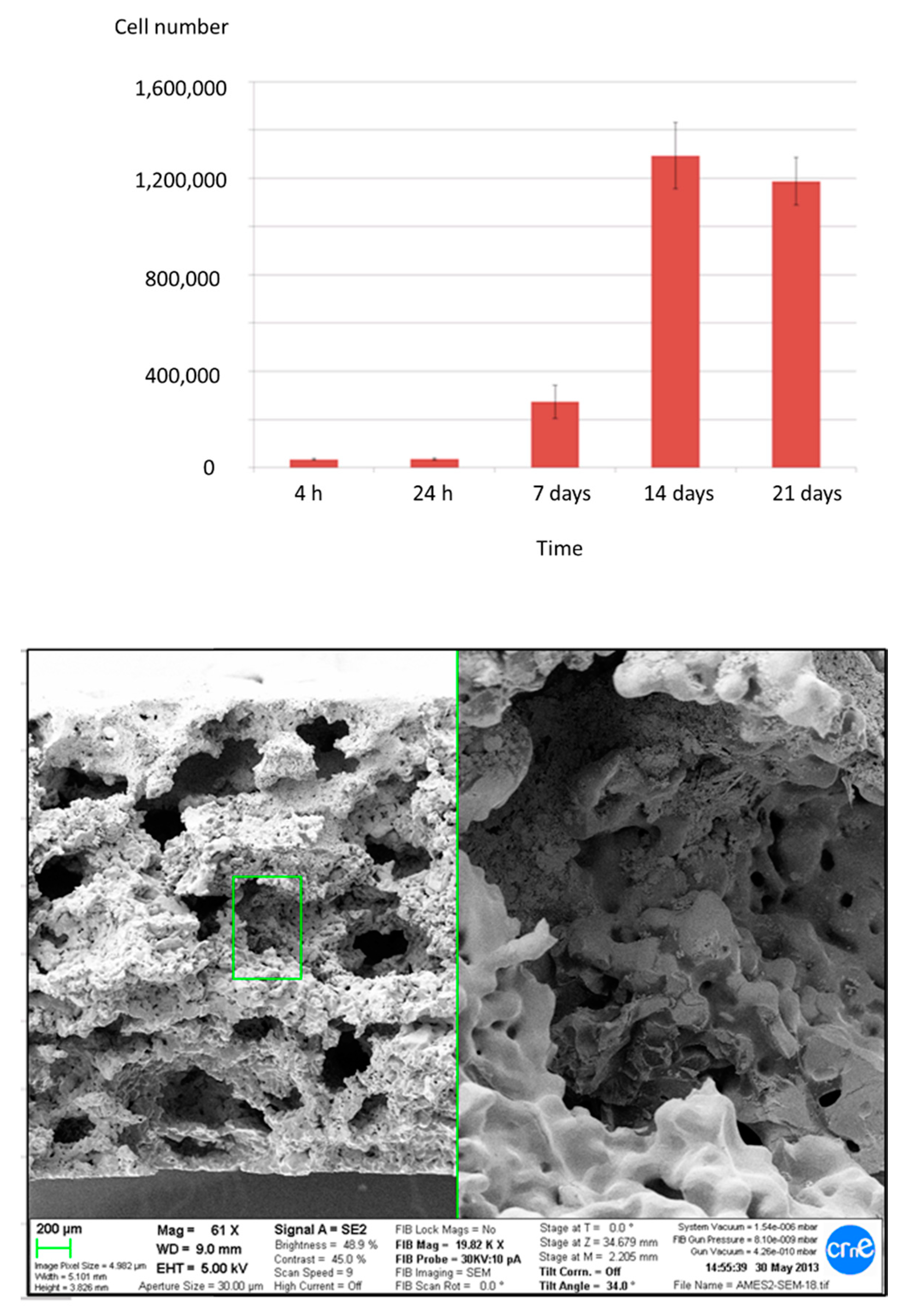
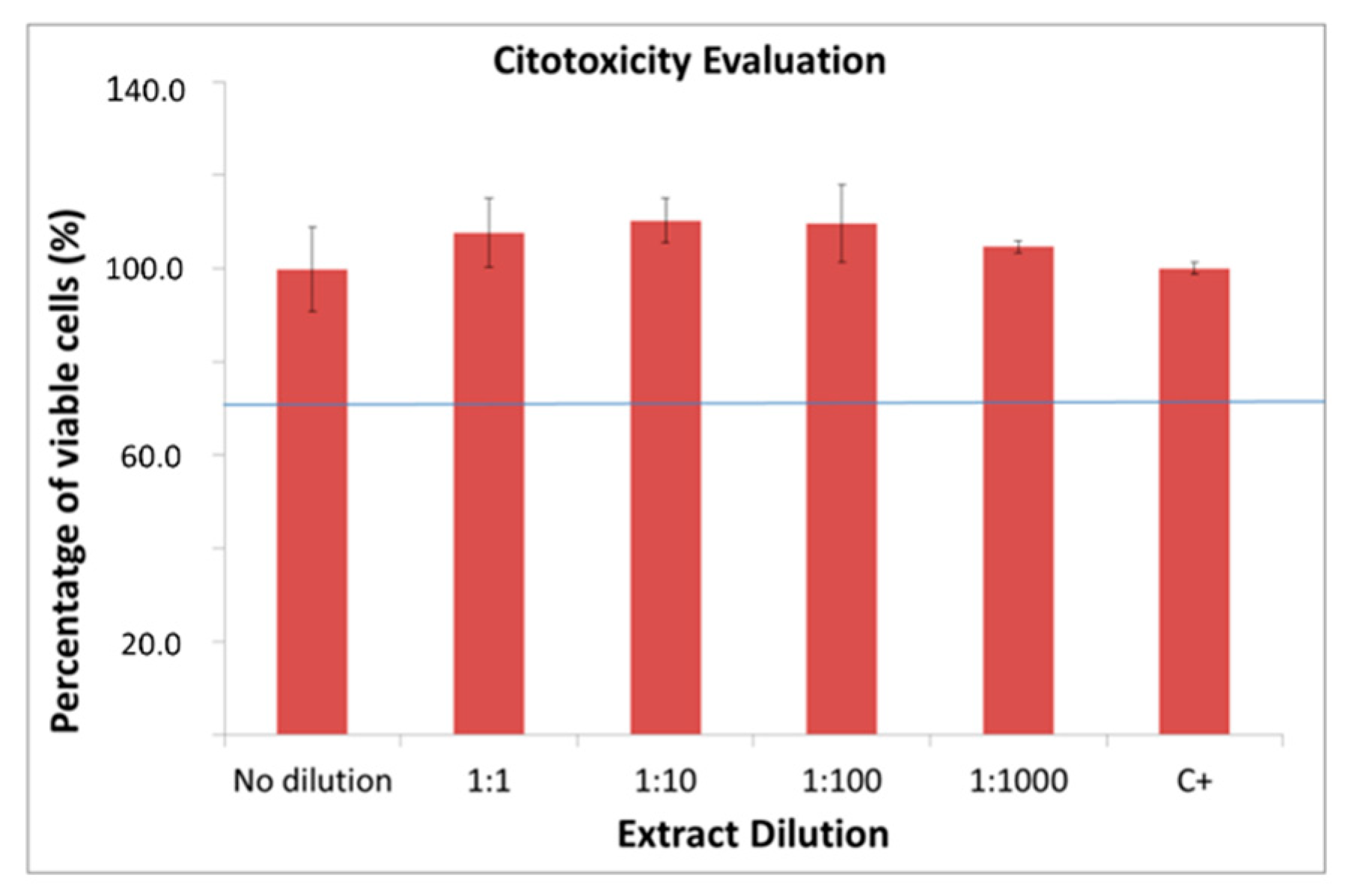
2.3. In Vitro Characterization
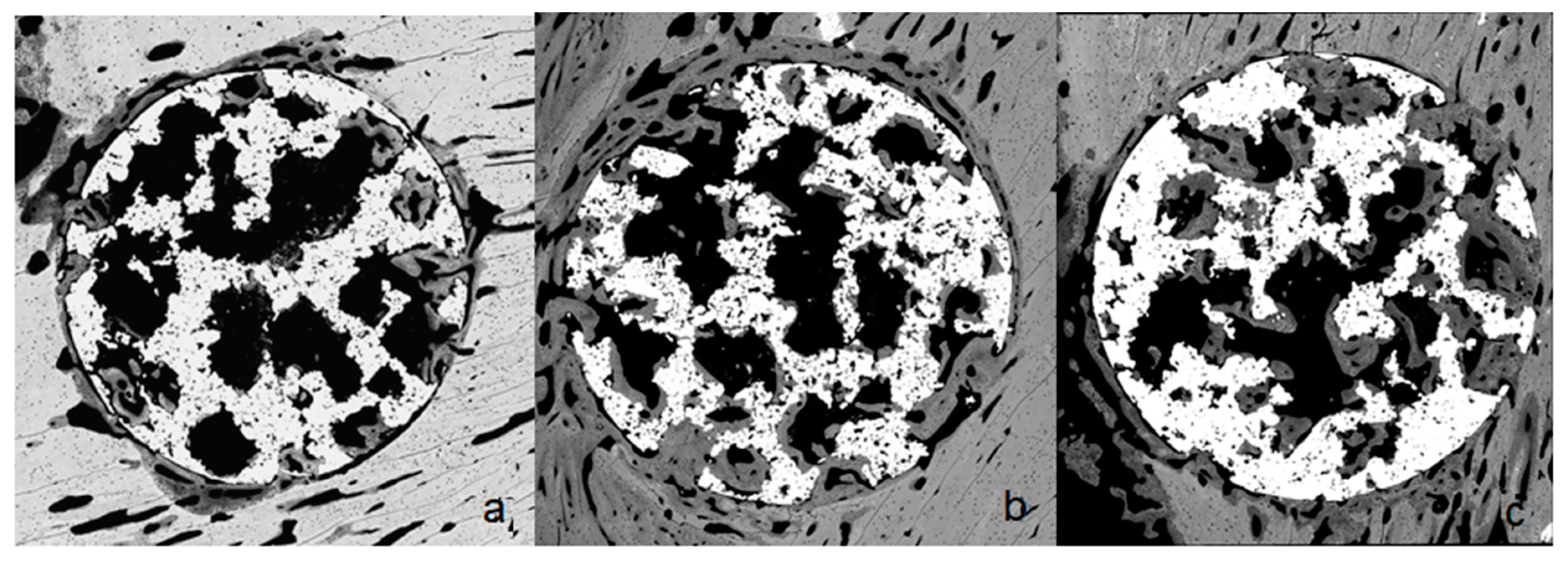
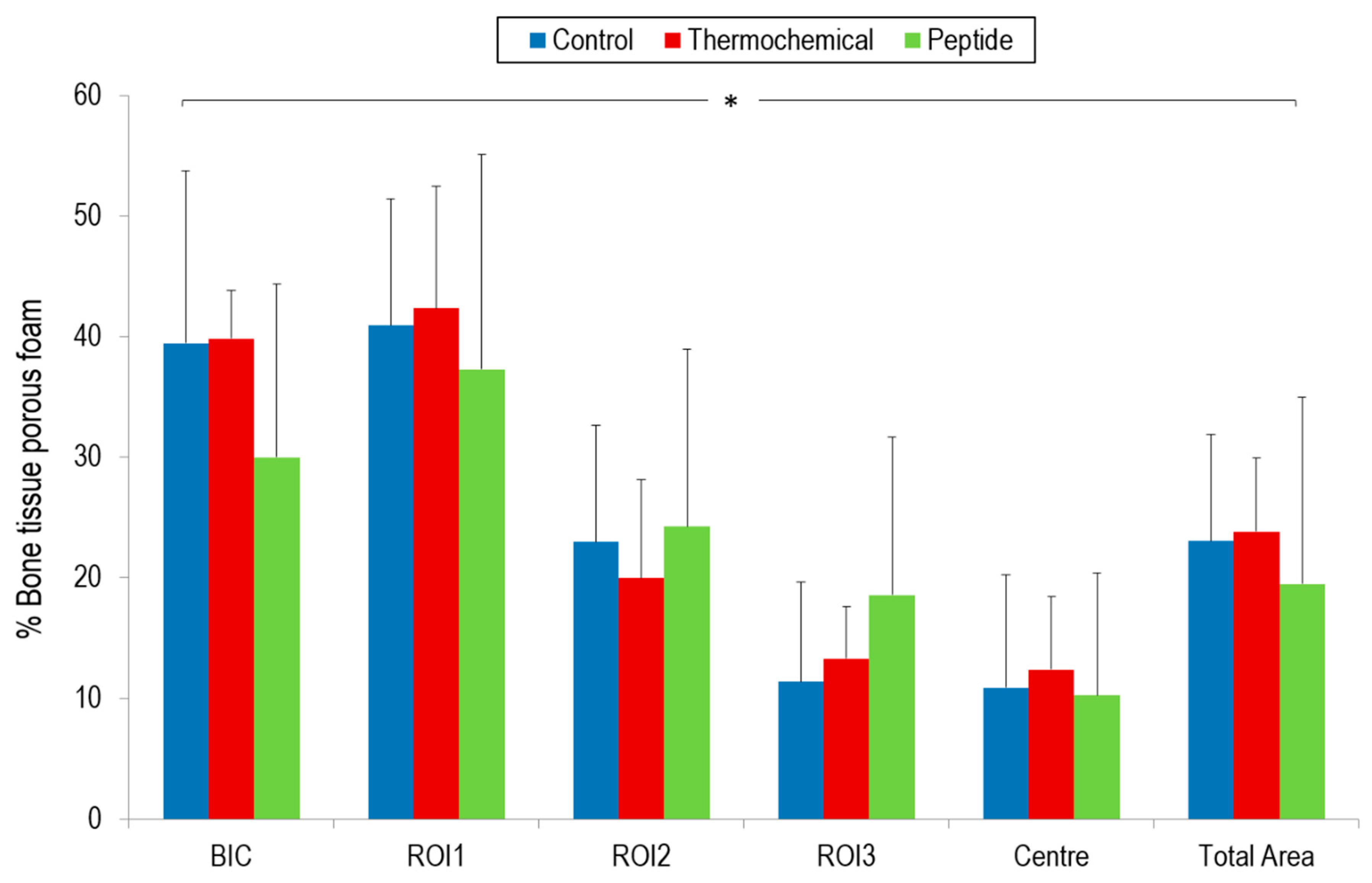
2.4. Osseointegration In Vivo
3. Discussion
4. Material and Methods
4.1. Scaffold Production
4.2. Surface Bioactivation
4.2.1. Thermo-Chemical Treatment
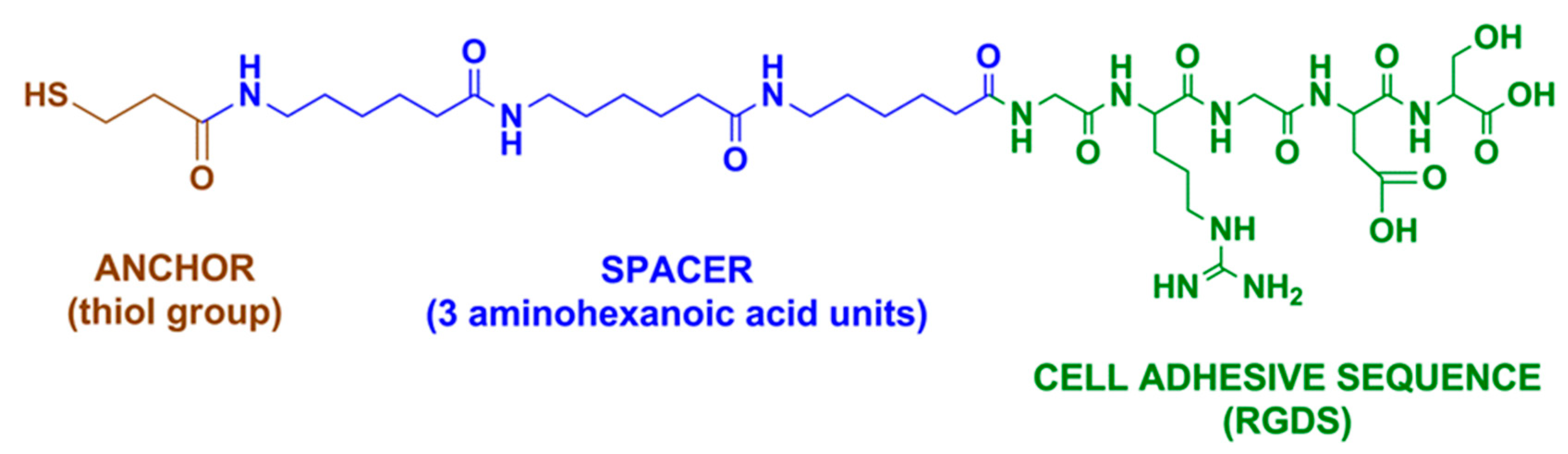
4.2.2. Peptide Adhesion
4.3. Mechanical Properties
4.4. In Vitro Biological Characterization
4.5. In Vivo Model of Osseointegration
4.6. Preparation of Histological Samples
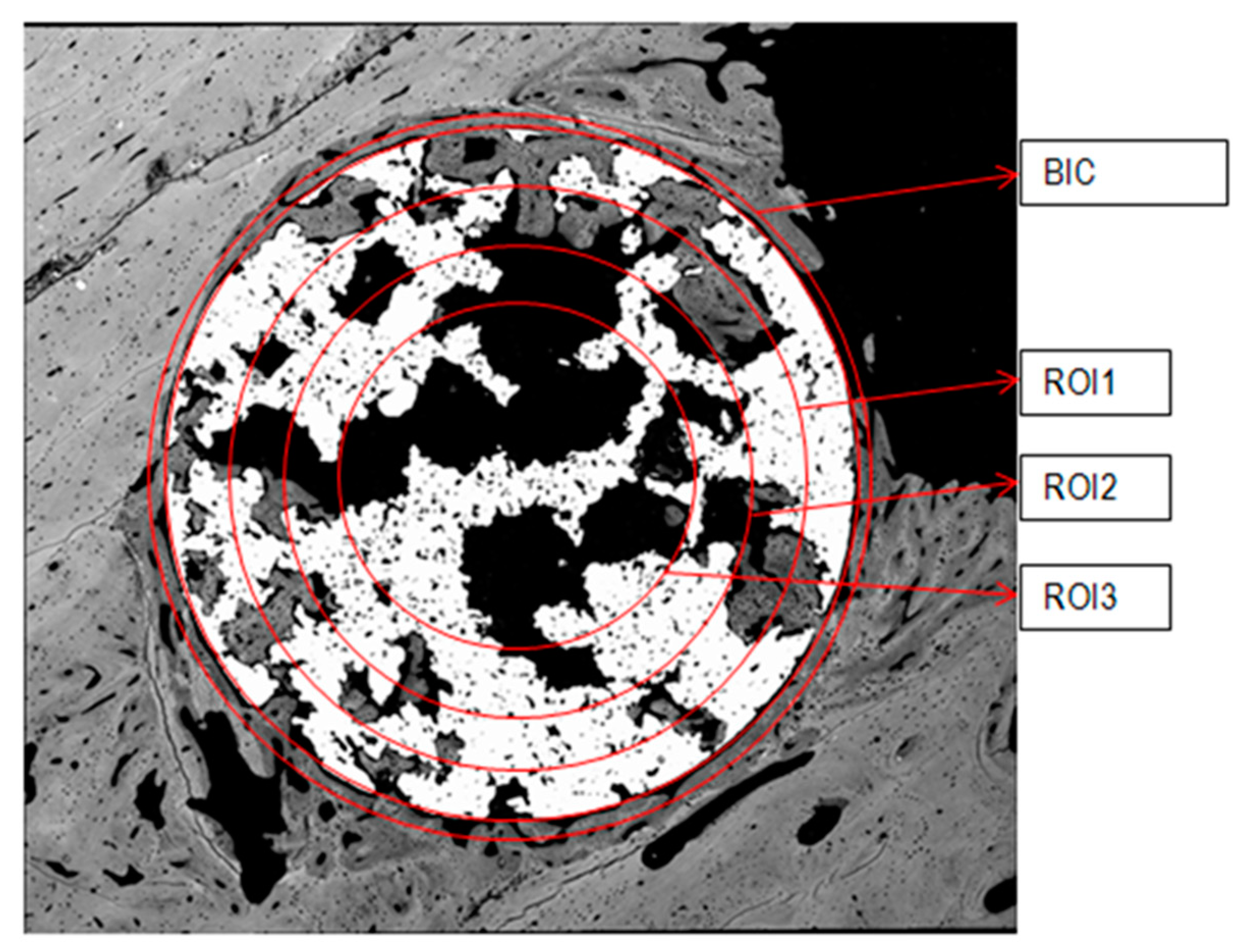
4.7. Histomorphometrical Characterization
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering biocompatible implant surfaces. Part I: Materials and surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Fernandez-Yague, M.A.; Abbah, S.A.; Mcnamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2015, 84, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Krishna, B.V.; Bose, S.; Bandyopadhyay, A. Low stiffness porous Ti structures for load-bearing implants. Acta Biomater. 2007, 3, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Li, S.H.; van Blitterswijk, C.A.; de Groot, K. A novel porous Ti6Al4V: Characterization and cell attachment. J. Biomed. Mater. Res. A 2005, 73, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.H.; Nomura, N.; Masahashi, N.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Scripta Mater. 2003, 49, 1197–1202. [Google Scholar] [CrossRef]
- Zhang, X.; Ayers, R.A.; Thorne, K.; Moore, J.J.; Schowengerdt, F. Combustion synthesis of porous materials for bone replacement. Biomed. Sci. Instrum. 2001, 37, 463–468. [Google Scholar] [PubMed]
- Heinl, P.; Müller, L.; Körner, C.; Singer, R.F.; Müller, F.A. Cellular Ti-6Al-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Ponader, S.; von Wilmowsky, C.; Widenmayer, M.; Lutz, R.; Heinl, P.; Körner, C.; Singer, R.F.; Nkenke, E.; Neukam, F.W.; Schlegel, K.A. In vivo performance of selective electron beam-melted Ti-6Al-4V structures. J. Biomed. Mater. Res. A 2010, 92, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Porous Ti6Al4V scaffold directly fabricating by rapid prototyping: Preparation and in vitro experiment. Biomaterials 2006, 27, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, M.M.; Lim, J.K. Properties of solid core and porous surface Ti-6Al-4V implants manufactured by powder metallurgy. J. Alloys Compd. 2008, 454, 442–446. [Google Scholar] [CrossRef]
- Vasconcellos, L.M.; Oliveira, M.V.; Alencastro Graça, M.L.; Vaconcellos, L.G.; Carvalho, Y.R.; Cairo, C.A. Porous titanium scaffolds produced by powder metallurgy for biomedical applications. Mater. Res. 2008, 11, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Liu, X.; Yeung, K.W.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mater. Sci. Eng. R 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Mullen, L.; Stamp, R.C.; Brooks, W.K.; Jones, E.; Sutcliffe, C.J. Selective laser melting: A regular unit cell approach for the manufacture of porous, titanium, bone ingrowth constructs, suitable for orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Osakada, K.; Shiomi, M.; Kitamura, Y.; Abe, F. Microstructure and mechanical properties of pure titanium models fabricated by selective laser melting. Proc. Inst. Mech. Eng. Part C 2004, 218, 711–719. [Google Scholar] [CrossRef]
- Shishkovsky, I.V.; Volova, L.T.; Kuznetsov, M.V.; Morozov, Y.G.; Parkin, I.P. Porous biocompatible implants and tissue scaffolds synthesized by selective laser sintering from Ti and NiTi. J. Mater. Chem. 2008, 18, 1309–1317. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T.; Mabuchi, M. Processing and mechanical properties of autogenous titanium implant materials. J. Mater. Sci. Mater. Med. 2002, 13, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Bram, M.; Stiller, C.; Buchkremer, P.H.; Stöver, D.; Baur, H. High-porosity titanium, stainless steel, and superalloy parts. Adv. Eng. Mater. 2000, 2, 196–199. [Google Scholar] [CrossRef]
- Ryan, G.; Pandit, A.; Apatsidis, D.P. Fabrication methods of porous metal for use in orthopedic applications. Biomaterials 2006, 27, 2651–2670. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Rao, P.J.; Walsh, W.R.; Pellitier, M.H.; Mobbs, R.J. Spine interbody implants: Material selection and modification, functionalization and bioactivation of surfaces to improve osseointegration. Orthop. Surg. 2014, 6, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.F. There is no such thing as a biocompatible material. Biomaterials 2014, 35, 10009–10014. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Heredia, M.A.; Sohier, J.; Gaillard, C.; Quillard, S. Rapid prototyping porous titanium coated with calcium phosphate as a scaffold for bone tissue engineering. Biomaterials 2008, 29, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Tamaddon, M.; Samizadeh, S.; Wang, L.; Blunn, G.; Liu, C. Intrinsic osteoinductivity of porous titanium scaffold for bone tissue engineering. Int. J. Biomater. 2017, 2017, 5093063. [Google Scholar] [CrossRef] [PubMed]
- Thalgott, J.S.; Giuffre, J.M.; Klezl, Z.; Timlin, M. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapatite, and demineralized bone matrix as part of a circumferential fusion. Spine J. 2002, 2, 63–69. [Google Scholar] [CrossRef]
- Kim, K.J.; Iwase, M.; Kotake, S.; Itoh, T. Effect of bone marrow grafting on the titanium porous-coated implant in bilateral total knee arthroplasty. Acta Orthop. 2007, 78, 116–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroese-Deutman, H.C.; Vehof, J.W.; Spauwen, P.H.; Stoelinga, P.J.; Jansen, J.A. Orthotopic bone formation in titanium fiber mesh loaded with platelet-rich plasma and placed in segmental defects. Int. J. Oral Maxillofac. Surg. 2008, 37, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.A.; Vehof, J.W.; Ruhé, P.Q.; Kroeze-Deutman, H.; Kuboki, Y.; Takita, H.; Hedberg, E.L.; Mikos, A.G. Growth factor-loaded scaffolds for bone engineering. J. Controll. Release 2005, 101, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, K.; Yoshinari, M.; Kokubu, E.; Shimono, M.; Yamada, Y.; Mabuchi, M.; Inoue, T. Bone formation in titanium porous scaffold with immobilization of BMP-2. J. Oral Tissue Eng. 2005, 2, 60–65. [Google Scholar] [CrossRef]
- De Peppo, G.M.; Palmquist, A.; Borchardt, P.; Lennerås, M.; Hyllner, J.; Snis, A.; Lausmaa, J.; Thomsen, P.; Karlsson, C. Free-form-fabricated commercially pure Ti and Ti6Al4V porous scaffolds support the growth of human embryonic stem cell-derived mesodermal progenitors. Sci. World J. 2012, 2012, 646417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; van Kuppevelt, T.H.; Daamen, W.F.; Bian, Z.; Jansen, J.A. The performance of human dental pulp stem cells on different three dimensional scaffolds materials. Biomaterials 2006, 27, 5658–5668. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Kapp, T.G.; Kessler, H. αvβ3- or α5β1-integrin-selective peptidomimetics for surface coating. Angew. Chem. Int. Ed. 2016, 55, 7048–7067. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C. Surface functionalization of biomaterials for bone tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Martins, C., Barbosa, M., Eds.; Elsevier: Oxford, UK, 2018; pp. 73–100. [Google Scholar]
- Karakoy, M.; Guttepe, E.; Pansdey, S.; Kharhab, M.; Gracias, D. Silane Surface modification for improved bioadhesion of esophageal stents. Appl. Surf. Sci. 2014, 311, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Vo Dinh, T. (Ed.) Nanotechnology in Biology and Medicine. Methoods, Devices and Applications; CRC Press Book: Natwick, MA, USA, 2010; ISBN 9781439893784. [Google Scholar]
- Yoon, S.T.; Konopka, J.A.; Wang, J.C.; Youssef, J.A.; Meisel, H.J.; Brodke, D.S.; Park, J.B. ACDF graft selection by surgeons: Survey of AOSpine members. Glob. Spine J. 2017, 7, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Caparrós, C.; Ortiz-Hernandez, M.; Molmeneu, M.; Punset, M.; Calero, J.A.; Aparicio, C.; Fernández-Fairén, M.; Perez, R.; Gil, F.J. Bioactive macroporous titanium implants highly interconected. J. Mater. Sci. Mater. Med. 2016, 27, 151. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Schenk, R.K.; Lussi, A.; Higginbottom, F.L.; Buser, D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: A histometric study in the canine mandible. J. Biomed. Mater. Res. 1998, 40, 1–11. [Google Scholar] [CrossRef]
- Ivanoff, C.J.; Widmark, G.; Hallgren, C.; Sennerby, L.; Wennerberg, A. Histologic evaluation of the bone integration of TiO2 blasted and turned titanium microimplants in humans. Clin. Oral Implants Res. 2001, 12, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Yamaguchi, S. Novel bioactive titanate layers formed on Ti metal and its alloys by chemical treatments. Materials 2010, 3, 48–63. [Google Scholar] [CrossRef]
- Takadama, C.H.; Kim, H.M.; Kokubo, T.; Nakamura, T. TEM-EDX study of mechanism of bonelike apatite formation on bioactive titanium metal in simulated body fluid. J. Biomed. Mater. Res. 2001, 57, 441–448. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Dorfner, P.M.; Manzenrieder, F.; Neubauer, S.; Reuning, U.; Burgkart, R.; Kessler, H. Behavior of primary human osteoblasts on trimmed and sandblasted Ti6Al4V surfaces functionalized with integrin αvβ3-selective cyclic RGD peptides. J. Biomed. Mater. Res. A 2013, 101, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Fraioli, R.; Albericio, F.; Manero, J.M.; Gil, F.J. Novel peptide-based platform for the dual presentation of biologically active peptide motifs on biomaterials. ACS Appl. Mater. Interfaces 2014, 6, 6525–6536. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruno, C.; Garrido, B.; Rodriguez, D.; Ruperez, E.; Gil, F.J. Biofunctionalization strategies on tantalum-based materials for osseointegrative applications. J. Mater. Sci. Mater. Med. 2015, 26, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraioli, R.; Rechenmacher, F.; Neubauer, S.; Manero, J.M.; Gil, J.; Kessler, H.; Mas-Moruno, C. Mimicking bone extracellular matrix: Integrin-binding peptidomimetics enhance osteoblast-like cells adhesion, proliferation, and differentiation on titanium. Colloids Surf. B Biointerfaces 2015, 128, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fairén, M.; Murcia, A.; Iglesias, R.; Sevilla, P.; Manero, J.M.; Gil, F.J. Analysis of tantalum implants used for avascular necrosis of the femoral head: A review of five retrieved specimens. J. Appl. Biomater. Funct. Mater. 2012, 10, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zardiackas, L.D.; Parsell, D.E.; Dillon, L.D.; Mitchell, D.W.; Nunnery, L.A.; Poggie, R. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J. Biomed. Mater. Res. 2001, 58, 180–187. [Google Scholar] [CrossRef]
- Goldstein, S.A. The mechanical properties of trabecular bone: Dependence of anatomic location and function. J. Biomech. 1987, 20, 1055–1061. [Google Scholar] [CrossRef]
- Sevilla, P.; Aparicio, C.; Planell, J.A.; Gil, F.J. Comparison of the mechanical properties between tantalum and nickel-titanium foams implant materials for bone ingrowth applications. J. Alloys Compd. 2007, 439, 67–73. [Google Scholar] [CrossRef]
- Biological Evaluation of Medical Devices; ISO 10993, parts 1–12; International Organization for Standardization: Geneva, Switzerland, 2009.
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-based biomaterials for preventing stress shielding between implant devices and bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Hedia, H.S.; Fouda, N. Design optimization of cementless hip prosthesis coating through functionally graded material. Comput. Mater. Sci. 2014, 87, 83–87. [Google Scholar] [CrossRef]
- Saravana Kumar, G.; George, S.P. Optimization of custom cementless stem using finite element analysis and elastic modulus distribution for reducing stress-shielding effect. Proc. Inst. Mech. Eng. H 2017, 231, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Barbas, A.; Bonnet, A.S.; Lipinski, P.; Pesci, R.; Dubois, G. Development and mechanical characterization of porous titanium bone substitutes. J. Mech. Behav. Biomed. Mater. 2012, 9, 34–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baril, E.; Lefebvre, L.P.; Hacking, S.A. Direct visualization and quantification of bone growth into porous titanium implants using micro computed tomography. J. Mater. Sci. Mater. Med. 2011, 22, 1321–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braem, A.; Chaudhari, A.; Cardoso, M.V.; Schrooten, J.; Duyck, J.; Vleugels, J. Peri- and intra-implant bone response to microporous Ti coatings with surface modification. Acta Biomater. 2014, 10, 986–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, Y.; Hodgson, P.D.; Wen, C. Biomimetic modification of porous TiNbZr alloy scaffold for bone tissue engineering. Tissue Eng. Part A 2010, 16, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, M.; Meratian, M.; Savabi, O.; Fathi, M. Comparative evaluation of the effect of different types of surface modifiers on bioactivity of porous titanium implants. Russ. J. Non-Ferrous Met. 2015, 56, 469–476. [Google Scholar] [CrossRef]
- Van Grunsven, W.; Goodall, R.; Reilly, G.C. Highly porous Titanium alloy: Fabrication and mechanical properties. J. Biomech. 2012, 45 (Suppl. 1), S339. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, C.R.; Jasty, M.; Greene, M.; Rubash, H.E.; Harris, W.H. Biologic fixation of total hip implants. Insights gained from a series of canine studies. J. Bone Joint Surg. 2004, 86 (Suppl. 2), 105–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Li, Y.C.; Hodgson, P.D.; Wen, P.C. The importance of particle size in porous titanium and nonporous counterparts for surface energy and its impact on apatite formation. Acta Biomater. 2009, 5, 2290–2302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Habibovic, P.; van, d.e.n.; Doel, M.; Wilson, C.E.; de Wijn, D.R.; van Blitterswijk, C.A.; De Groot, K. Bone ingrowth in porous titanium implants produced by 3D fiber deposition. Biomaterials 2007, 28, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- Mediasvanti, K.; Wen, C.; Ivanova, E.P.; Berndt, C.C.; Malherbe, F.; Pham, V.T.; Wang, J. A review on bioactive porous metallic biomaterials. J. Biomim. Biomater Tissue Eng. 2013, 18, 1. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous Titanium implants: A review. Adv. Eng. Mater. 2018, 1700648. [Google Scholar] [CrossRef]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; So, K.; Akiyama, N.; Matsushita, T.; Kokubo, T.; Nakamura, T. A porous bioactive titanium implant for spinal interbody fusion: An experimental study using a canine model. J. Neurosurg. Spine 2007, 7, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Güden, M.; Çelik, E.; Hizal, A.; Altindiş, M.; Çetiner, S. Effects of compaction pressure and particle shape on the porosity and compression mechanical properties of sintered Ti6Al4V powder compacts for hard tissue implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jäger, M.; Böge, C.; Janissen, R.; Rohrbeck, D.; Hülsen, T.; Lensing-Höhn, S.; Krauspe, R.; Herten, M. Osteoblastic potency of bone marrow cells cultivated on functionalized biometals with cyclic RGD-peptide. J. Biomed. Mater. Res. A 2013, 101, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
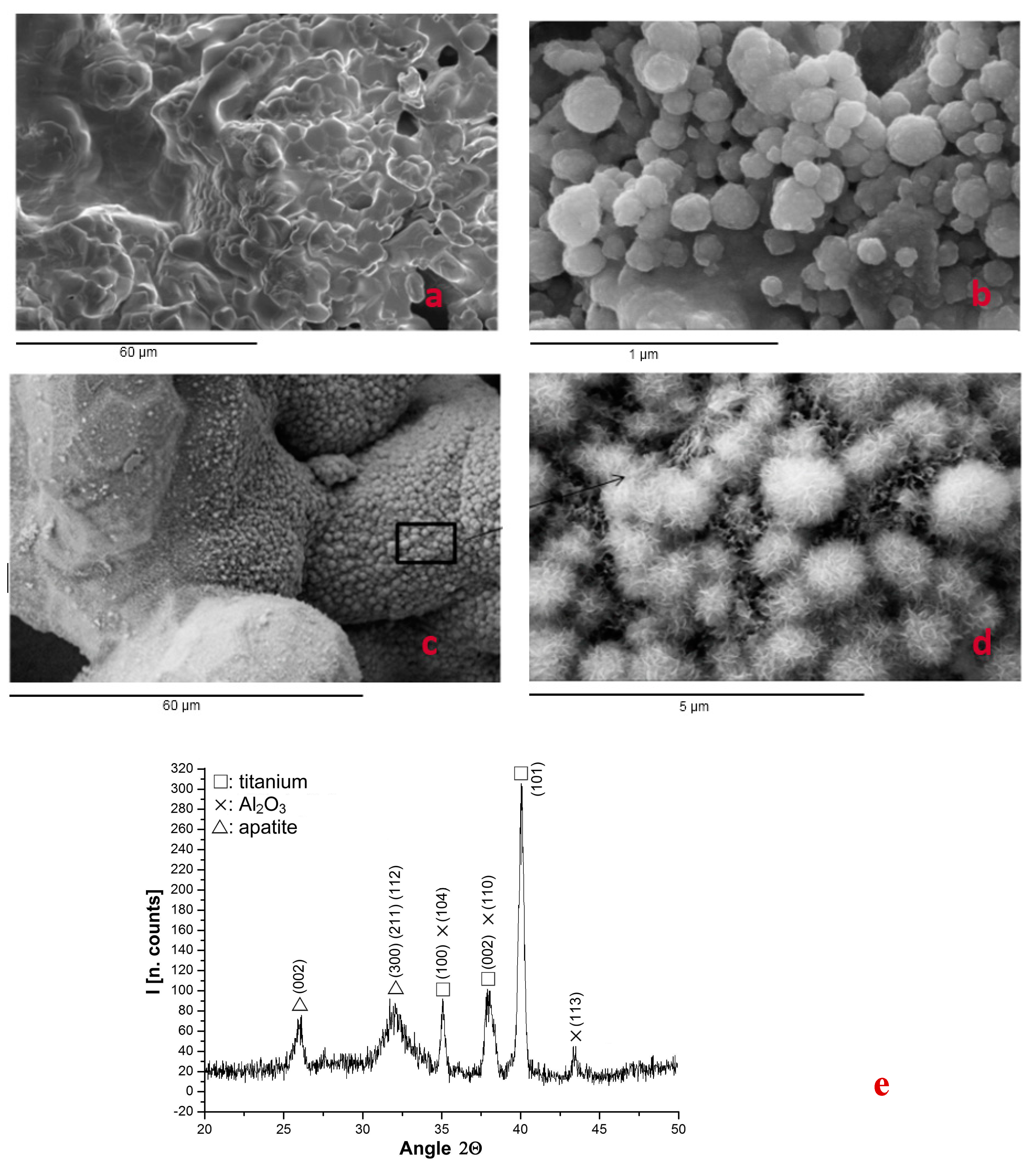
| Porous Material | P (μm) | I (%) | Ra (μm) | References |
|---|---|---|---|---|
| Ti porous | 210 ± 9 | 57 ± 3 | 1.1 ± 0.1 | |
| Ti porous thermochemical | 208 ± 10 | 57 ± 2 | 1.1 ± 0.2 | |
| Ti porous with peptides | 210 ± 8 | 56 ± 3 | 1.3 ± 0.4 | |
| Ta porous | 370 ± 15 | 65 ± 5 | 1.4 ± 0.2 | [47,48] |
| NiTi porous | 350 ± 12 | 63 ± 6 | 1.1 ± 0.1 | [49,50] |
| Porous Material | E (GPa) | σ0 (MPa) | σmax (MPa) | ε (%) | σf (MPa) | References |
|---|---|---|---|---|---|---|
| Ti porous | 0.61 ± 0.22 | 105.2 ± 10.8 | 170 ± 20.06 | 30.9 ± 4.6 | 16.4 ± 3.0 | |
| Ti porous with thermochem. | 0.66 ± 0.12 | 116.2 ± 9.7 | 177 ± 15.22 | 27.0 ± 4.6 | 15.4 ± 3.2 | |
| Ti porous with peptides | 0.63 ± 0.24 | 101.1 ± 9.8 | 165 ± 22.16 | 25.1 ± 4.6 | 13.5 ± 2.7 | |
| Ta porous | 1.15 ± 0.86 | 35.2 ± 0.8 | 71.2 ± 15.6 | 8.1 ± 1.8 | 7.5 ± 3.6 | [47,48] |
| NiTi porous | 1.21 ± 0.31 | 101.3 ± 14.3 | 142.5 ± 29.3 | 23.0 ± 4.1 | 13.2 ± 4.2 | [49,50] |
| Cancellous bone | 0.55 ± 0.48 | 15.2 ± 8.0 | 25.0 ± 8.1 | 7.1 ± 3.0 | [48] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Hernandez, M.; Rappe, K.S.; Molmeneu, M.; Mas-Moruno, C.; Guillem-Marti, J.; Punset, M.; Caparros, C.; Calero, J.; Franch, J.; Fernandez-Fairen, M.; et al. Two Different Strategies to Enhance Osseointegration in Porous Titanium: Inorganic Thermo-Chemical Treatment Versus Organic Coating by Peptide Adsorption. Int. J. Mol. Sci. 2018, 19, 2574. https://doi.org/10.3390/ijms19092574
Ortiz-Hernandez M, Rappe KS, Molmeneu M, Mas-Moruno C, Guillem-Marti J, Punset M, Caparros C, Calero J, Franch J, Fernandez-Fairen M, et al. Two Different Strategies to Enhance Osseointegration in Porous Titanium: Inorganic Thermo-Chemical Treatment Versus Organic Coating by Peptide Adsorption. International Journal of Molecular Sciences. 2018; 19(9):2574. https://doi.org/10.3390/ijms19092574
Chicago/Turabian StyleOrtiz-Hernandez, Monica, Katrin S. Rappe, Meritxell Molmeneu, Carles Mas-Moruno, Jordi Guillem-Marti, Miquel Punset, Cristina Caparros, Jose Calero, Jordi Franch, Mariano Fernandez-Fairen, and et al. 2018. "Two Different Strategies to Enhance Osseointegration in Porous Titanium: Inorganic Thermo-Chemical Treatment Versus Organic Coating by Peptide Adsorption" International Journal of Molecular Sciences 19, no. 9: 2574. https://doi.org/10.3390/ijms19092574
APA StyleOrtiz-Hernandez, M., Rappe, K. S., Molmeneu, M., Mas-Moruno, C., Guillem-Marti, J., Punset, M., Caparros, C., Calero, J., Franch, J., Fernandez-Fairen, M., & Gil, J. (2018). Two Different Strategies to Enhance Osseointegration in Porous Titanium: Inorganic Thermo-Chemical Treatment Versus Organic Coating by Peptide Adsorption. International Journal of Molecular Sciences, 19(9), 2574. https://doi.org/10.3390/ijms19092574






