The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease
Abstract
1. Introduction
2. Classification, Structure, and Molecular Characteristics
2.1. Classification
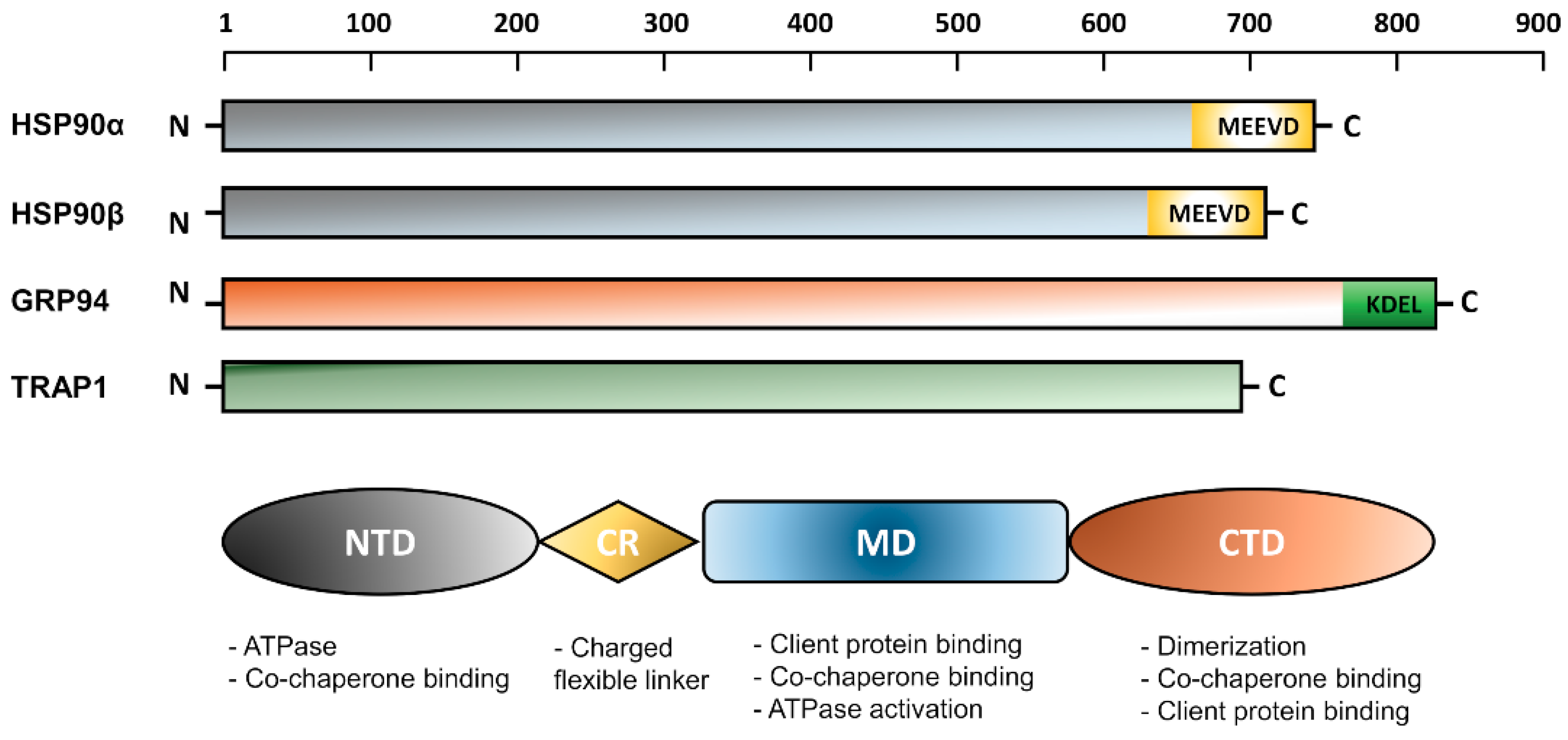
2.2. General Structure of HSP90 Isoforms
2.2.1. Molecular Characteristics of the Cytoplasmic Isoforms: Hsp90α and HSP90β
The N-Terminal Domain (NTD)
The Charged Linker Region (CR)
The Middle Domain (MD)
The C-Terminal Domain (CTD)
2.2.2. Molecular Characteristics of the ER Isoform; GRP94
2.2.3. Molecular Characteristics of the Mitochondrial Isoform; TRAP1
3. Regulation of HSP90
3.1. Transcriptional Regulation
3.1.1. Cytoplasmic HSP90
3.1.2. GRP94
3.1.3. TRAP1
3.2. Regulation by Posttranslational Modification
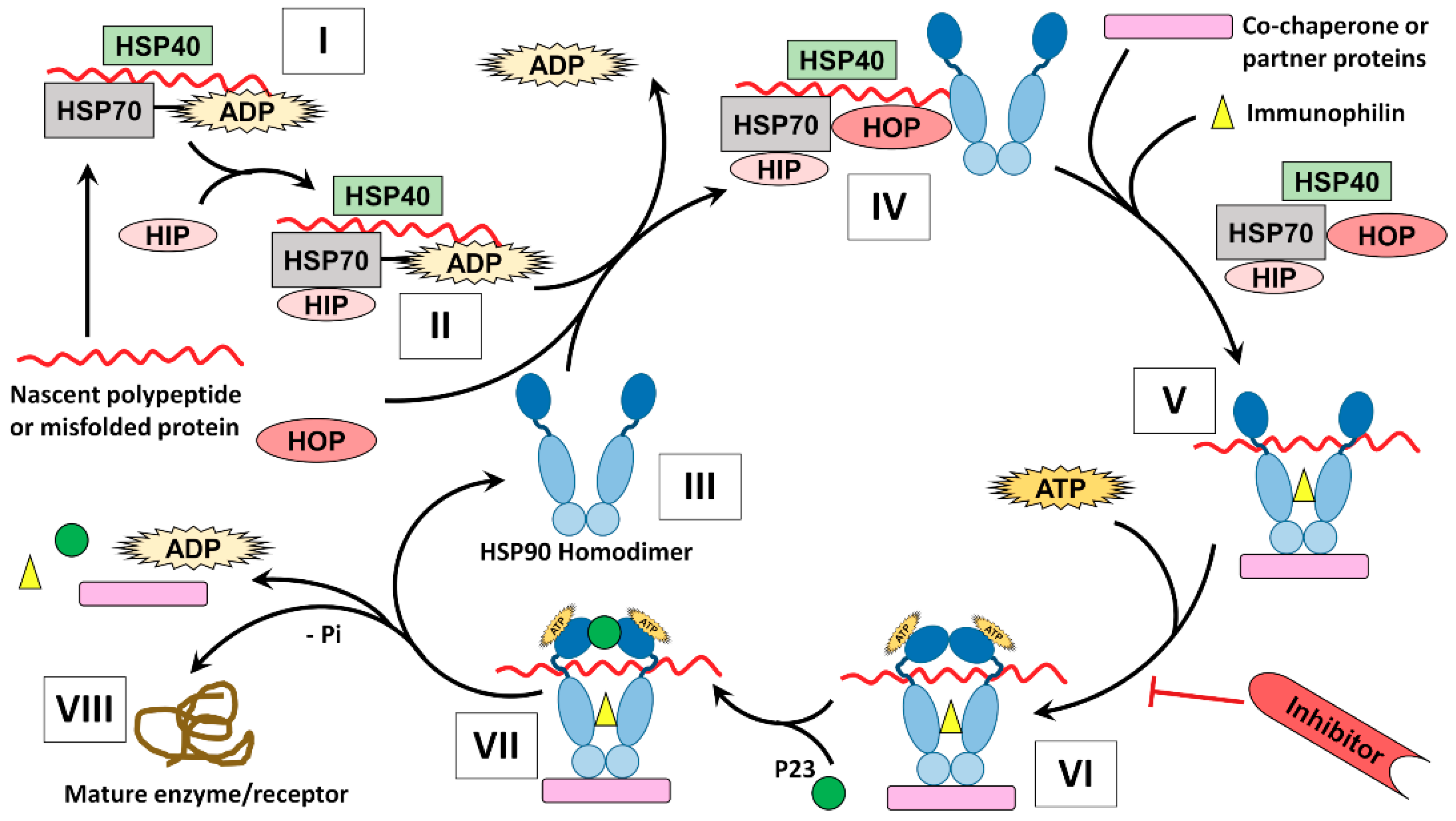
3.3. Co-Chaperones
4. Client Proteins
4.1. HSP90
4.2. GRP94 and TRAP1
5. Mode of Action
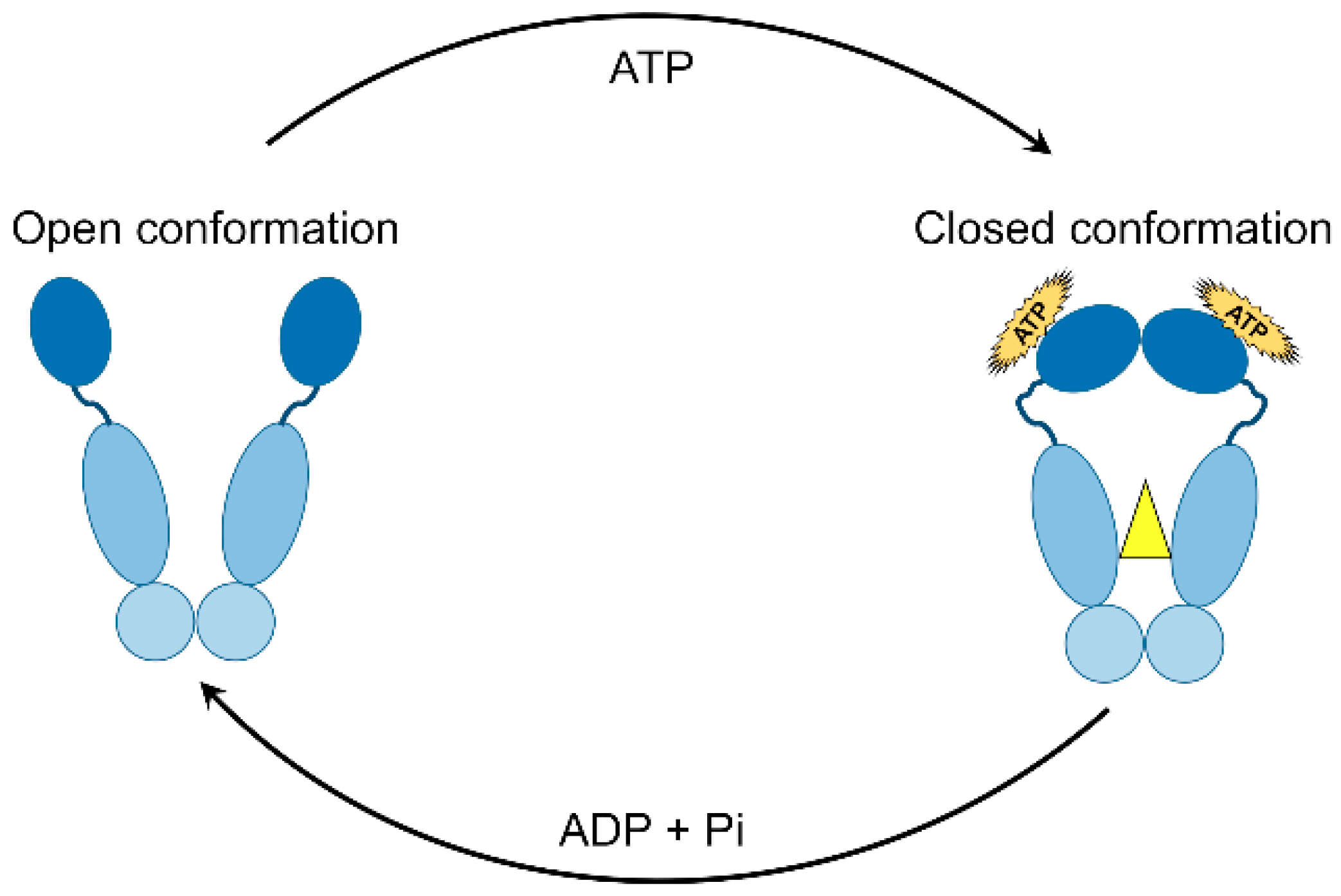
5.1. ATPase Activity
5.2. Chaperone Cycle
6. Roles of HSP90 Isoforms in Cellular Physiology
6.1. Cytosolic HSP90
6.1.1. Chaperone Function
6.1.2. Cell Signaling
6.1.3. Cytoskeleton
6.1.4. Nuclear Functions
6.1.5. Cell Cycle and Cellular Differentiation
6.1.6. Extracellular HSP90 or Secreted HSP90
6.2. GRP94
6.2.1. Protein Folding
6.2.2. Calcium Homeostasis
6.2.3. ER Quality Control
6.2.4. ER Stress
6.2.5. Extracellular GRP94
6.3. TRAP1
6.3.1. Role of TRAP1 in Mitochondria
6.3.2. How Does TRAP1 Protect Against Oxidative Stress?
6.3.3. Extra-Mitochondrial Roles
7. Role of HSP90 Isoforms in Pathological Conditions
7.1. Cytosolic HSP90
7.1.1. Cancer
HSP90 as a Target for Anti-Cancer Drugs
7.1.2. Neurodegenerative Diseases
7.1.3. Infectious Diseases
Viral Diseases
Parasitic Diseases
7.1.4. Other Diseases
7.2. GRP94
7.2.1. Cancer
GRP94 as a Target for Anti-Cancer Drugs
7.2.2. Neurodegenerative Diseases
7.2.3. Infectious Diseases
Viral Diseases
Parasitic Diseases
7.2.4. Other Diseases
7.3. TRAP1
7.3.1. Cancer
7.3.2. Neurodegenerative Diseases
7.3.3. Kidney Diseases
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aha1 | Activator of Hsp90 ATPase protein 1 |
| Apaf-1 | Apoptotic protease-activating factor 1 |
| ARNT | Aryl hydrocarbon receptor nuclear translocator |
| ARRB1 | Beta-arrestin-1 |
| Cdc37 | Hsp90 co-chaperone Cdc37, cell division cycle 37 |
| CDK4 | Cyclin-dependent kinase 4 |
| CHIP | Carboxyl terminus of Hsp70-interacting protein |
| CLAVATA | Receptor-like protein CLAVATA |
| c-MET | Proto-oncogene c-Met, hepatocyte growth factor receptor |
| CYP-D | Cyclophilin or Peptidyl-prolyl cis-trans isomerase F, mitochondrial |
| ERBB2 | Receptor tyrosine-protein kinase erbB-2 |
| FANCA | Fanconi anemia group A protein |
| FKBP51 and FKBP52 | Peptidyl-prolyl cis-trans isomerase or 51 kDa FK506-binding protein |
| Flt-3 | Receptor-type tyrosine-protein kinase FLT3 |
| HIF1α | Hypoxia-inducible factor 1-alpha |
| HIP | Hsc70-interacting protein |
| HMG | High mobility group protein |
| HOP | Co-chaperone Hsp70/Hsp90-organizing protein |
| HSP | Heat shock protein |
| IRAK | Interleukin-1 receptor-associated kinase |
| MAFG | Transcription factor MafG |
| MHC class II | Major Histocompatibility Complex Class II |
| MMP2 | Matrix metalloproteinase-2 |
| Myt1 | Membrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase |
| NEK8 | Serine/threonine-protein kinase Nek8, Never in mitosis A-related kinase 8 |
| NF-κB | Nuclear factor NF-kappa |
| p23 | Co-chaperone protein 23 |
| P53 | Cellular tumor antigen p53 |
| PI3K | Phosphatidylinositol 3-kinase |
| Plk | Serine/threonine-protein kinase PLK1 |
| PP5 | Serine/threonine-protein phosphatase 5 |
| RTK | Receptor Tyrosine Kinase |
| Sgt1 | Protein SGT1 homolog, Suppressor of G2 allele of SKP1 homolog |
| SREBF | Sterol regulatory element-binding protein |
| SSDF-1 | Stromal cell derived factor -1 |
| TLR | Toll-like receptor |
| TNF | Tumor Necrosis Factor |
| Tom70 | Mitochondrial import receptor subunit TOM70 |
| VEGFR | Vascular endothelial growth factor receptor |
| Wee1 | Wee1-like protein kinase |
| WISp39 | Waf1 Cip1 stabilizing protein 39 |
References
- Ritossa, F. A new puffing pattern induced by temperature shock and DNP in drosophila. Experientia 1962, 18, 571–573. [Google Scholar] [CrossRef]
- Malyshev, I. A General Description of HSPs, The Molecular Structure of HSP70 and The HSP70 Cycle. In Immunity, Tumors and Aging: The Role of HSP70; Springer: Dordrecht, The Netherlands, 2013; pp. 1–13. ISBN 978-94-007-5943-5. [Google Scholar]
- Lindquist, S. The heat-shock response. Annu. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [CrossRef] [PubMed]
- Pirkkala, L.; Sistonen, L. Heat Shock Proteins (HSPs): Structure, Function and Genetics. Encycl. Life Sci. 2006, 1–7. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhong, D.; Monteiro, A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genom. 2006, 7, 156. [Google Scholar] [CrossRef]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Sun, W.; Taldone, T.; Rodina, A.; Chiosis, G. Heat shock protein 90 in neurodegenerative diseases. Mol. Neurodegener. 2010, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Geller, R.; Taguwa, S.; Frydman, J. Broad action of Hsp90 as a host chaperone required for viral replication. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Buchner, J. Structure, function and regulation of the hsp90 machinery. Biomed. J. 2012, 36, 106–117. [Google Scholar] [CrossRef]
- Langer, T.; Rosmus, S.; Fasold, H. Intracellular localization of the 90 kDA heat shock protein (HSP90α) determined by expression of a EGFP-HSP90α-fusion protein in unstressed and heat stressed 3T3 cells. Cell Biol. Int. 2003, 27, 47–52. [Google Scholar] [CrossRef]
- Prodromou, C. Mechanisms of Hsp90 regulation. Biochem. J. 2016, 473, 2439–2452. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Soroka, J.; Buchner, J. The Hsp90 chaperone machinery: Conformational dynamics and regulation by co-chaperones. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Didenko, T.; Duarte, A.M.S.; Karagöz, G.E.; Rüdiger, S.G.D. Hsp90 structure and function studied by NMR spectroscopy. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.E. Hsp90: Structure and function. Top. Curr. Chem. 2013, 328, 155–240. [Google Scholar] [CrossRef] [PubMed]
- Lavery, L.A.; Partridge, J.R.; Ramelot, T.A.; Elnatan, D.; Kennedy, M.A.; Agard, D.A. Structural Asymmetry in the Closed State of Mitochondrial Hsp90 (TRAP1) Supports a Two-Step ATP Hydrolysis Mechanism. Mol. Cell 2014, 53, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.S.; Kalmár, É.; Csermely, P.; Shen, Y.F. Hsp90 isoforms: Functions, expression and clinical importance. FEBS Lett. 2004, 562, 11–15. [Google Scholar] [CrossRef]
- Csermely, P.; Schnaider, T.; Soti, C.; Prohászka, Z.; Nardai, G. The 90-kDa Molecular Chaperone Family: Structure, Function, and Clinical Applications. A Comprehensive Review. Pharmacol. Ther. 1998, 79, 129–168. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Mollapour, M.; Prodromou, C.; Lee, C.-T.; Panaretou, B.; Yoshida, S.; Mayer, M.P.; Neckers, L.M. Charged linker sequence modulates eukaryotic heat shock protein 90 (Hsp90) chaperone activity. Proc. Natl. Acad. Sci. USA 2012, 109, 2937–2942. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol. Biol. Evol. 1995, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Arrigo, A.P.; Calderwood, S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch. Toxicol. 2013, 87, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.J.; Feramisco, J.R. Purification of the major mammalian heat shock proteins. J. Biol. Chem. 1982, 257, 14949–14959. [Google Scholar] [PubMed]
- Rose, D.W.; Wettenhall, R.E.H.; Kudlicki, W.; Kramer, G.; Hardesty, B. The 90-kilodalton peptide of the heme-regulated eIF-2 alpha kinase has sequence similarity with the 90-kilodalton heat shock protein. Biochemistry 1987, 26, 6583–6587. [Google Scholar] [CrossRef] [PubMed]
- Koyasu, S.; Nishida, E.; Kadowaki, T.; Matsuzaki, F.; Iida, K.; Harada, F.; Kasuga, M.; Sakai, H.; Yahara, I. Two mammalian heat shock proteins, HSP90 and HSP100, are actin-binding proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 8054–8058. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, Y.; Inano, K.; Horigome, T.; Sugano, H. Characterization of the hydrophobic region of heat shock protein 90. J. Biochem. 1991, 110, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Nardai, G.; Schnaider, T.; Söti, C.; Ryan, M.T.; Hoj, P.B.; Somogyi, J.; Csermely, P. Characterization of the 90 kDa heat shock protein (HSP90)-associated ATP/GTPase. J. Biosci. 1996, 21, 179–190. [Google Scholar] [CrossRef]
- Prodromou, C.; Roe, S.M.; Piper, P.W.; Pearl, L.H. A molecular clamp in the crystal structure of the N-terminal domain of the yeast Hsp90 chaperone. Nat. Struct. Biol. 1997, 4, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Inouye, M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci. 2000, 25, 24–28. [Google Scholar] [CrossRef]
- Panaretou, B.; Prodromou, C.; Roe, S.M.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998, 17, 4829–4836. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Mollapour, M.; Graf, C.; Lee, C.T.; Scroggins, B.T.; Xu, W.; Haslerova, L.; Hessling, M.; Konstantinova, A.A.; Trepel, J.B.; et al. Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain. Nat. Struct. Mol. Biol. 2009, 16, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Shiau, A.K.; Harris, S.F.; Southworth, D.R.; Agard, D.A. Structural Analysis of E. coli hsp90 Reveals Dramatic Nucleotide-Dependent Conformational Rearrangements. Cell 2006, 127, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, T.; Siegmund, H.I.; Jaenicke, R.; Ganz, P.; Lilie, H.; Buchner, J. The charged region of Hsp90 modulates the function of the N-terminal domain. Proc. Natl. Acad. Sci. USA 1999, 96, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Louvion, J.F.; Warth, R.; Picard, D. Two eukaryote-specific regions of Hsp82 are dispensable for its viability and signal transduction functions in yeast. Proc. Natl. Acad. Sci. USA 1996, 93, 13937–13942. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.; Prodromou, C.; Hu, B.; Vaughan, C.; Roe, S.M.; Panaretou, B.; Piper, P.W.; Pearl, L.H. Structural and functional analysis of the middle segment of hsp90: Implications for ATP hydrolysis and client protein and cochaperone interactions. Mol. Cell 2003, 11, 647–658. [Google Scholar] [CrossRef]
- Huai, Q.; Wang, H.; Liu, Y.; Kim, H.Y.; Toft, D.; Ke, H. Structures of the N-terminal and middle domains of E. coli Hsp90 and conformation changes upon ADP binding. Structure 2005, 13, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Kawasaki, H.; Suzuki, K.; Yahara, I. The calmodulin-binding domain of the mouse 90-kDa heat shock protein. J. Biol. Chem. 1993, 268, 9604–9610. [Google Scholar] [PubMed]
- Toft, D.; Meng, X.; Devin, J.; Sullivan, W.; Baulieu, E.; Catelli, M. Mutational analysis of Hsp90 alpha dimerization and subcellular localization: Dimer disruption does not impede “in vivo’’ interaction with estrogen receptor. J. Cell Sci. 1996, 109, 1677–1687. [Google Scholar]
- Soti, C.; Vermes, Á.; Haystead, T.A.J.; Csermely, P. Comparative analysis of the ATP-binding sites of Hsp90 by nucleotide affinity cleavage: A distinct nucleotide specificity of the C-terminal ATP-binding site. Eur. J. Biochem. 2003, 270, 2421–2428. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.G.; Schulte, T.W.; Neckers, L. Novobiocin and Related Coumarins and Depletion of Heat Shock Protein 90-Dependent Signaling Proteins. JNCI J. Natl. Cancer Inst. 2000, 92, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Henry, E.C.; Gasiewicz, T.A. (−)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry 2009, 48, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Schneider, C.; Hartl, F.U. In vitro evidence that hsp90 contains two independent chaperone sites. FEBS Lett. 1997, 418, 139–143. [Google Scholar] [CrossRef]
- Sullivan, W.P.; Toft, D.O. Mutational analysis of hsp90 binding to the progesterone receptor. J. Biol. Chem. 1993, 268, 20373–20379. [Google Scholar] [PubMed]
- Garg, G.; Khandelwal, A.; Blagg, B.S.J. Chapter Three—Anticancer Inhibitors of Hsp90 Function: Beyond the Usual Suspects. In Hsp90 in Cancer: Beyond the Usual Suspects; Isaacs, J., Whitesell, L., Eds.; Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2016; Volume 129, pp. 51–88. [Google Scholar]
- Yang, Y.; Li, Z. Roles of heat shock protein gp96 in the ER quality control: Redundant or unique function? Mol. Cells 2005, 20, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Smith, M.; Macer, D.; Webster, P.; Mortara, R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J. Cell Sci. 1986, 86, 217–232. [Google Scholar] [PubMed]
- Hoter, A.; Amiri, M.; Warda, M.; Naim, H.Y. Molecular cloning, cellular expression and characterization of Arabian camel (Camelus dromedarius) endoplasmin. Int. J. Biol. Macromol. 2018, 117, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Soldano, K.L.; Jivan, A.; Nicchitta, C.V.; Gewirth, D.T. Structure of the N-terminal domain of GRP94. Basis for ligand specificity and regulation. J. Biol. Chem. 2003, 278, 48330–48338. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Eletto, D.; Argon, Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta 2012, 1823, 774–787. [Google Scholar] [CrossRef] [PubMed]
- Immormino, R.M.; Dollins, D.E.; Shaffer, P.L.; Soldano, K.L.; Walker, M.A.; Gewirth, D.T. Ligand-induced conformational shift in the N-terminal domain of GRP94, an Hsp90 chaperone. J. Biol. Chem. 2004, 279, 46162–46171. [Google Scholar] [CrossRef] [PubMed]
- Dollins, D.E.; Immormino, R.M.; Gewirth, D.T. Structure of unliganded GRP94, the endoplasmic reticulum Hsp90: Basis for nucleotide-induced conformational change. J. Biol. Chem. 2005, 280, 30438–30447. [Google Scholar] [CrossRef] [PubMed]
- Hainzl, O.; Lapina, M.C.; Buchner, J.; Richter, K. The charged linker region is an important regulator of Hsp90 function. J. Biol. Chem. 2009, 284, 22559–22567. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Ostrovsky, O.; Makarewich, C.A.; Wanderling, S.; Gidalevitz, T.; Argon, Y. The peptide-binding activity of GRP94 is regulated by calcium. Biochem. J. 2007, 405, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Hsp90 regulation of mitochondrial protein folding: From organelle integrity to cellular homeostasis. Cell. Mol. Life Sci. 2013, 70, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C.; Stein, G.S.; Lian, J.B.; Languino, L.R. TRAP-1, the mitochondrial Hsp90. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Song, H.Y.; Dunbar, J.D.; Zhang, Y.X.; Guo, D.; Donner, D.B. Identification of a protein with homology to hsp90 that binds the type 1 tumor necrosis factor receptor. J. Biol. Chem. 1995, 270, 3574–3581. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Piel, W.H.; Gui, L.; Bruford, E.; Monteiro, A. The HSP90 family of genes in the human genome: Insights into their divergence and evolution. Genomics 2005, 86, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Leskovar, A.; Wegele, H.; Werbeck, N.D.; Buchner, J.; Reinstein, J. The ATPase cycle of the mitochondrial Hsp90 analog trap1. J. Biol. Chem. 2008, 283, 11677–11688. [Google Scholar] [CrossRef] [PubMed]
- Elnatan, D.; Agard, D.A. Modulation of mitochondrial Hsp90 (TRAP1) ATPase activity by calcium and magnesium. bioRxiv 2018, 90. [Google Scholar] [CrossRef]
- Kang, B.H. TRAP1 regulation of mitochondrial life or death decision in cancer cells and mitochondria-targeted TRAP1 inhibitors. BMB Rep. 2012, 45, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Masgras, I.; Sanchez-Martin, C.; Colombo, G.; Rasola, A. The Chaperone TRAP1 As a Modulator of the Mitochondrial Adaptations in Cancer Cells. Front. Oncol. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Felts, S.J.; Owen, B.A.; Nguyen, P.; Trepel, J.; Donner, D.B.; Toft, D.O. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J. Biol. Chem. 2000, 275, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Voellmy, R.; Boellmann, F. Chaperone regulation of the heat shock protein response. Adv. Exp. Med. Biol. 2007, 594, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Hickey, E.; Brandon, S.E.; Smale, G.; Lloyd, D.; Weber, L.A. Sequence and regulation of a gene encoding a human 89-kilodalton heat shock protein. Mol. Cell. Biol. 1989, 9, 2615–2626. [Google Scholar] [CrossRef] [PubMed]
- Mollapour, M.; Neckers, L. Post-translational modifications of Hsp90 and their contributions to chaperone regulation. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, A.D.; Beebe, K.; Neckers, L.; Prince, T. Regulation and function of the human HSP90AA1 gene. Gene 2015, 570, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, J.W.; Olzmann, J.A.; Chin, L.S.; Li, L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007, 5, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Zuehlke, A.; Johnson, J.L. Hsp90 and co-chaperones twist the functions of diverse client proteins. Biopolymers 2010, 93, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Rasola, A.; Neckers, L.; Picard, D. Mitochondrial oxidative phosphorylation TRAP(1)ped in tumor cells. Trends Cell Biol. 2014, 24, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Andreani, V.; Kapoor, T.; Herp, S.; Flach, H.; Duchniewicz, M.; Grosschedl, R. MZB1 is a GRP94 cochaperone that enables proper immunoglobulin heavy chain biosynthesis upon ER stress. Genes Dev. 2014, 28, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Schaiff, W.T.; Hruska, K.A.; McCourt, D.W.; Green, M.; Schwartz, B.D. HLA-DR associates with specific stress proteins and is retained in the endoplasmic reticulum in invariant chain negative cells. J. Exp. Med. 1992, 176, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Chavany, C.; Mimnaugh, E.; Miller, P.; Bitton, R.; Nguyen, P.; Trepel, J.; Whitesell, L.; Schnur, R.; Moyer, J.; Neckers, L. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J. Biol. Chem. 1996, 271, 4974–4977. [Google Scholar] [PubMed]
- Chen, C.F.; Chen, Y.; Dai, K.; Chen, P.L.; Riley, D.J.; Lee, W.H. A new member of the hsp90 family of molecular chaperones interacts with the retinoblastoma protein during mitosis and after heat shock. Mol. Cell. Biol. 1996, 16, 4691–4699. [Google Scholar] [CrossRef] [PubMed]
- Ostrovsky, O.; Ahmed, N.T.; Argon, Y. The chaperone activity of GRP94 toward insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol. Biol. Cell 2009, 20, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Simmons, A.D.; Musy, M.M.; Lopes, C.S.; Hwang, L.Y.; Yang, Y.P.; Lovett, M. A direct interaction between EXT proteins and glycosyltransferases is defective in hereditary multiple exostoses. Hum. Mol. Genet. 1999, 8, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Pennell, C.A. Heat shock proteins in immune response in the Fall of 2004. Immunology 2005, 114, 297–300. [Google Scholar] [CrossRef]
- Ishiguro, S.; Watanabe, Y.; Ito, N.; Nonaka, H.; Takeda, N.; Sakai, T.; Kanaya, H.; Okada, K. SHEPHERD is the Arabidopsis GRP94 responsible for the formation of functional CLAVATA proteins. EMBO J. 2002, 21, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Landriscina, M.; Laudiero, G.; Maddalena, F.; Amoroso, M.R.; Piscazzi, A.; Cozzolino, F.; Monti, M.; Garbi, C.; Fersini, A.; Pucci, P.; et al. Mitochondrial chaperone trap1 and the calcium binding protein sorcin interact and protect cells against apoptosis induced by antiblastic agents. Cancer Res. 2010, 70, 6577–6586. [Google Scholar] [CrossRef] [PubMed]
- Nganga, A.; Bruneau, N.; Sbarra, V.; Lombardo, D.; Le Petit-Thevenin, J. Control of pancreatic bile-salt-dependent-lipase secretion by the glucose-regulated protein of 94 kDa (Grp94). Biochem. J. 2000, 352, 865–874. [Google Scholar] [PubMed]
- Amoroso, M.R.; Matassa, D.S.; Laudiero, G.; Egorova, A.V.; Polishchuk, R.S.; Maddalena, F.; Piscazzi, A.; Paladino, S.; Sarnataro, D.; Garbi, C.; et al. TRAP1 and the proteasome regulatory particle TBP7/Rpt3 interact in the endoplasmic reticulum and control cellular ubiquitination of specific mitochondrial proteins. Cell Death Differ. 2012, 19, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Brunati, A.M.; Contri, A.; Muenchbach, M.; James, P.; Marin, O.; Pinna, L.A. GRP94 (endoplasmin) co-purifies with and is phosphorylated by Golgi apparatus casein kinase. FEBS Lett. 2000, 471, 151–155. [Google Scholar] [CrossRef]
- Hecht, J.T.; Hayes, E.; Snuggs, M.; Decker, G.; Montufar-Solis, D.; Doege, K.; Mwalle, F.; Poole, R.; Stevens, J.; Duke, P.J. Calreticulin, PDI, Grp94 and BiP chaperone proteins are associated with retained COMP in pseudoachondroplasia chondrocytes. Matrix Biol. 2001, 20, 251–262. [Google Scholar] [CrossRef]
- Schmidt, B.Z.; Perlmutter, D.H. Grp78, Grp94, and Grp170 interact with alpha1-antitrypsin mutants that are retained in the endoplasmic reticulum. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G444–G455. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, G.; Chen, L.B.; Nigam, S.K. Several endoplasmic reticulum stress proteins, including ERp72, interact with thyroglobulin during its maturation. J. Biol. Chem. 1994, 269, 22990–22995. [Google Scholar] [PubMed]
- Ramakrishnan, M.; Tugizov, S.; Pereira, L.; Lee, A.S. Conformation-defective herpes simplex virus 1 glycoprotein B activates the promoter of the grp94 gene that codes for the 94-kD stress protein in the endoplasmic reticulum. DNA Cell Biol. 1995, 14, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Linnik, K.M.; Herscovitz, H. Multiple molecular chaperones interact with apolipoprotein B during its maturation. The network of endoplasmic reticulum-resident chaperones (ERp72, GRP94, calreticulin, and BiP) interacts with apolipoprotein b regardless of its lipidation state. J. Biol. Chem. 1998, 273, 21368–21373. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.R.; Norris, K.; Smith, T.; Hebert, C.; Sauk, J.J. Association of Hsp47, Grp78, and Grp94 with procollagen supports the successive or coupled action of molecular chaperones. J. Cell. Biochem. 1994, 56, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Katsumi, A.; Senda, T.; Yamashita, Y.; Yamazaki, T.; Hamaguchi, M.; Kojima, T.; Kobayashi, S.; Saito, H. Protein C Nagoya, an elongated mutant of protein C, is retained within the endoplasmic reticulum and is associated with GRP78 and GRP94. Blood 1996, 87, 4164–4175. [Google Scholar] [PubMed]
- Randow, F.; Seed, B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 2001, 3, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Melnick, J.; Aviel, S.; Argon, Y. The endoplasmic reticulum stress protein GRP94, in addition to BiP, associates with unassembled immunoglobulin chains. J. Biol. Chem. 1992, 267, 21303–21306. [Google Scholar] [PubMed]
- Foy, S.P.; Matsuuchi, L. Association of B lymphocyte antigen receptor polypeptides with multiple chaperone proteins. Immunol. Lett. 2001, 78, 149–160. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Chen, L.B.; Nigam, S.K. Multiple molecular chaperones complex with misfolded large oligomeric glycoproteins in the endoplasmic reticulum. J. Biol. Chem. 1997, 272, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, M.; McAndrew, C.; Prodromou, C.; Pearl, L.; Kalusa, A.; Jones, K.; Workman, P.; Aherne, W. Detection of the ATPase Activity of the Molecular Chaperones Hsp90 and Hsp72 Using the TranscreenerTM ADP Assay Kit. J. Biomol. Screen. 2010, 15, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Wandinger, S.K.; Richter, K.; Buchner, J. The Hsp90 chaperone machinery. J. Biol. Chem. 2008, 283, 18473–18477. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.U.; Mark Roe, S.; Vaughan, C.K.; Meyer, P.; Panaretou, B.; Piper, P.W.; Prodromou, C.; Pearl, L.H. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 2006, 440, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Scheibel, T.; Weikl, T.; Buchner, J. Two chaperone sites in Hsp90 differing in substrate specificity and ATP dependence. Proc. Natl. Acad. Sci. USA 1998, 95, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Roe, S.M.; Prodromou, C.; O’Brien, R.; Ladbury, J.E.; Piper, P.W.; Pearl, L.H. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999, 42, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Sharp, S.; Workman, P. Inhibitors of the HSP90 Molecular Chaperone: Current Status. Adv. Cancer Res. 2006, 95, 323–348. [Google Scholar] [CrossRef] [PubMed]
- Blagg, B.S.J.; Kerr, T.D. Hsp90 inhibitors: Small molecules that transform the Hsp90 protein folding machinery into a catalyst for protein degradation. Med. Res. Rev. 2006, 26, 310–338. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, A.; Blagg, B. Novobiocin and Additional Inhibitors of the Hsp90 C-Terminal Nucleotide-binding Pocket. Curr. Med. Chem. 2008, 15, 2702–2717. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.; Buchner, J. Molecular Chaperones—Cellular Machines for Protein Folding. Angew. Chem. Int. Ed. 2002, 41, 1098–1113. [Google Scholar] [CrossRef]
- Chaudhury, S.; Welch, T.R.; Blagg, B.S.J. Hsp90 as a Target for Drug Development. ChemMedChem 2006, 1, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.J.M.; Kanelakis, K.C.; Galigniana, M.D.; Morishima, Y.; Pratt, W.B. Stoichiometry, Abundance, and Functional Significance of the hsp90/hsp70-based Multiprotein Chaperone Machinery in Reticulocyte Lysate. J. Biol. Chem. 2001, 276, 30092–30098. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.J.; Mandal, A.K.; Theodoraki, M.A. Molecular chaperones and protein kinase quality control. Trends Cell Biol. 2007, 17, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kosano, H.; Stensgard, B.; Charlesworth, M.C.; McMahon, N.; Toft, D. The assembly of progesterone receptor-hsp90 complexes using purified proteins. J. Biol. Chem. 1998, 273, 32973–32979. [Google Scholar] [CrossRef] [PubMed]
- Prodromou, C.; Panaretou, B.; Chohan, S.; Siligardi, G.; O’Brien, R.; Ladbury, J.E.; Roe, S.M.; Piper, P.W.; Pearl, L.H. The ATPase cycle of Hsp90 drives a molecular “clamp” via transient dimerization of the N-terminal domains. EMBO J. 2000, 19, 4383–4392. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Yahara, I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J. Biol. Chem. 1992, 267, 7042–7047. [Google Scholar] [PubMed]
- Daturpalli, S.; Waudby, C.A.; Meehan, S.; Jackson, S.E. Hsp90 inhibits α-synuclein aggregation by interacting with soluble oligomers. J. Mol. Biol. 2013, 425, 4614–4628. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.G.; Wisén, S.; Gestwicki, J.E. Heat shock proteins 70 and 90 inhibit early stages of amyloid β-(1-42) aggregation in vitro. J. Biol. Chem. 2006, 281, 33182–33191. [Google Scholar] [CrossRef] [PubMed]
- Jakob, U.; Lilie, H.; Meyer, I.; Buchner, J. Transient interaction of Hsp90 with early unfolding intermediates of citrate synthase: Implications for heat shock in vivo. J. Biol. Chem. 1995, 270, 7288–7294. [Google Scholar] [CrossRef] [PubMed]
- Wiech, H.; Buchner, J.; Zimmermann, R.; Jakob, U. Hsp90 chaperones protein folding in vitro. Nature 1992, 358, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Yahara, I. Interaction between Casein Kinase II and the 90-kDa Stress Protein, HSP90. Biochemistry 1995, 34, 8123–8129. [Google Scholar] [CrossRef] [PubMed]
- Yonehara, M.; Minami, Y.; Kawata, Y.; Nagai, J.; Yahara, I. Heat-induced chaperone activity of HSP90. J. Biol. Chem. 1996, 271, 2641–2645. [Google Scholar] [CrossRef] [PubMed]
- Jakob, U.; Buchner, J. Assisting spontaneity: The role of Hsp90 and small Hsps as molecular chaperones. Trends Biochem. Sci. 1994, 19, 205–211. [Google Scholar] [CrossRef]
- Buchner, J. Supervising the fold: Functional principles of molecular chaperones. FASEB J. 1996, 10, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, A.; Sundström, S.; Poellinger, L.; Pongratz, I. The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol. Cell. Biol. 2001, 21, 2594–2607. [Google Scholar] [CrossRef] [PubMed]
- Holley, S.J.; Yamamoto, K.R. A role for Hsp90 in retinoid receptor signal transduction. Mol. Biol. Cell 1995, 6, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Boczek, E.E.; Reefschläger, L.G.; Dehling, M.; Struller, T.J.; Häusler, E.; Seidl, A.; Kaila, V.R.I.; Buchner, J. Conformational processing of oncogenic v-Src kinase by the molecular chaperone Hsp90. Proc. Natl. Acad. Sci. USA 2015, 112, E3189–E3198. [Google Scholar] [CrossRef] [PubMed]
- Brugge, J.S.; Erikson, E.; Erikson, R.L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell 1981, 25, 363–372. [Google Scholar] [CrossRef]
- Schulte, T.W.; Blagosklonny, M.V.; Ingui, C.; Neckers, L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J. Biol. Chem. 1995, 270, 24585–24588. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Fujita, N.; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl. Acad. Sci. USA 2000, 97, 10832–10837. [Google Scholar] [CrossRef] [PubMed]
- Stancato, L.F.; Silverstein, A.M.; Owens-Grillo, J.K.; Chow, Y.H.; Jove, R.; Pratt, W.B. The hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase. J. Biol. Chem. 1997, 272, 4013–4020. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.A.; Caplan, A.J. Quality control and fate determination of Hsp90 client proteins. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Akner, G.; Mossberg, K.; Sundqvist, K.G.; Gustafsson, J.A.; Wikström, A.C. Evidence for reversible, non-microtubule and non-microfilament-dependent nuclear translocation of hsp90 after heat shock in human fibroblasts. Eur. J. Cell Biol. 1992, 58, 356–364. [Google Scholar] [PubMed]
- Fostinis, Y.; Theodoropoulos, P.A.; Gravanis, A.; Stournaras, C. Heat shock protein HSP90 and its association with the cytoskeleton: A morphological study. Biochem. Cell Biol. 1992, 70, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Suetsugu, S.; Sagara, H.; Takenawa, T. HSP90 cross-links branched actin filaments induced by N-WASP and the Arp2/3 complex. Genes Cells 2007, 12, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Weis, F.; Moullintraffort, L.; Heichette, C.; Chrétien, D.; Garnier, C. The 90-kDa heat shock protein Hsp90 protects tubulin against thermal denaturation. J. Biol. Chem. 2010, 285, 9525–9534. [Google Scholar] [CrossRef] [PubMed]
- Krtková, J.; Zimmermann, A.; Schwarzerová, K.; Nick, P. Hsp90 binds microtubules and is involved in the reorganization of the microtubular network in angiosperms. J. Plant Physiol. 2012, 169, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Etard, C.; Roostalu, U.; Strähle, U. Shuttling of the chaperones Unc45b and Hsp90a between the A band and the Z line of the myofibril. J. Cell Biol. 2008, 180, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.P.; Fakan, S.; Tissières, A. Localization of the heat shock-induced proteins in Drosophila melanogaster tissue culture cells. Dev. Biol. 1980, 78, 86–103. [Google Scholar] [CrossRef]
- Carbajal, M.E.; Valet, J.P.; Charest, P.M.; Tanguay, R.M. Purification of Drosophila hsp 83 and immunoelectron microscopic localization. Eur. J. Cell Biol. 1990, 52, 147–156. [Google Scholar] [PubMed]
- Galigniana, M.D.; Echeverría, P.C.; Erlejman, A.G.; Piwien-Pilipuk, G. Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore. Nucleus 2010, 1, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Chambraud, B.; Berry, M.; Redeuilh, G.; Chambon, P.; Baulieu, E.E. Several regions of human estrogen receptor are involved in the formation of receptor-heat shock protein 90 complexes. J. Biol. Chem. 1990, 265, 20686–20691. [Google Scholar] [PubMed]
- Mikolajczyk, M.; Nelson, M.A. Regulation of stability of cyclin-dependent kinase CDK11p110 and a caspase-processed form, CDK11p46, by Hsp90. Biochem. J. 2004, 384, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Aligue, R.; Akhavan-Niak, H.; Russell, P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994, 13, 6099–6106. [Google Scholar] [CrossRef] [PubMed]
- Jérôme, V.; Vourc’h, C.; Baulieu, E.E.; Catelli, M.G. Cell cycle regulation of the chicken hsp90 alpha expression. Exp. Cell Res. 1993, 205, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Szent-Gyorgyi, C. A bipartite operator interacts with a heat shock element to mediate early meiotic induction of Saccharomyces cerevisiae HSP82. Mol. Cell. Biol. 1995, 15, 6754–6769. [Google Scholar] [CrossRef] [PubMed]
- Marcussen, M.; Larsen, P.J. Cell Cycle-Dependent Regulation of Cellular ATP Concentration, and Depolymerization of the Interphase Microtubular Network Induced by Elevated Cellular ATP Concentration in Whole Fibroblasts. Cell Motil. Cytoskelet. 1996, 35, 94–99. [Google Scholar] [CrossRef]
- Kohda, T.; Kondo, K.; Oishi, M. Cellular HSP90 (HSP86) mRNA level and in vitro differentiation of mouse embryonal carcinoma (F9) cells. FEBS Lett. 1991, 290, 107–110. [Google Scholar] [CrossRef]
- Shakoori, A.R.; Oberdorf, A.M.; Owen, T.A.; Weber, L.A.; Hickey, E.; Stein, J.L.; Lian, J.B.; Stein, G.S. Expression of heat shock genes during differentiation of mammalian osteoblasts and promyelocytic leukemia cells. J. Cell. Biochem. 1992, 48, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Jing, R.; Duncan, C.B.; Duncan, S.A. A small-molecule screen reveals that HSP90β promotes the conversion of induced pluripotent stem cell-derived endoderm to a hepatic fate and regulates HNF4A turnover. Development 2017, 144, 1764–1774. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Csermely, P.; Sodblacti, C. Hsp90 chaperones PPARγ and regulates differentiation and survival of 3T3-L1 adipocytes. Cell Death Differ. 2013, 20, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, P.C.; Briand, P.-A.; Picard, D. A Remodeled Hsp90 Molecular Chaperone Ensemble with the Novel Cochaperone Aarsd1 Is Required for Muscle Differentiation. Mol. Cell. Biol. 2016, 36, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Hightower, L.E.; Noonan, E.J. Discovery of the Cellular Secretion of Cell Stress Proteins. In Cellular Trafficking of Cell Stress Proteins in Health and Disease; Henderson, B., Pockley, A.G., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 1–11. ISBN 978-94-007-4740-1. [Google Scholar]
- Hightower, L.E.; Guidon, P.T. Selective release from cultured mammalian cells of heat-shock (stress) proteins that resemble glia-axon transfer proteins. J. Cell. Physiol. 1989, 138, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Gong, J.; Murshid, A. Extracellular HSPs: The complicated roles of extracellular HSPs in immunity. Front. Immunol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sahu, D.; Tsen, F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-F.; Fan, J.; Fedesco, M.; Guan, S.; Li, Y.; Bandyopadhyay, B.; Bright, A.M.; Yerushalmi, D.; Liang, M.; Chen, M.; et al. Transforming Growth Factor (TGF)-Stimulated Secretion of HSP90: Using the Receptor LRP-1/CD91 To Promote Human Skin Cell Migration against a TGF—Rich Environment during Wound Healing. Mol. Cell. Biol. 2008, 28, 3344–3358. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, Y.; Guan, S.; Fan, J.; Cheng, C.F.; Bright, A.M.; Chinn, C.; Chen, M.; Woodley, D.T. Extracellular heat shock protein-90α: Linking hypoxia to skin cell motility and wound healing. EMBO J. 2007, 26, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Woodley, D.T.; Fan, J.; Cheng, C.-F.; Li, Y.; Chen, M.; Bu, G.; Li, W. Participation of the lipoprotein receptor LRP1 in hypoxia-HSP90 autocrine signaling to promote keratinocyte migration. J. Cell Sci. 2009, 122, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Sahu, D.; Tsen, F.; Zhao, Z.; Fan, J.; Wang, X.; Kim, R.; Kuang, Y.; Chen, M.; Woodley, D.; et al. A fragment of secreted Hsp90alpha carries unique properties to accelerate acute and chronic wound healing. J. Clin. Investig. 2011, 121. [Google Scholar] [CrossRef] [PubMed]
- Gidalevitz, T.; Stevens, F.; Argon, Y. Orchestration of secretory protein folding by ER chaperones. Biochim. Biophys. Acta 2013, 1833, 2410–2424. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, N.; Lombardo, D.; Bendayan, M. Participation of GRP94-related protein in secretion of pancreatic bile salt-dependent lipase and in its internalization by the intestinal epithelium. J. Cell Sci. 1998, 111, 2665–2679. [Google Scholar] [PubMed]
- Bruneau, N.; Lombardo, D. Chaperone function of a Grp 94-related protein for folding and transport of the pancreatic bile salt-dependent lipase. J. Biol. Chem. 1995, 270, 13524–13533. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, B.; Dai, J.; Srivastava, P.K.; Zammit, D.J.; Lefrançois, L.; Li, Z. Heat Shock Protein gp96 Is a Master Chaperone for Toll-like Receptors and Is Important in the Innate Function of Macrophages. Immunity 2007, 26, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.; Wu, S.; Yang, Y.; Hao, B.; Li, Z. Drosophila glycoprotein 93 Is an ortholog of mammalian heat shock protein gp96 (grp94, HSP90b1, HSPC4) and retains disulfide bond-independent chaperone function for TLRs and integrins. J. Immunol. 2009, 183, 5121–5128. [Google Scholar] [CrossRef] [PubMed]
- Staron, M.; Wu, S.; Hong, F.; Stojanovic, A.; Du, X.; Bona, R.; Liu, B.; Li, Z. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood 2011, 117, 7136–7144. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Van, P.; Peter, F.; Soling, H.D. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J. Biol. Chem. 1989, 264, 17494–17501. [Google Scholar]
- Barton, E.R.; Park, S.; James, J.K.; Makarewich, C.A.; Philippou, A.; Eletto, D.; Lei, H.; Brisson, B.; Ostrovsky, O.; Li, Z.; et al. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 2012, 26, 3691–3702. [Google Scholar] [CrossRef] [PubMed]
- Eletto, D.; Dersh, D.; Argon, Y. GRP94 in ER quality control and stress responses. Semin. Cell Dev. Biol. 2010, 21, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Wiest, D.L.; Burkhardt, J.K.; Hester, S.; Hortsch, M.; Meyer, D.I.; Argon, Y. Membrane biogenesis during B cell differentiation: Most endoplasmic reticulum proteins are expressed coordinately. J. Cell Biol. 1990, 110, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappaB pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Berwin, B.; Reed, R.C.; Nicchitta, C.V. Virally Induced Lytic Cell Death Elicits the Release of Immunogenic GRP94/gp96. J. Biol. Chem. 2001, 276, 21083–21088. [Google Scholar] [CrossRef] [PubMed]
- Evdokimovskaya, Y.; Skarga, Y.; Vrublevskaya, V.; Morenkov, O. Release of the glucose-regulated protein 94 by baby hamster kidney cells. Cell Biochem. Funct. 2012, 30, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Spee, P.; Neefjes, J. TAP-translocated peptides specifically bind proteins in the endoplasmic reticulum, including gp96, protein disulfide isomerase and calreticulin. Eur. J. Immunol. 1997, 27, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Lammert, E.; Arnold, D.; Nijenhuis, M.; Momburg, F.; Hämmerling, G.J.; Brunner, J.; Stevanović, S.; Rammensee, H.G.; Schild, H. The endoplasmic reticulum-resident stress protein gp96 binds peptides translocated by TAP. Eur. J. Immunol. 1997, 27, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Altmeyer, A.; Maki, R.G.; Feldweg, A.M.; Heike, M.; Protopopov, V.P.; Masur, S.K.; Srivastava, P.K. Tumor-specific cell surface expression of the-KDEL containing, endoplasmic reticular heat shock protein gp96. Int. J. Cancer 1996, 69, 340–349. [Google Scholar] [CrossRef]
- Srivastava, P. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2002, 2, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Patten, D.A.; Germain, M.; Kelly, M.A.; Slack, R.S. Reactive oxygen species: Stuck in the middle of neurodegeneration. J. Alzheimers Dis. 2010, 20. [Google Scholar] [CrossRef] [PubMed]
- Gesualdi, N.M.; Chirico, G.; Pirozzi, G.; Costantino, E.; Landriscina, M.; Esposito, F. Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress 2007, 10, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Shima, G.; Aiuchi, T.; Horie, M.; Hori, K.; Nakajo, S.; Kajimoto, S.; Shibayama-Imazu, T.; Nakaya, K. Involvement of tumor necrosis factor receptor-associated protein 1 (TRAP1) in apoptosis induced by beta-hydroxyisovalerylshikonin. J. Biol. Chem. 2004, 279, 42503–42515. [Google Scholar] [CrossRef] [PubMed]
- Hua, G.; Zhang, Q.; Fan, Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J. Biol. Chem. 2007, 282, 20553–20560. [Google Scholar] [CrossRef] [PubMed]
- Im, C.-N.; Lee, J.-S.; Zheng, Y.; Seo, J.-S. Iron chelation study in a normal human hepatocyte cell line suggests that tumor necrosis factor receptor-associated protein 1 (TRAP1) regulates production of reactive oxygen species. J. Cell. Biochem. 2007, 100, 474–486. [Google Scholar] [CrossRef] [PubMed]
- Siegelin, M.D.; Dohi, T.; Raskett, C.M.; Orlowski, G.M.; Powers, C.M.; Gilbert, C.A.; Ross, A.H.; Plescia, J.; Altieri, D.C. Exploiting the mitochondrial unfolded protein response for cancer therapy in mice and human cells. J. Clin. Investig. 2011, 121, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Kroemer, G. Mitochondria in cell death: Novel targets for neuroprotection and cardioprotection. Trends Mol. Med. 2003, 9, 196–205. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial Membrane Permeabilization in Cell Death. Physiol. Rev. 2007, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Nakagawa, T.; Shimizu, S. Mitochondrial membrane permeability transition and cell death. Biochim. Biophys. Acta 2006, 1757, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Kaiser, R.A.; Sheiko, T.; Craigen, W.J.; Molkentin, J.D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007, 9, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka, J.E.; Waymire, K.G.; Levy, S.E.; Sligh, J.E.; Cai, J.; Jones, D.P.; MacGregor, G.R.; Douglas, C.W. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004, 427, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Shimizu, S.; Watanabe, T.; Yamaguchi, O.; Otsu, K.; Yamagata, H.; Inohara, H.; Kubo, T.; Tsujimoto, Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 2005, 434, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Basso, E.; Fante, L.; Fowlkes, J.; Petronilli, V.; Forte, M.A.; Bernardi, P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J. Biol. Chem. 2005, 280, 18558–18561. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.; Khan, A.; Virji, S.; Ward, J.M.; Crompton, M. Import and processing of heart mitochondrial cyclophilin D. Eur. J. Biochem. 1999, 263, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Altieri, D.C. Compartmentalized cancer drug discovery targeting mitochondrial Hsp90 chaperones. Oncogene 2009, 28, 3681–3688. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Plescia, J.; Dohi, T.; Rosa, J.; Doxsey, S.J.; Altieri, D.C. Regulation of Tumor Cell Mitochondrial Homeostasis by an Organelle-Specific Hsp90 Chaperone Network. Cell 2007, 131, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Deocaris, C.C.; Kaul, S.C.; Wadhwa, R. On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 2006, 11, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Nakamoto, H.; Neckers, L. The therapeutic target Hsp90 and cancer hallmarks. Curr. Pharm. Des. 2013, 19, 347–365. [Google Scholar] [CrossRef] [PubMed]
- Vartholomaiou, E.; Echeverría, P.C.; Picard, D. Chapter One—Unusual Suspects in the Twilight Zone Between the Hsp90 Interactome and Carcinogenesis. In Hsp90 in Cancer: Beyond the Usual Suspects; Isaacs, J., Whitesell, L., Eds.; Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2016; Volume 129, pp. 1–30. [Google Scholar]
- Sidera, K.; Patsavoudi, E. HSP90 inhibitors: Current development and potential in cancer therapy. Recent Pat. Anticancer Drug Discov. 2014, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Burns, T.F. Targeting heat shock proteins in cancer: A promising therapeutic approach. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Grenert, J.P.; Sullivan, W.P.; Fadden, P.; Haystead, T.A.; Clark, J.; Mimnaugh, E.; Krutzsch, H.; Ochel, H.J.; Schulte, T.W.; Sausville, E.; et al. The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation. J. Biol. Chem. 1997, 272, 23843–23850. [Google Scholar] [CrossRef] [PubMed]
- Castagnola, P.; Bellese, G.; Birocchi, F.; Gagliani, M.C.; Tacchetti, C.; Cortese, K. Identification of an HSP90 modulated multi-step process for ERBB2 degradation in breast cancer cells. Oncotarget 2016, 7, 85411–85429. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.D.; De Mazière, A.M.; Pisacane, P.I.; van Dijk, S.M.; Eigenbrot, C.; Sliwkowski, M.X.; Klumperman, J.; Scheller, R.H. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol. Biol. Cell 2004, 15, 5268–5282. [Google Scholar] [CrossRef] [PubMed]
- Soga, S.; Shiotsu, Y.; Akinaga, S.; Sharma, S.V. Development of radicicol analogues. Curr. Cancer Drug Targets 2003, 3, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.W.; Neckers, L.M. The benzoquinone ansamycin 17-allylamino-17-demethoxygeldanamycin binds to HSP90 and shares important biologic activities with geldanamycin. Cancer Chemother. Pharmacol. 1998, 42, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Neckers, L.; Workman, P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin. Cancer Res. 2012, 18, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Woodford, M.R.; Dunn, D.; Miller, J.B.; Jamal, S.; Neckers, L.; Mollapour, M. Chapter Two—Impact of Posttranslational Modifications on the Anticancer Activity of Hsp90 Inhibitors. In Hsp90 in Cancer: Beyond the Usual Suspects; Isaacs, J., Whitesell, L., Eds.; Advances in Cancer Research; Academic Press: Cambridge, MA, USA, 2016; Volume 129, pp. 31–50. [Google Scholar]
- Dickey, C.A.; Kamal, A.; Lundgren, K.; Klosak, N.; Bailey, R.M.; Dunmore, J.; Ash, P.; Shoraka, S.; Zlatkovic, J.; Eckman, C.B.; et al. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Investig. 2007, 117, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Klucken, J.; Shin, Y.; Masliah, E.; Hyman, B.T.; McLean, P.J. Hsp70 reduces α-synuclein aggregation and toxicity. J. Biol. Chem. 2004, 279, 25497–25502. [Google Scholar] [CrossRef] [PubMed]
- McLean, P.J.; Kawamata, H.; Shariff, S.; Hewett, J.; Sharma, N.; Ueda, K.; Breakefield, X.O.; Hyman, B.T. TorsinA and heat shock proteins act as molecular chaperones: Suppression of a-synuclein aggregation. J. Neurochem. 2002, 83, 846–854. [Google Scholar] [CrossRef] [PubMed]
- McLean, P.J.; Klucken, J.; Shin, Y.; Hyman, B.T. Geldanamycin induces Hsp70 and prevents α-synuclein aggregation and toxicity in vitro. Biochem. Biophys. Res. Commun. 2004, 321, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Anselmo, D. In vitro reconstitution of a functional duck hepatitis B virus reverse transcriptase: Posttranslational activation by Hsp90. J. Virol. 2000, 74, 11447–11455. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Toft, D.; Anselmo, D.; Wang, X. In vitro reconstitution of functional hepadnavirus reverse transcriptase with cellular chaperone proteins. J. Virol. 2002, 76, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Toft, D.O.; Seeger, C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 1997, 16, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Nageshan, R.K.; Ranade, S.; Tatu, U. Heat shock protein 90 from neglected protozoan parasites. Biochim. Biophys. Acta 2012, 1823, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Shonhai, A.; Maier, A.G.; Przyborski, J.M.; Blatch, G.L. Intracellular protozoan parasites of humans: The role of molecular chaperones in development and pathogenesis. Protein Pept. Lett. 2011, 18, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Graefe, S.E.B.; Wiesgigl, M.; Gaworski, I.; Macdonald, A.; Clos, J. Inhibition of HSP90 in Trypanosoma cruzi induces a stress response but no stage differentiation. Eukaryot. Cell 2002, 1, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Bentel, M.; Harder, S.; Wiesgigl, M.; Heukeshoven, J.; Gelhaus, C.; Krause, E.; Clos, J.; Bruchhaus, I. Developmentally induced changes of the proteome in the protozoan parasite Leishmania donovani. Proteomics 2003, 3, 1811–1829. [Google Scholar] [CrossRef] [PubMed]
- Sieriebriennikov, B.; Markov, G.V.; Witte, H.; Sommer, R.J. The Role of DAF-21/Hsp90 in Mouth-Form Plasticity in Pristionchus pacificus. Mol. Biol. Evol. 2017, 34, 1644–1653. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Venable, J.; LaPointe, P.; Hutt, D.M.; Koulov, A.V.; Coppinger, J.; Gurkan, C.; Kellner, W.; Matteson, J.; Plutner, H.; et al. Hsp90 Cochaperone Aha1 Downregulation Rescues Misfolding of CFTR in Cystic Fibrosis. Cell 2006, 127, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jang, S.-W.; Park, E.; Oh, M.; Park, S.; Ko, J. The role of heat shock protein 90 in migration and proliferation of vascular smooth muscle cells in the development of atherosclerosis. J. Mol. Cell. Cardiol. 2014, 72, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Businaro, R.; Profumo, E.; Tagliani, A.; Buttari, B.; Leone, S.; D’Amati, G.; Ippoliti, F.; Leopizzi, M.; D’Arcangelo, D.; Capoano, R.; et al. Heat-shock protein 90: A novel autoantigen in human carotid atherosclerosis. Atherosclerosis 2009, 207, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, G.; Guindon, L.; Perez, L.; Evans-Molina, C.; Blum, J. Heat shock protein 90, a potential biomarker for type I diabetes, is secreted by human pancreatic beta cells in response to cytokine stress (BA7P.161). J. Immunol. 2015, 194, 115.21. [Google Scholar]
- Lazaro, I.; Oguiza, A.; Recio, C.; Mallavia, B.; Madrigal-Matute, J.; Blanco, J.; Egido, J.; Martin-Ventura, J.L.; Gomez-Guerrero, C. Targeting HSP90 ameliorates nephropathy and atherosclerosis through suppression of NF-κB and STAT signaling pathways in diabetic mice. Diabetes 2015, 64, 3600–3613. [Google Scholar] [CrossRef] [PubMed]
- Ansa-Addo, E.A.; Thaxton, J.; Hong, F.; Wu, B.X.; Zhang, Y.; Fugle, C.W.; Metelli, A.; Riesenberg, B.; Williams, K.; Gewirth, D.T.; et al. Clients and Oncogenic Roles of Molecular Chaperone gp96/grp94. Curr. Top. Med. Chem. 2016, 16, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Staron, M.; Hong, F.; Wu, B.X.; Sun, S.; Morales, C.; Crosson, C.E.; Tomlinson, S.; Kim, I.; Wu, D.; et al. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 6877–6882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.X.; Metelli, A.; Thaxton, J.E.; Hong, F.; Rachidi, S.; Ansa-Addo, E.; Sun, S.; Vasu, C.; Yang, Y.; et al. GP96 is a GARP chaperone and controls regulatory T cell functions. J. Clin. Investig. 2015, 125, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Wanderling, S.; Simen, B.B.; Ostrovsky, O.; Ahmed, N.T.; Vogen, S.M.; Gidalevitz, T.; Argon, Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol. Biol. Cell 2007, 18, 3764–3775. [Google Scholar] [CrossRef] [PubMed]
- Staron, M.; Yang, Y.; Liu, B.; Li, J.; Shen, Y.; Zúñiga-Pflücker, J.C.; Aguila, H.L.; Goldschneider, I.; Li, Z. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood 2010, 115, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Liu, B.; Chiosis, G.; Gewirth, D.T.; Li, Z. α7 helix region of αI domain is crucial for integrin binding to endoplasmic reticulum chaperone gp96: A potential therapeutic target for cancer metastasis. J. Biol. Chem. 2013, 288, 18243–18248. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; DeLeo, A.B.; Old, L.J. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc. Natl. Acad. Sci. USA 1986, 83, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.C.; Lin, C.Y.; Lee, L.Y.; Chen, Y.J.; Lu, Y.C.; Wang, H.M.; Liao, C.T.; Chang, J.T.C.; Cheng, A.J. Molecular chaperones as a common set of proteins that regulate the invasion phenotype of head and neck cancer. Clin. Cancer Res. 2011, 17, 4629–4641. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Ma, C.; Sun, S.; Zhang, J.; Sun, Y. Expression of heat-shock protein gp96 in gallbladder cancer and its prognostic clinical significance. Int. J. Clin. Exp. Pathol. 2015, 8, 1946–1953. [Google Scholar] [PubMed]
- Haverty, A.A.; Harmey, J.H.; Redmond, H.P.; Bouchier-Hayes, D.J. Interleukin-6 upregulates GP96 expression in breast cancer. J. Surg. Res. 1997, 69, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Rachidi, S.; Sun, S.; Wu, B.X.; Jones, E.; Drake, R.R.; Ogretmen, B.; Cowart, L.A.; Clarke, C.J.; Hannun, Y.A.; Chiosis, G.; et al. Endoplasmic reticulum heat shock protein gp96 maintains liver homeostasis and promotes hepatocellular carcinogenesis. J. Hepatol. 2015, 62, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; White-Gilbertson, S.; Kellner, J.; Rachidi, S.; Usmani, S.Z.; Chiosis, G.; Depinho, R.; Li, Z.; Liu, B. Molecular chaperone gp96 is a novel therapeutic target of multiple myeloma. Clin. Cancer Res. 2013, 19, 6242–6251. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.; Rachidi, S.; Hong, F.; Sun, S.; Ouyang, X.; Wallace, C.; Zhang, Y.; Garret-Mayer, E.; Wu, J.; Liu, B.; et al. Immune chaperone gp96 drives the contributions of macrophages to inflammatory colon tumorigenesis. Cancer Res. 2014, 74, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Ménoret, A.; Cohen, N. Cell surface expression of the endoplasmic reticular heat shock protein gp96 is phylogenetically conserved. J. Immunol. 1999, 163, 4133–4139. [Google Scholar] [PubMed]
- Dai, J.; Liu, B.; Caudill, M.M.; Zheng, H.; Qiao, Y.; Podack, E.R.; Li, Z. Cell surface expression of heat shock protein gp96 enhances cross-presentation of cellular antigens and the generation of tumor-specific T cell memory. Cancer Immun. 2003, 3, 1–11. [Google Scholar] [PubMed]
- Linderoth, N.A.; Popowicz, A.; Sastry, S. Identification of the peptide-binding site in the heat shock chaperone/tumor rejection antigen gp96 (Grp94). J. Biol. Chem. 2000, 275, 5472–5477. [Google Scholar] [CrossRef] [PubMed]
- Kovalchin, J.T.; Murthy, A.S.; Horattas, M.C.; Guyton, D.P.; Chandawarkar, R.Y. Determinants of efficacy of immunotherapy with tumor-derived heat shock protein gp96. Cancer Immun. Arch. 2001, 1, 7. [Google Scholar]
- Sabbatino, F.; Favoino, E.; Wang, Y.; Wang, X.; Villani, V.; Cai, L.; Yang, L.; Ferrone, S.; Ferrone, C. Grp94-specific monoclonal antibody to counteract BRAF inhibitor resistance in BRAFV600E melanoma. J. Transl. Med. 2015, 13, K12. [Google Scholar] [CrossRef]
- Taldone, T.; Ochiana, S.O.; Patel, P.D.; Chiosis, G. Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol. Sci. 2014, 35, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Duerfeldt, A.S.; Peterson, L.B.; Maynard, J.C.; Ng, C.L.; Eletto, D.; Ostrovsky, O.; Shinogle, H.E.; Moore, D.S.; Argon, Y.; Nicchitta, C.V.; et al. Development of a Grp94 inhibitor. J. Am. Chem. Soc. 2012, 134, 9796–9804. [Google Scholar] [CrossRef] [PubMed]
- Immormino, R.M.; Metzger, L.E.; Reardon, P.N.; Dollins, D.E.; Blagg, B.S.J.; Gewirth, D.T. Different poses for ligand and chaperone in inhibitor-bound Hsp90 and GRP94: Implications for paralog-specific drug design. J. Mol. Biol. 2009, 388, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.D.; Yan, P.; Seidler, P.M.; Patel, H.J.; Sun, W.; Yang, C.; Que, N.S.; Taldone, T.; Finotti, P.; Stephani, R.A.; et al. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat. Chem. Biol. 2013, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, L.; Tabas, I. Role of endoplasmic reticulum stress in metabolic disease and other disorders. Annu. Rev. Med. 2012, 63, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Wang, Y.; Zhang, H.; Han, F. The role of endoplasmic reticulum stress in neurodegenerative disease. Apoptosis 2017, 22, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Viana, R.J.S.; Steer, C.J.; Rodrigues, C.M.P. Amyloid-β peptide-induced secretion of endoplasmic reticulum chaperone glycoprotein GRP94. J. Alzheimers Dis. 2011, 27, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Villadiego, J.; Labrador-Garrido, A.; Franco, J.M.; Leal-Lasarte, M.; De Genst, E.J.; Dobson, C.M.; Pozo, D.; Toledo-Aral, J.J.; Roodveldt, C. Immunization with α-synuclein/Grp94 reshapes peripheral immunity and suppresses microgliosis in a chronic Parkinsonism model. Glia 2018, 66, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hong, F.; Gewirth, D.; Guo, B.; Liu, B.; Li, Z. The molecular chaperone gp96/GRP94 interacts with toll-like receptors and integrins via its C-terminal hydrophobic domain. J. Biol. Chem. 2012, 287, 6735–6742. [Google Scholar] [CrossRef] [PubMed]
- Xagorari, A.; Chlichlia, K. Toll-Like Receptors and Viruses: Induction of Innate Antiviral Immune Responses. Open Microbiol. J. 2008, 2, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Bloor, S.; Maelfait, J.; Krumbach, R.; Beyaert, R.; Randow, F. Endoplasmic reticulum chaperone gp96 is essential for infection with vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 2010, 107, 6970–6975. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Bellamy, R.; Taylor, J. BiP (GRP78) and endoplasmin (GRP94) are induced following rotavirus infection and bind transiently to an endoplasmic reticulum-localized virion component. J. Virol. 1998, 72, 9865–9872. [Google Scholar] [PubMed]
- Fan, H.; Yan, X.; Zhang, Y.; Zhang, X.; Gao, Y.; Xu, Y.; Wang, F.; Meng, S. Increased Expression of Gp96 by HBx-Induced NF-κB Activation Feedback Enhances Hepatitis B Virus Production. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Li, C.L.; Lang, Z.W.; Gao, G.F.; Tien, P. Significant correlation between expression level of HSP gp96 and progression of hepatitis B virus induced diseases. World J. Gastroenterol. 2004, 10, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Wong, J.; Luo, H. Viral interaction with molecular chaperones: Role in regulating viral infection. Arch. Virol. 2010, 155, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Shin, H.J.; Cho, Y.H.; Rho, H.M. Expression of stable hepatitis B viral polymerase associated with GRP94 in E. coli. Arch. Virol. 2000, 145, 1305–1320. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.Y.; Tsai, M.C.; Wu, Y.P.; Wang, R.Y.L. Identification of heat-shock protein 90 beta in Japanese encephalitis virus-induced secretion proteins. J. Gen. Virol. 2011, 92, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Strbo, N.; Romero, L.; Podack, E.R.; Villasante, E.F.; Edgel, K.A.; Strbo, N. Secreted heat shock protein gp96-Ig vaccine for malaria prophylaxis. J. Immunol. 2016, 196, 146.10. [Google Scholar]
- Kim, D.; Song, L.; Wang, J.; Wu, H.; Gu, G.; Sugi, Y.; Li, Z.; Wang, H. GRP94 Is an Essential Regulator of Pancreatic β-Cell Development, Mass, and Function in Male Mice. Endocrinology 2018, 159, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, M.R.; Matassa, D.S.; Sisinni, L.; Lettini, G.; Landriscina, M.; Esposito, F. TRAP1 revisited: Novel localizations and functions of a “next-generation” biomarker (Review). Int. J. Oncol. 2014, 45, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Matassa, D.S.; Agliarulo, I.; Avolio, R.; Landriscina, M.; Esposito, F. TRAP1 Regulation of Cancer Metabolism: Dual Role as Oncogene or Tumor Suppressor. Genes 2018, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Costantino, E.; Maddalena, F.; Calise, S.; Piscazzi, A.; Tirino, V.; Fersini, A.; Ambrosi, A.; Neri, V.; Esposito, F.; Landriscina, M. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptotis in human colorectal carcinoma cells. Cancer Lett. 2009, 279, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Pak, M.G.; Koh, H.J.; Roh, M.S. Clinicopathologic significance of TRAP1 expression in colorectal cancer: A large scale study of human colorectal adenocarcinoma tissues. Diagn. Pathol. 2017, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Maddalena, F.; Sisinni, L.; Lettini, G.; Condelli, V.; Matassa, D.S.; Piscazzi, A.; Amoroso, M.R.; La Torre, G.; Esposito, F.; Landriscina, M. Resistance to paclitxel in breast carcinoma cells requires a quality control of mitochondrial antiapoptotic proteins by TRAP1. Mol. Oncol. 2013, 7, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, J.; Huang, Z.; Wei, P.; Liu, Y.; Hao, J.; Zhao, L.; Zhang, F.; Tu, Y.; Wei, T. Aberrantly upregulated TRAP1 is required for tumorigenesis of breast cancer. Oncotarget 2015, 6, 44495–44508. [Google Scholar] [CrossRef] [PubMed]
- Leav, I.; Plescia, J.; Goel, H.L.; Li, J.; Jiang, Z.; Cohen, R.J.; Languino, L.R.; Altieri, D.C. Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer. Am. J. Pathol. 2010, 176, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Lisanti, S.; Garlick, D.S.; Bryant, K.G.; Tavecchio, M.; Mills, G.B.; Lu, Y.; Kossenkov, A.V.; Showe, L.C.; Languino, L.R.; Altieri, D.C. Transgenic expression of the mitochondrial chaperone TNFR-associated protein 1 (TRAP1) accelerates prostate cancer development. J. Biol. Chem. 2016, 291, 25247–25254. [Google Scholar] [CrossRef] [PubMed]
- Im, C.N.; Seo, J.S. Overexpression of tumor necrosis factor receptor-associated protein 1 (TRAP1), leads to mitochondrial aberrations in mouse fibroblast NIH/3T3 cells. BMB Rep. 2014, 47, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Siegelin, M.D.; Plescia, J.; Raskett, C.M.; Gilbert, C.A.; Ross, A.H.; Altieri, D.C. Global Targeting of Subcellular Heat Shock Protein-90 Networks for Therapy of Glioblastoma. Mol. Cancer Ther. 2010, 9, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Aust, S.; Bachmayr-Heyda, A.; Pateisky, P.; Tong, D.; Darb-Esfahani, S.; Denkert, C.; Chekerov, R.; Sehouli, J.; Mahner, S.; Van Gorp, T.; et al. Role of TRAP1 and estrogen receptor alpha in patients with ovarian cancer—A study of the OVCAD consortium. Mol. Cancer 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Matassa, D.S.; Amoroso, M.R.; Lu, H.; Avolio, R.; Arzeni, D.; Procaccini, C.; Faicchia, D.; Maddalena, F.; Simeon, V.; Agliarulo, I.; et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 2016, 23, 1542–1554. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Tsutsumi, S.; Muhlebach, G.; Sourbier, C.; Lee, M.-J.; Lee, S.; Vartholomaiou, E.; Tatokoro, M.; Beebe, K.; Miyajima, N.; et al. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc. Natl. Acad. Sci. USA 2013, 110, E1604–E1612. [Google Scholar] [CrossRef] [PubMed]
- Takamura, H.; Koyama, Y.; Matsuzaki, S.; Yamada, K.; Hattori, T.; Miyata, S.; Takemoto, K.; Tohyama, M.; Katayama, T. TRAP1 Controls Mitochondrial Fusion/Fission Balance through Drp1 and Mff Expression. PLoS ONE 2012, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Karsten, P.; Hamm, S.; Pogson, J.H.; Müller-Rischart, A.K.; Exner, N.; Haass, C.; Whitworth, A.J.; Winklhofer, K.F.; Schulz, J.B.; et al. TRAP1 rescues PINK1 loss-of-function phenotypes. Hum. Mol. Genet. 2013, 22, 2829–2841. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Loh, S.H.; Martins, L.M. Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson’s disease. Cell Death Dis. 2013, 4, e467. [Google Scholar] [CrossRef] [PubMed]
- Butler, E.K.; Voigt, A.; Lutz, A.K.; Toegel, J.P.; Gerhardt, E.; Karsten, P.; Falkenburger, B.; Reinartz, A.; Winklhofer, K.F.; Schulz, J.B. The mitochondrial chaperone protein TRAP1 mitigates α-synuclein toxicity. PLoS Genet. 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Casadei, N.; Sood, P.; Ulrich, T.; Fallier-Becker, P.; Kieper, N.; Helling, S.; May, C.; Glaab, E.; Chen, J.; Nuber, S.; et al. Mitochondrial defects and neurodegeneration in mice overexpressing wild-type or G399S mutant HtrA2. Hum. Mol. Genet. 2016, 25, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.C.; Zimprich, A.; Berrio, D.A.C.; Schindler, K.M.; Maurer, B.; Schulte, C.; Bus, C.; Hauser, A.K.; Kübler, M.; Lewin, R.; et al. Metformin reverses TRAP1 mutation-associated alterations in mitochondrial function in Parkinson’s disease. Brain 2017, 140, 2444–2459. [Google Scholar] [CrossRef] [PubMed]
- Fismen, S.; Thiyagarajan, D.; Seredkina, N.; Nielsen, H.; Jacobsen, S.; Elung-Jensen, T.; Kamper, A.L.; Johansen, S.D.; Mortensen, E.S.; Rekvig, O.P. Impact of the tumor necrosis factor receptor-associated protein 1 (Trap1) on renal DNaseI shutdown and on progression of murine and human lupus nephritis. Am. J. Pathol. 2013, 182, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Saisawat, P.; Kohl, S.; Hilger, A.C.; Hwang, D.Y.; Yung Gee, H.; Dworschak, G.C.; Tasic, V.; Pennimpede, T.; Natarajan, S.; Sperry, E.; et al. Whole-exome resequencing reveals recessive mutations in TRAP1 in individuals with CAKUT and VACTERL association. Kidney Int. 2014, 85, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Protein Name | Alternative Name | Human Gene ID |
|---|---|---|---|
| HSPC1 | HSPC1 | HSP90AA1; HSPN; LAP2; HSP86; HSPC1; HSPCA; HSP89; HSP90; HSP90A; HSP90N; HSPCAL1; HSPCAL4; FLJ31884 | 3320 |
| HSPC2 | HSPC2 | HSP90AA2; HSPCA; HSPCAL3; HSP90ALPHA | 3324 |
| HSPC3 | HSPC3 | HSP90AB1; HSPC2; HSPCB; D6S182; HSP90B; FLJ26984; HSP90-BETA | 3326 |
| HSPC4 | HSPC4 | HSP90B1; ECGP; GP96; TRA1; GRP94; endoplasmin | 7184 |
| HSPC5 | HSPC5 | TRAP1; HSP75; HSP90L | 10131 |
| Co-Chaperone or Protein Partner | Role in Carcinogenesis |
|---|---|
| Aha1 | Stimulates ATPase activity |
| Cdc37 | Mediates activation of protein kinase substrates |
| CHIP | Involved in degradation of unfolded client proteins |
| Cyclophilin-40 | Peptidyl propyl isomerase |
| FKBP51 and 52 | Peptidyl propyl isomerase |
| HOP | Mediates interaction between Hsp90 and Hsp70 |
| Hsp40 | Stabilizes and delivers client proteins to Hsp90 complex |
| Hsp70 | Stabilizes and delivers client proteins to Hsp90 |
| p23 | Stabilizes closed, clamped substrate bound |
| HIP | Inhibits ATPase activity of Hsp70 |
| PP5 | Protein phosphatase 5 |
| Tom70 | Facilitates translocation of pre-proteins into mitochondrial matrix |
| Sgt1 | Client adaptor, involved in client recruitment |
| WISp39 | Regulates p21 stability |
| GRP94 Client Protein | Citation | TRAP1 Client Protein | Citation |
|---|---|---|---|
| MHC class II | [72] | TNF receptor | [56] |
| ErbB2 | [73] | retinoblastoma protein | [74] |
| Insulin-like growth factor -II | [75] | Tumour suppressor EXT proteins | [76] |
| P43 | [77] | CYP-D | [60] |
| CLAVATA complex | [78] | Calcium-binding protein, Sorcin | [79] |
| Bile salt dependent lipase | [80] | Proteasome regulatory particle TBP7 | [81] |
| Golgi apparatus casein kinase | [82] | ||
| Cartilage oligomeric matrix protein (COMP) | [83] | ||
| α1-antitrypsin 1 | [84] | ||
| Thyroglobulin | [85] | ||
| HSV-1 glycoprotein B 1 | [86] | ||
| Apolipoprotein B | [87] | ||
| Collagen | [88] | ||
| Protein C 1 | [89] | ||
| Protein apparatus casein Kinase | [82] | ||
| TLR 1,2,4 | [90] | ||
| Ig heavy chain and light chain | [91] | ||
| Ig α chain | [92] | ||
| Integrins | [90] | ||
| Thrombospondin | [93] |
| Protein Client | Role in Carcinogenesis |
|---|---|
| Receptor tyrosine kinases, serine/threonine kinases, steroid hormone receptors | Uncontrolled proliferation |
| Telomerases | Immortalization |
| AKT, NF-κB, p53, c-MET, Apaf-1, Survivin | Anti-apoptotic |
| HIF1α, VEGFR, PI3K/AKT, RTKs, flt-3 | Angiogenesis |
| IRAK3 | Escaping immune destruction |
| ARNT, ARRB1, HIF-1α, HMG1, SREBF1 | Modification of cellular energetics |
| FANCA, MAFG, NEK8, NEK9, NEK11 | Genome instability and mutation |
| IL-6, IL-8, IRAK1, IRAK2, IRAK3 | Tumor-promoting inflammation |
| Plk, Wee1, Myc1, CDK4, CDK6, Myt1 | Evading growth suppressors |
| MMP2,c-MET, SSDF-1 | Invasion and metastasis |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoter, A.; El-Sabban, M.E.; Naim, H.Y. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. Int. J. Mol. Sci. 2018, 19, 2560. https://doi.org/10.3390/ijms19092560
Hoter A, El-Sabban ME, Naim HY. The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. International Journal of Molecular Sciences. 2018; 19(9):2560. https://doi.org/10.3390/ijms19092560
Chicago/Turabian StyleHoter, Abdullah, Marwan E. El-Sabban, and Hassan Y. Naim. 2018. "The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease" International Journal of Molecular Sciences 19, no. 9: 2560. https://doi.org/10.3390/ijms19092560
APA StyleHoter, A., El-Sabban, M. E., & Naim, H. Y. (2018). The HSP90 Family: Structure, Regulation, Function, and Implications in Health and Disease. International Journal of Molecular Sciences, 19(9), 2560. https://doi.org/10.3390/ijms19092560







