The Na,K-ATPase-Dependent Src Kinase Signaling Changes with Mesenteric Artery Diameter
Abstract
1. Introduction
2. Results
3. Discussion
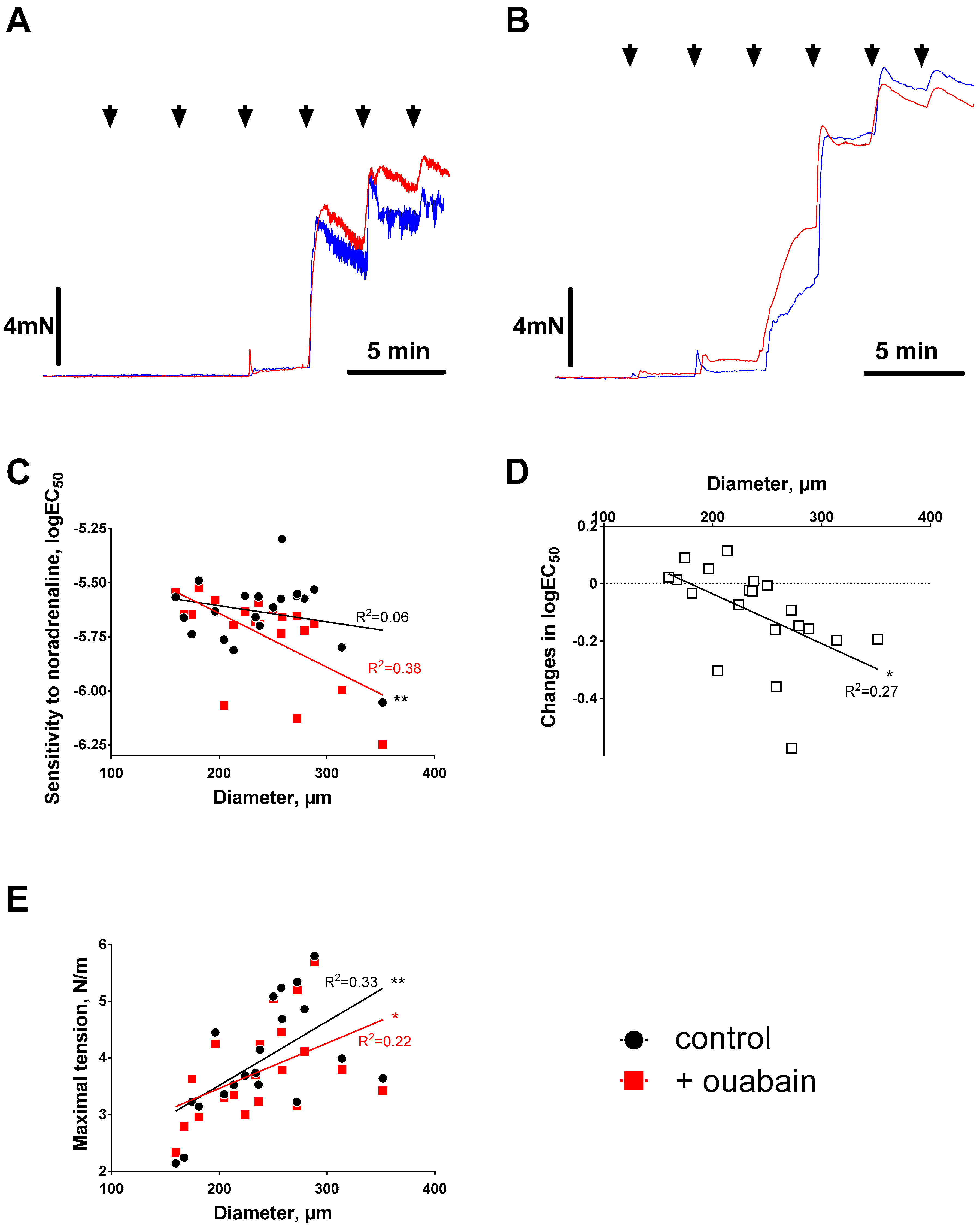
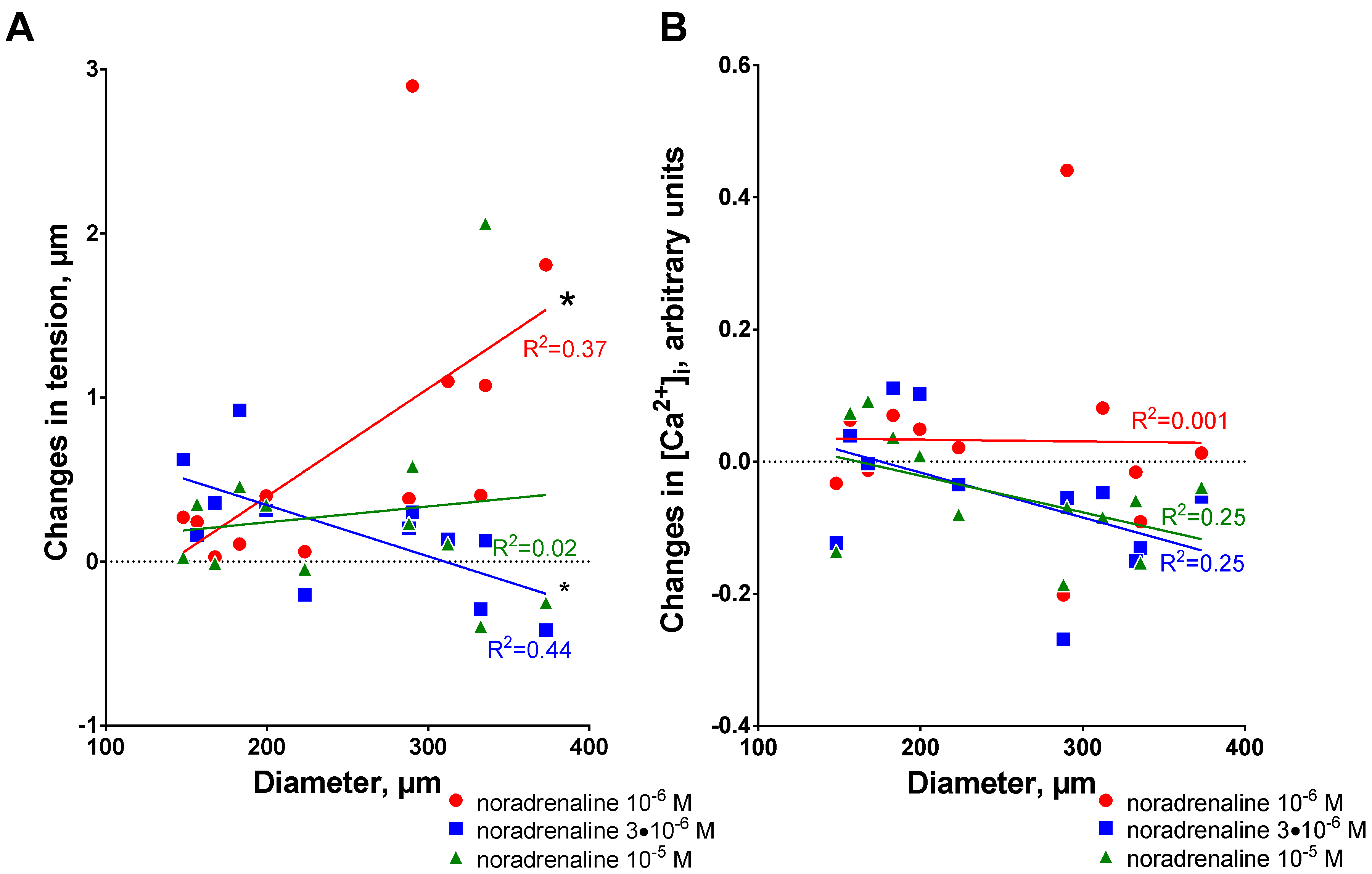
3.1. Ouabain-Induced Potentiation of Contraction Increases with Arterial Diameter
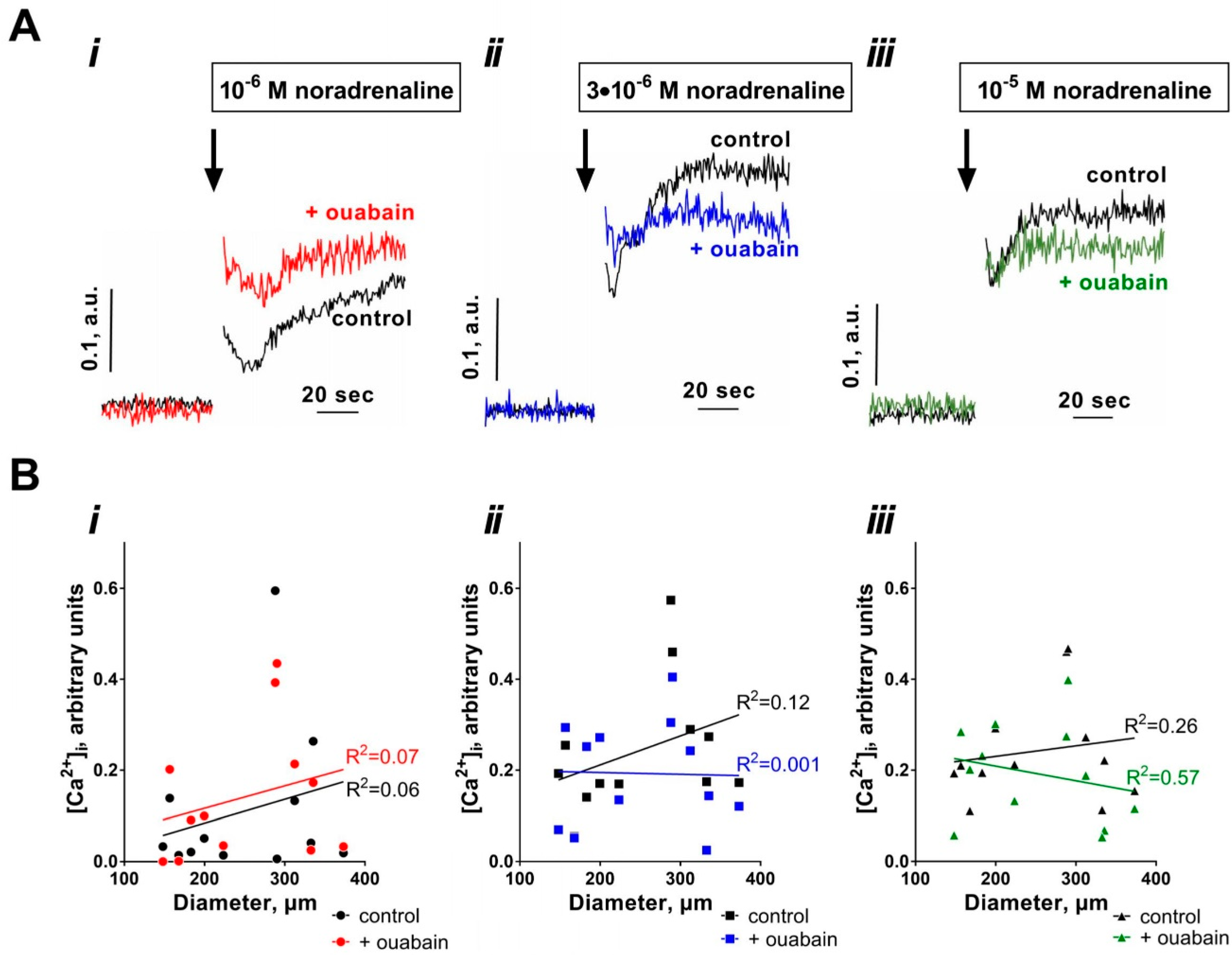
3.2. Sensitization to [Ca2+]i Increases with Arterial Diameter
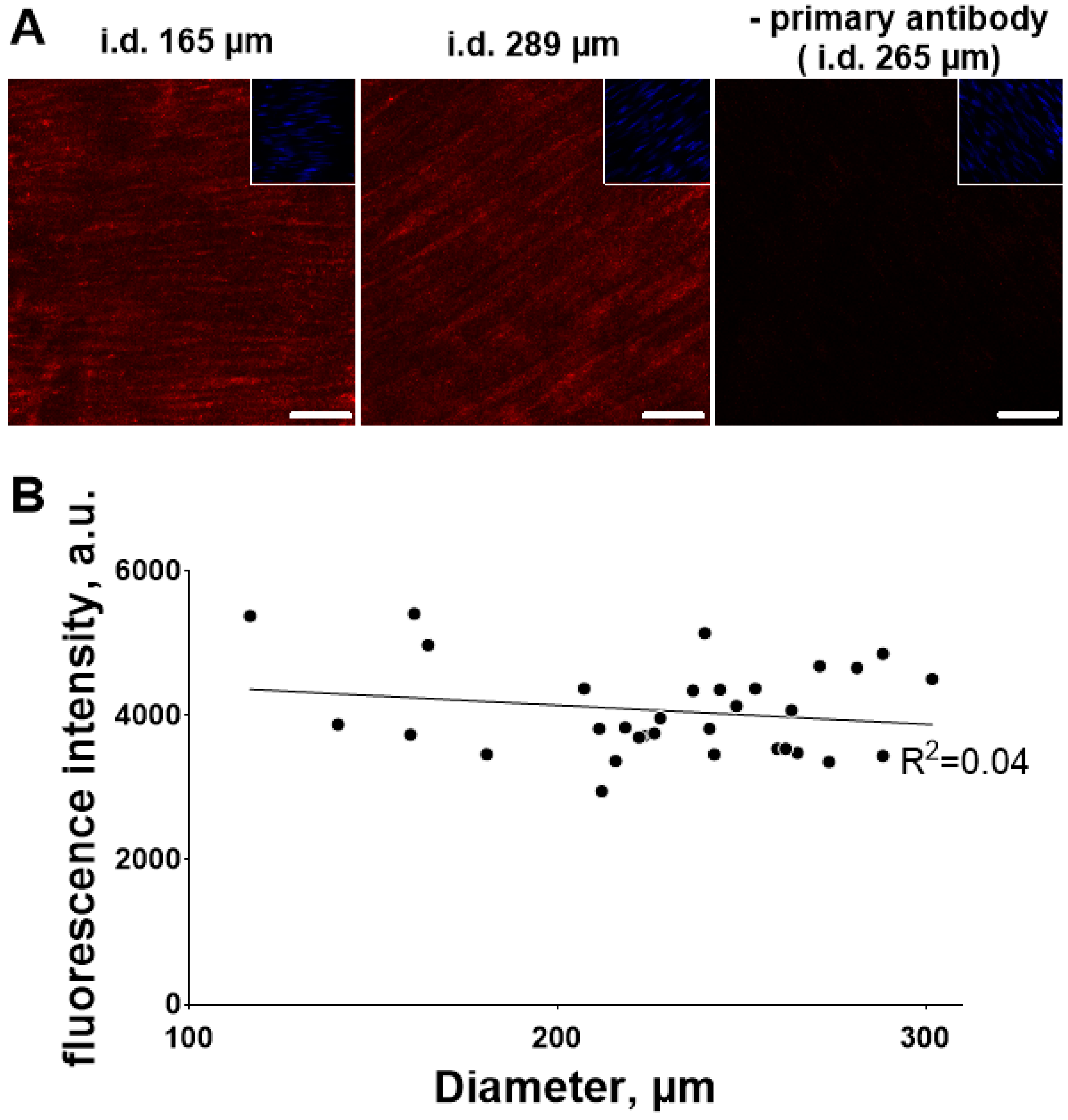
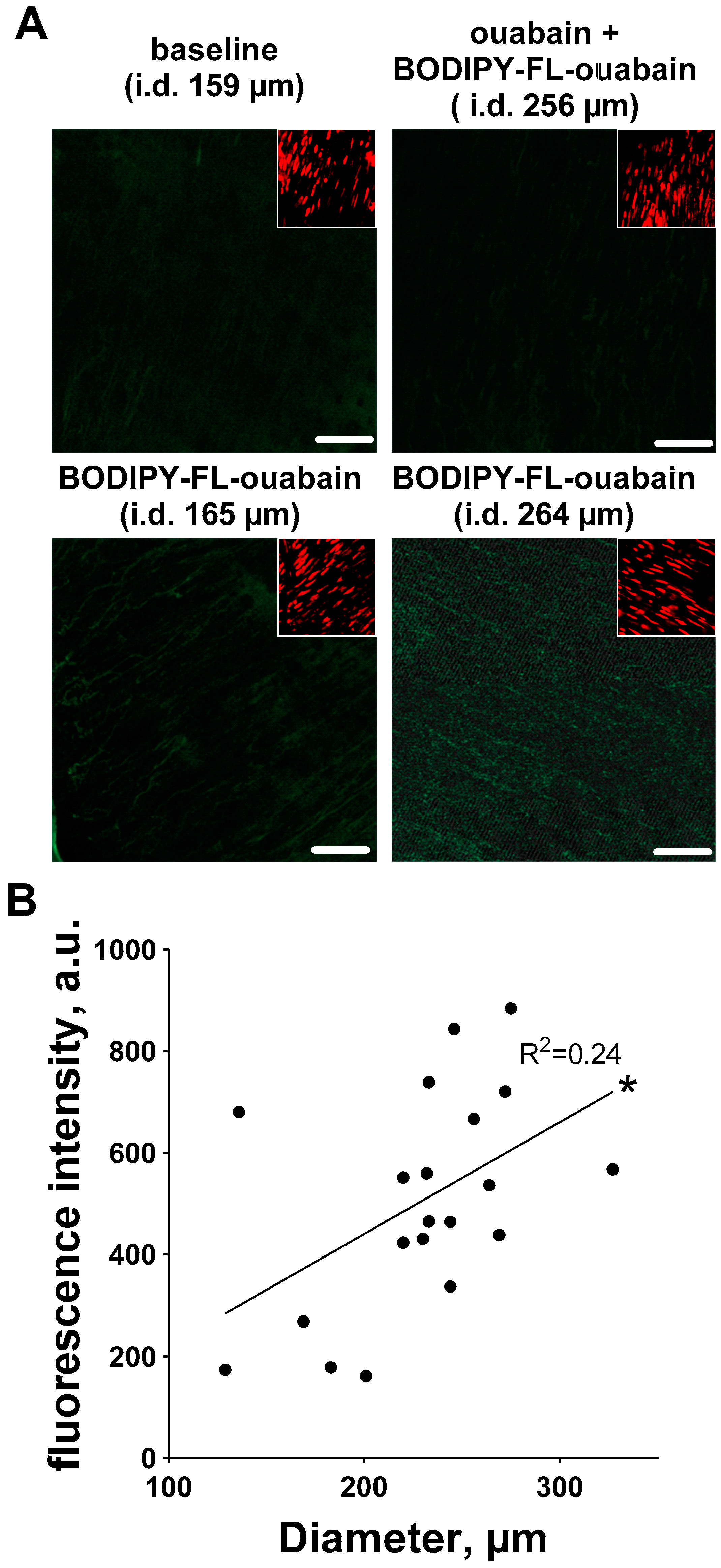
3.3. Ouabain-Induced Src Phosphorylation Increases with Arterial Diameter and Is Associated with an Increase in High-Affinity Ouabain Binding Sites
4. Significance
5. Methods
5.1. Isometric Force and [Ca2+]i Measurements
5.2. Semi-Quantification of Total Src and Phosphorylated Src in Mesenteric Artery Wall
5.3. Semi-Quantification of Ouabain-Binding Sites in the Arterial Wall
5.4. Solutions and Chemicals
6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blaustein, M.P.; Chen, L.; Hamlyn, J.M.; Leenen, F.H.; Lingrel, J.B.; Wier, W.G.; Zhang, J. Pivotal role of alpha2 Na+ pumps and their high affinity ouabain binding site in cardiovascular health and disease. J. Physiol. 2016, 594, 6079–6103. [Google Scholar] [CrossRef] [PubMed]
- Manunta, P.; Hamilton, J.; Rogowski, A.C.; Hamilton, B.P.; Hamlyn, J.M. Chronic hypertension induced by ouabain but not digoxin in the rat: Antihypertensive effect of digoxin and digitoxin. Hypertens. Res. 2000, 23, 77–85. [Google Scholar] [CrossRef]
- Pulgar, V.M.; Jeffers, A.B.; Rashad, H.M.; Diz, D.I.; Aileru, A.A. Increased constrictor tone induced by ouabain treatment in rats. J. Cardiovasc. Pharmacol. 2013, 62, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Briones, A.M.; Xavier, F.E.; Arribas, S.M.; Gonzalez, M.C.; Rossoni, L.V.; Alonso, M.J.; Salaices, M. Alterations in structure and mechanics of resistance arteries from ouabain-induced hypertensive rats. Am. J. Physiol. 2006, 291, H193–H201. [Google Scholar] [CrossRef] [PubMed]
- Dostanic, I.; Paul, R.J.; Lorenz, J.N.; Theriault, S.; Van Huysse, J.W.; Lingrel, J.B. The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am. J. Physiol. 2005, 288, H477–H485. [Google Scholar]
- Yuan, C.M.; Manunta, P.; Hamlyn, J.M.; Chen, S.; Bohen, E.; Yeun, J.; Haddy, F.J.; Pamnani, M.B. Long-term ouabain administration produces hypertension in rats. Hypertension 1993, 22, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Dostanic-Larson, I.; Van Huysse, J.W.; Lorenz, J.N.; Lingrel, J.B. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc. Natl. Acad. Sci. USA 2005, 102, 15845–15850. [Google Scholar] [CrossRef] [PubMed]
- Manunta, P.; Hamilton, B.P.; Hamlyn, J.M. Salt intake and depletion increase circulating levels of endogenous ouabain in normal men. A J. Physiol. 2006, 290, R553–R559. [Google Scholar] [CrossRef] [PubMed]
- Hamlyn, J.M.; Blaustein, M.P. Endogenous Ouabain: Recent Advances and Controversies. Hypertension 2016, 68, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Karashima, E.; Hamlyn, J.M.; Blaustein, M.P. Ouabain-digoxin antagonism in rat arteries and neurones. J. Physiol. 2014, 592, 941–969. [Google Scholar] [CrossRef] [PubMed]
- Pierdomenico, S.D.; Bucci, A.; Manunta, P.; Rivera, R.; Ferrandi, M.; Hamlyn, J.M.; Lapenna, D.; Cuccurullo, F.; Mezzetti, A. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am. J. Hypertens. 2001, 14, 44–50. [Google Scholar] [CrossRef]
- Manunta, P.; Stella, P.; Rivera, R.; Ciurlino, D.; Cusi, D.; Ferrandi, M.; Hamlyn, J.M.; Bianchi, G. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension 1999, 34, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Matchkov, V.V. Mechanisms of cellular synchronization in the vascular wall. Mechanisms of vasomotion. Dan. Med. Bull. 2010, 57, B4191. [Google Scholar] [PubMed]
- Blanco, G.; Mercer, R.W. Isozymes of the Na-K-ATPase: Heterogeneity in structure, diversity in function. Am. J. Physiol. 1998, 275, F633–F650. [Google Scholar] [CrossRef] [PubMed]
- Matchkov, V.V.; Krivoi, I.I. Specialized Functional Diversity and Interactions of the Na,K-ATPase. Front. Physiol. 2016, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Arnon, A.; Hamlyn, J.M.; Blaustein, M.P. Ouabain augments Ca2+ transients in arterial smooth muscle without raising cytosolic Na+. Am. J. Physiol. 2000, 279, H679–H691. [Google Scholar] [CrossRef] [PubMed]
- Shelly, D.A.; He, S.; Moseley, A.; Weber, C.; Stegemeyer, M.; Lynch, R.M.; Lingrel, J.; Paul, R.J. Na+ pump alpha 2-isoform specifically couples to contractility in vascular smooth muscle: Evidence from gene-targeted neonatal mice. Am. J. Physiol. 2004, 286, C813–C820. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, H.; Wang, Y.; Lee, J.C.; Kotlikoff, M.I.; Pritchard, T.J.; Paul, R.J.; Zhang, J.; Blaustein, M.P. Arterial alpha2-Na+ pump expression influences blood pressure: Lessons from novel, genetically engineered smooth muscle-specific alpha2 mice. Am. J. Physiol. 2015, 309, H958–H968. [Google Scholar]
- Pritchard, T.J.; Bullard, D.P.; Lynch, R.M.; Lorenz, J.N.; Paul, R.J. Transgenic Mice Expressing Na+-K+ ATPase in Smooth Muscle Decreases Blood Pressure. Am. J. Physiol. 2007, 293, H1172–H1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lee, M.Y.; Cavalli, M.; Chen, L.; Berra-Romani, R.; Balke, C.W.; Bianchi, G.; Ferrari, P.; Hamlyn, J.M.; Iwamoto, T.; et al. Sodium pump alpha2 subunits control myogenic tone and blood pressure in mice. J. Physiol. 2005, 569, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Kita, S.; Zhang, J.; Blaustein, M.P.; Arai, Y.; Yoshida, S.; Wakimoto, K.; Komuro, I.; Katsuragi, T. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat. Med. 2004, 10, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Bouzinova, E.V.; Hangaard, L.; Staehr, C.; Mazur, A.; Ferreira, A.; Chibalin, A.; Sandow, S.L.; Xie, Z.; Aalkjaer, C.; Matchkov, V.V. The α2 isoform Na,K-ATPase modulates contraction of rat mesenteric small artery via cSrc–dependent Ca2+ sensitization. Acta Physiol. 2018. accepted. [Google Scholar] [CrossRef] [PubMed]
- Aalkjaer, C.; Mulvany, M.J. Effect of ouabain on tone, membrane potential and sodium efflux compared with [3H]ouabain binding in rat resistance vessels. J. Physiol. 1985, 362, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Matchkov, V.V.; Moeller-Nielsen, N.; Secher, D.V.; Nourian, Z.; Bodtkjer, D.M.; Aalkjaer, C. The alpha2 isoform of the Na,K-pump is important for intercellular communication, agonist-induced contraction and EDHF-like response in rat mesenteric arteries. Am. J. Physiol. 2012, 303, H36–H46. [Google Scholar]
- Staehr, C.; Hangaard, L.; Bouzinova, E.V.; Kim, S.; Rajanathan, R.; Boegh Jessen, P.; Luque, N.; Xie, Z.; Lykke-Hartmann, K.; Sandow, S.L.; et al. Smooth muscle Ca(2+) sensitization causes hypercontractility of middle cerebral arteries in mice bearing the familial hemiplegic migraine type 2 associated mutation. J. Cereb. Blood Flow. Metab. 2018, 271678X18761712. [Google Scholar]
- Vassallo, D.V.; Songu-Mize, E.; Rossoni, L.V.; Amaral, S.M. Effects of ouabain on vascular reactivity. Braz. J. Med. Biol. Res. 1997, 30, 545–552. [Google Scholar] [PubMed]
- Weiss, D.N.; Podberesky, D.J.; Heidrich, J.; Blaustein, M.P. Nanomolar ouabain augments caffeine-evoked contractions in rat arteries. Am. J. Physiol. 1993, 265, C1443–C1448. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Aalkjaer, C.; Petersen, T.T. Intracellular sodium, membrane potential, and contractility of rat mesenteric small arteries. Circ. Res. 1984, 54, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Zulian, A.; Linde, C.I.; Pulina, M.V.; Baryshnikov, S.G.; Papparella, I.; Hamlyn, J.M.; Golovina, V.A. Activation of c-SRC underlies the differential effects of ouabain and digoxin on Ca2+ signaling in arterial smooth muscle cells. Am. J. Physiol. 2013, 304, C324–C333. [Google Scholar] [CrossRef] [PubMed]
- Matchkov, V.V.; Gustafsson, H.; Rahman, A.; Boedtkjer, D.M.; Gorintin, S.; Hansen, A.K.; Bouzinova, E.V.; Praetorius, H.A.; Aalkjaer, C.; Nilsson, H. Interaction between Na+/K+-pump and Na+/Ca2+-exchanger modulates intercellular communication. Circ. Res. 2007, 100, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Hangaard, L.; Bouzinova, E.V.; Staehr, C.; Dam, V.S.; Kim, S.; Xie, Z.; Aalkjaer, C.; Matchkov, V.V. Na,K-ATPase regulates intercellular communication in the vascular wall via cSrc kinase dependent connexin43 phosphorylation. Am. J. Physiol. 2017, 312, C385–C397. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Cai, T.; Yuan, Z.; Wang, H.; Liu, L.; Haas, M.; Maksimova, E.; Huang, X.Y.; Xie, Z.J. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol. Biol. Cell 2006, 17, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Lai, F.; Banerjee, M.; Duan, Q.; Li, Z.; Si, S.; Xie, Z. Expression of mutant alpha1 Na/K-ATPase defective in conformational transition attenuates Src-mediated signal transduction. J. Biol. Chem. 2013, 288, 5803–5814. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Duan, Q.; Xie, Z. SH2 Ligand-Like Effects of Second Cytosolic Domain of Na/K-ATPase alpha1 Subunit on Src Kinase. PLoS ONE 2015, 10, e0142119. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Cai, T.; Tian, J.; Qu, W.; Xie, Z.J. Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J. Biol. Chem. 2006, 281, 9709–19719. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Madan, N.; Ye, Q.; Duan, Q.; Li, Z.; Wang, S.; Si, S.; Xie, Z. Identification of a Mutant alpha1 Na/K-ATPase That Pumps but Is Defective in Signal Transduction. J. Biol. Chem. 2013, 288, 13295–13304. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Askari, A.; Xie, Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J. Biol. Chem. 2000, 275, 27832–27837. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cai, T.; Tian, J.; Xie, J.X.; Zhao, X.; Liu, L.; Shapiro, J.I.; Xie, Z. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J. Biol. Chem. 2009, 284, 21066–21076. [Google Scholar] [CrossRef] [PubMed]
- Toma, C.; Jensen, P.E.; Prieto, D.; Hughes, A.; Mulvany, M.J.; Aalkjaer, C. Effects of tyrosine kinase inhibitors on the contractility of rat mesenteric resistance arteries. Br. J. Pharmacol. 1995, 114, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Toda, N. Mechanisms of ouabain-induced arterial muscle contraction. Am. J. Physiol. 1980, 239, H199–H205. [Google Scholar] [CrossRef] [PubMed]
- Matchkov, V.V.; Aalkjaer, C. Reply from Vladimir, V. Matchkov and Christian Aalkjaer. J. Physiol. 2017, 595, 6785–6787. [Google Scholar] [PubMed]
- Raina, H.; Zhang, Q.; Rhee, A.Y.; Pallone, T.L.; Wier, W.G. Sympathetic nerves and the endothelium influence the vasoconstrictor effect of low concentrations of ouabain in pressurized small arteries. Am. J. Physiol. 2010, 298, H2093–H2101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hamlyn, J.M.; Karashima, E.; Raina, H.; Mauban, J.R.; Izuka, M.; Berra-Romani, R.; Zulian, A.; Wier, W.G.; Blaustein, M.P. Low-dose ouabain constricts small arteries from ouabain-hypertensive rats: Implications for sustained elevation of vascular resistance. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1140–H1150. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Aalkjaer, C. Structure and function of small arteries. Physiol. Rev. 1990, 70, 921–961. [Google Scholar] [CrossRef] [PubMed]
- Matchkov, V.V.; Kudryavtseva, O.; Aalkjaer, C. Intracellular Ca(2)(+) signalling and phenotype of vascular smooth muscle cells. Basic Clin. Pharmacol. Toxicol. 2012, 110, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Hamlyn, J.M. Signaling mechanisms that link salt retention to hypertension: Endogenous ouabain, the Na+ pump, the Na+/Ca2+ exchanger and TRPC proteins. Biochim. Biophys. Acta 2010, 1802, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.; Mulvany, M.J. Prolonged exposure to ouabain eliminates the greater norepinephrine-dependent calcium sensitivity of resistance vessels in spontaneously hypertensive rats. Hypertension 1981, 3, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Nilsson, H.; Flatman, J.A.; Korsgaard, N. Potentiating and depressive effects of ouabain and potassium-free solutions on rat mesenteric resistance vessels. Circ. Res. 1982, 51, 14–524. [Google Scholar] [CrossRef]
- Tian, J.; Xie, Z.J. The Na-K-ATPase and calcium-signaling microdomains. Physiology 2008, 23, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Knock, G.A.; Shaifta, Y.; Snetkov, V.A.; Vowles, B.; Drndarski, S.; Ward, J.P.; Aaronson, P.I. Interaction between src family kinases and rho-kinase in agonist-induced Ca2+-sensitization of rat pulmonary artery. Cardiovasc. Res. 2008, 77, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Miriel, V.A.; Mauban, J.R.; Blaustein, M.P.; Wier, W.G. Local and cellular Ca2+ transients in smooth muscle of pressurized rat resistance arteries during myogenic and agonist stimulation. J. Physiol. 1999, 518, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ye, Q.; Cui, X.; Madan, N.; Yi, Q.; Pierre, S.V.; Xie, Z. Expression of Rat Na/K-ATPase alpha2 Enables Ion Pumping but not Ouabain-Induced Signaling in alpha1-Deficient Porcine Renal Epithelial Cells. Am. J. Physiol. 2015, 309, C373–C382. [Google Scholar] [CrossRef] [PubMed]
- Kotova, O.; Al-Khalili, L.; Talia, S.; Hooke, C.; Fedorova, O.V.; Bagrov, A.Y.; Chibalin, A.V. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J. Biol. Chem. 2006, 281, 20085–20094. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, P.L.; Hakansson, K.O.; Karlish, S.J. Structure and mechanism of Na,K-ATPase: Functional sites and their interactions. Annu. Rev. Physiol. 2003, 65, 817–849. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. Arrangement of blood vessels and their relation with adrenergic nerves in the rat mesentery. J. Anat. 1973, 115, 347–364. [Google Scholar] [PubMed]
- Furness, J.B. The physiological relevance of constriction of mesenteric arteries by topically applied noradrenaline. J. Physiol. 2017, 595, 6783–6784. [Google Scholar] [CrossRef] [PubMed]
- Fenger-Gron, J.; Mulvany, M.J.; Christensen, K.L. Intestinal blood flow is controlled by both feed arteries and microcirculatory resistance vessels in freely moving rats. J. Physiol. 1997, 498, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, M.P.; Leenen, F.H.; Chen, L.; Golovina, V.A.; Hamlyn, J.M.; Pallone, T.L.; Van Huysse, J.W.; Zhang, J.; Wier, W.G. How NaCl raises blood pressure: A new paradigm for the pathogenesis of salt-dependent hypertension. Am. J. Physiol. 2012, 302, H1031–H1049. [Google Scholar] [CrossRef] [PubMed]
- Mulvany, M.J.; Halpern, W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977, 41, 19–26. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Aalkjaer, C.; Matchkov, V.V. The Na,K-ATPase-Dependent Src Kinase Signaling Changes with Mesenteric Artery Diameter. Int. J. Mol. Sci. 2018, 19, 2489. https://doi.org/10.3390/ijms19092489
Zhang L, Aalkjaer C, Matchkov VV. The Na,K-ATPase-Dependent Src Kinase Signaling Changes with Mesenteric Artery Diameter. International Journal of Molecular Sciences. 2018; 19(9):2489. https://doi.org/10.3390/ijms19092489
Chicago/Turabian StyleZhang, Lin, Christian Aalkjaer, and Vladimir V. Matchkov. 2018. "The Na,K-ATPase-Dependent Src Kinase Signaling Changes with Mesenteric Artery Diameter" International Journal of Molecular Sciences 19, no. 9: 2489. https://doi.org/10.3390/ijms19092489
APA StyleZhang, L., Aalkjaer, C., & Matchkov, V. V. (2018). The Na,K-ATPase-Dependent Src Kinase Signaling Changes with Mesenteric Artery Diameter. International Journal of Molecular Sciences, 19(9), 2489. https://doi.org/10.3390/ijms19092489



