Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury
Abstract
1. Introduction
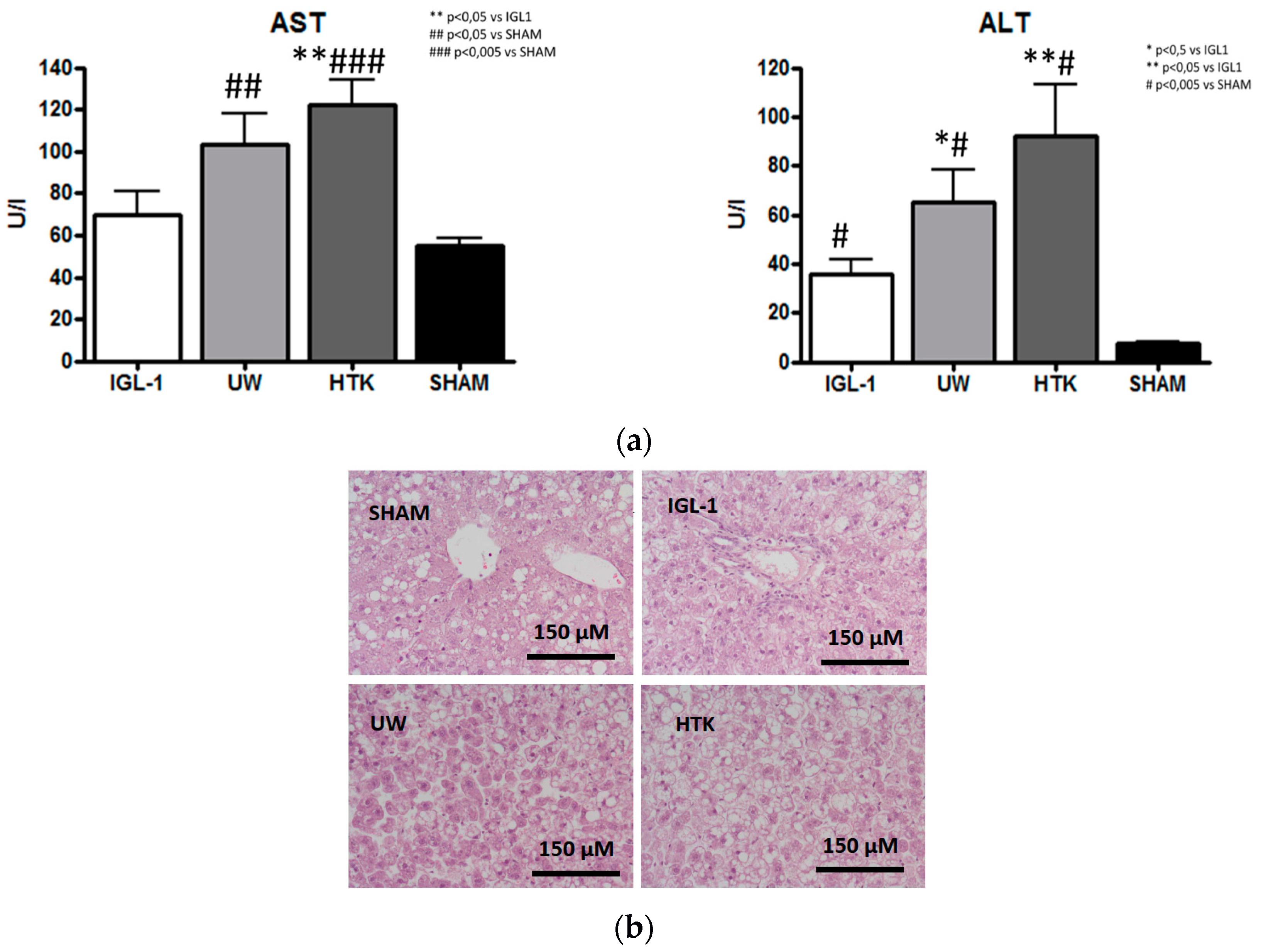
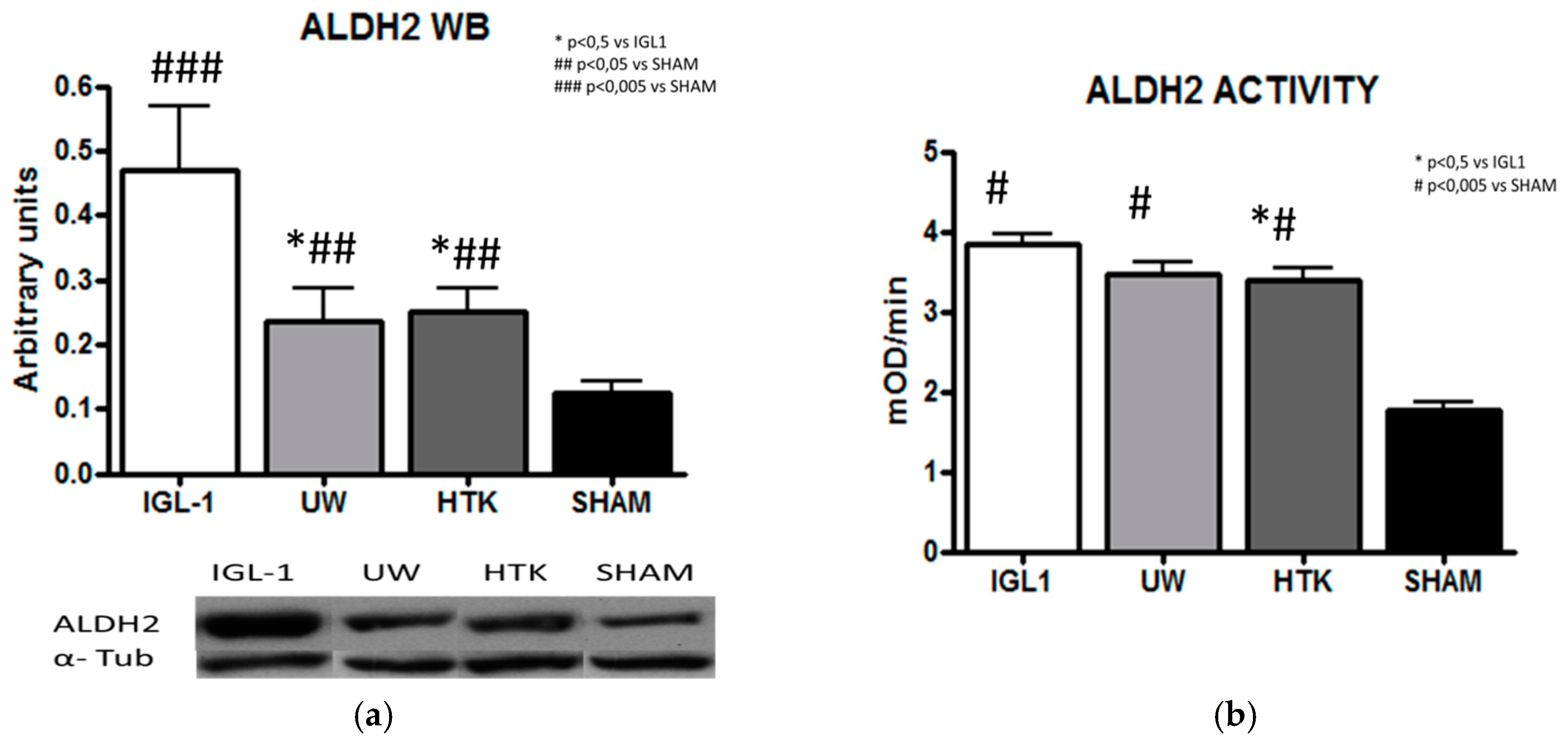
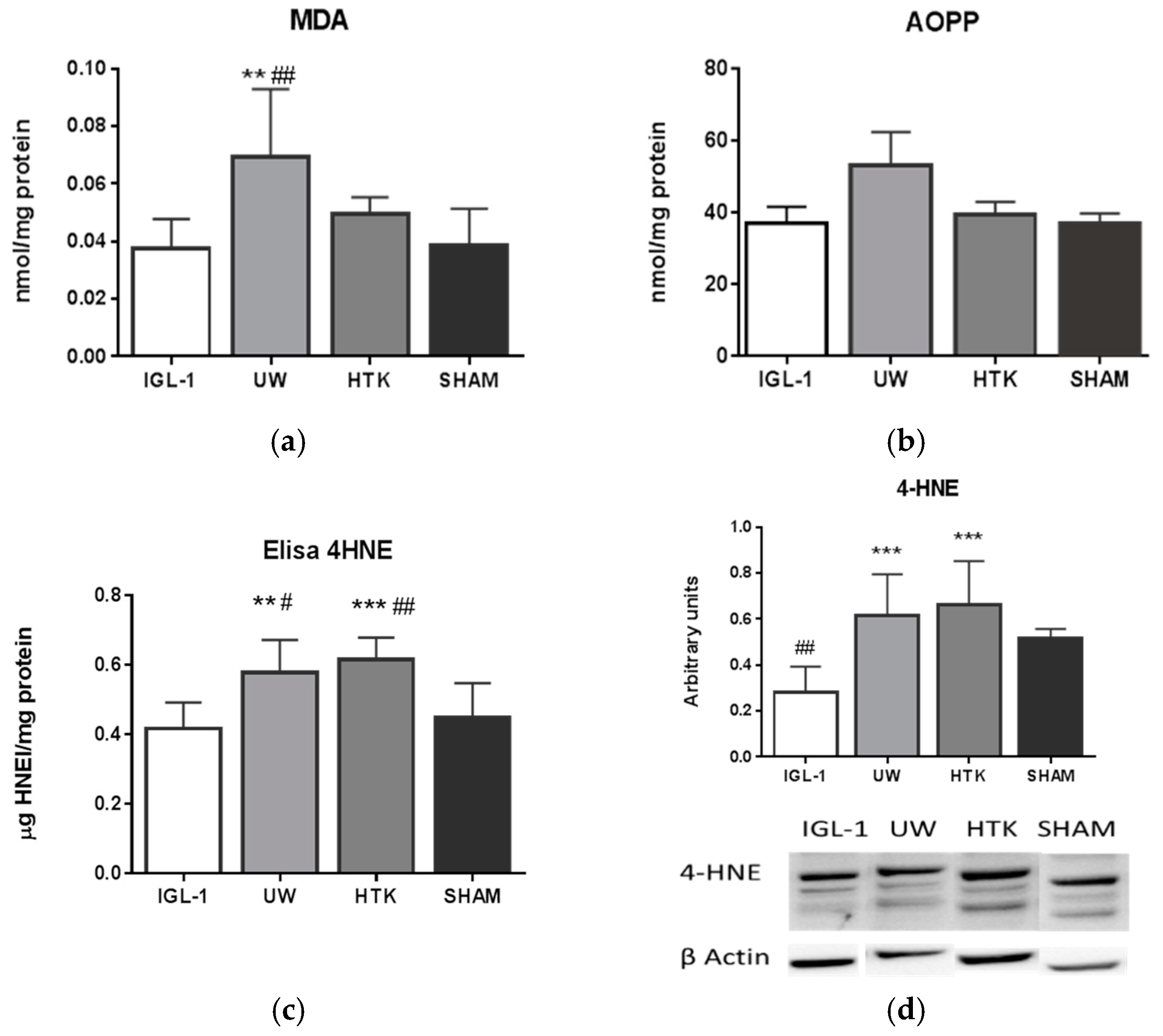
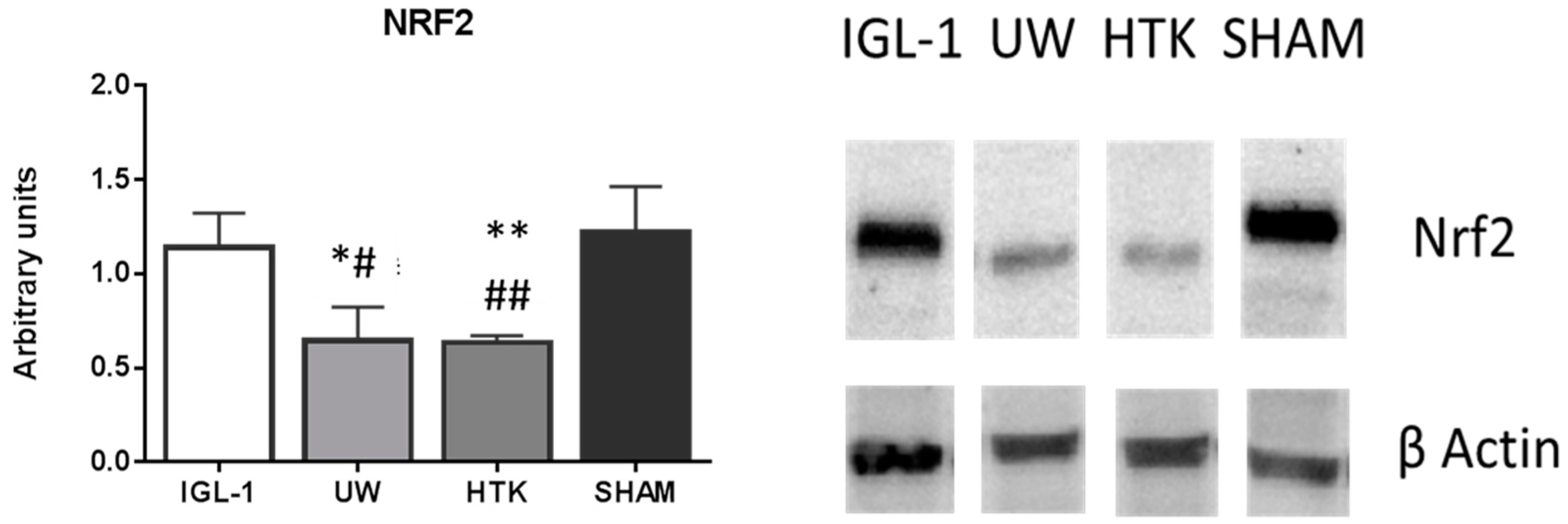
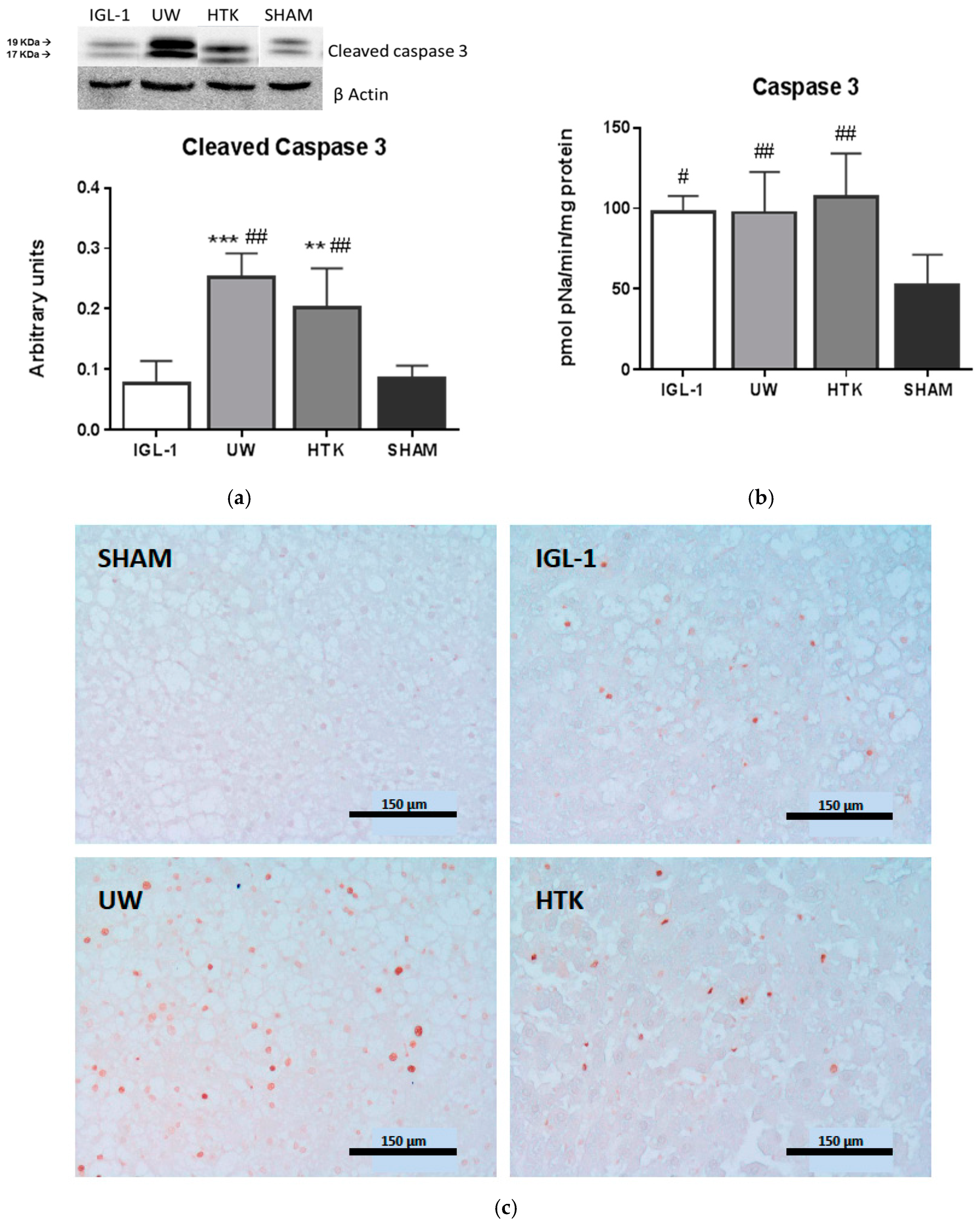
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Groups
4.3. Biochemical Determinations
4.3.1. Transaminase Assay
4.3.2. Histology
4.3.3. ALDH2 Activity
4.3.4. 4-Hydroxynonenal
4.3.5. Malondialdehyde
4.3.6. Advanced Oxidation Protein Products
4.3.7. Caspase 3 Activity
4.3.8. ATP Measurements
4.3.9. Western-Blotting Analysis of ALDH2, 4-HNE, Caspase 3 and Caspase 3 Activity
4.3.10. TUNEL Assay
4.4. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belzer, F.O.; Southard, J.H. Principles of solid organ preservation by cold storage. Transplantation 1988, 45, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Southard, J.H.; Belzer, F.O. Organ Preservation. Annu. Rev. Med. 1995, 46, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Abdennebi, H.B.; Padrissa-Altés, S.; Mahfoudh-Boussaid, A.; Roselló-Catafau, J. Pharmacological strategies against cold ischemia reperfusion injury. Expert Opin. Pharmacother. 2010, 11, 537–555. [Google Scholar] [CrossRef] [PubMed]
- Casillas-Ramírez, A.; Mosbah, I.B.; Ramalho, F.; Roselló-Catafau, J.; Peralta, C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 2006, 79, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Serracino-Inglott, F.; Habib, N.A.; Mathie, R.T. Hepatic ischemia-reperfusion injury. Am. J. Surg. 2001, 181, 160–166. [Google Scholar] [CrossRef]
- Tashiro, H.; Kuroda, S.; Mikuriya, Y.; Ohdan, H. Ischemia–reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery. Surg. Today 2014, 44, 1611–1625. [Google Scholar] [CrossRef] [PubMed]
- Selzner, M.; Clavien, P.A. Fatty liver in liver transplantation and surgery. Semin. Liver Dis. 2001, 21, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Delvart, V.; Karam, V.; Ducerf, C.; Navarro, F.; Letoublon, C.; Belghiti, J.; Pezet, D.; Castaign, D.; LeTreut, Y.; et al. The European liver, intestine trasplant association ELITA. Am. J. Trasplant. 2015, 15, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ preservation: Current concepts and new strategies for the next decade. Transfus. Med. Hemother. 2011, 38, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Cipak Gasparovic, A.; Zarkovic, N. Overview on major lipid peroxidation bioactive factor 4-hydoxynonenal as pluripotent growth-regulating factor. Free Radic. Res. 2015, 49, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Porter, N.A.; Brash, A.R. Routes to 4-hydroxynonenal: Fundamental issue in mechanisms of lipid peroxidation. J. Biol. Chem. 2008, 283, 15539–15543. [Google Scholar] [CrossRef] [PubMed]
- Kristal, B.S.; Park, B.K.; Yu, B.P. 4-Hydroxynonenal is a potent inducer of mitochondrial permability transition. J. Biol. Chem. 1996, 271, 6033–6038. [Google Scholar] [CrossRef] [PubMed]
- Kruman, I.; Bruce-Keller, A.J.; Bredesen, D.; Waeg, G.; Matson, M.P. Evidence that 4-hydroxynonenal mediates oxidative-stress-induced neuronal apoptosis. J. Neurosci. 1997, 17, 5089–5100. [Google Scholar] [CrossRef] [PubMed]
- Baskol, M.; Baskol, G.; Koçer, D.; Ozbakir, O.; Yucesoy, M. Advanced oxidation protein products: A novel marker of oxidative stress in ulcerative colitis. J. Clin. Gastroenterol. 2008, 42, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhong, Z.M.; Pan, Y.; Zeng, J.H.; Zheng, S.; Zhu, S.Y.; Chen, J.T. Advanced Oxidation Protein Products as a Novel Marker of Oxidative Stress in Postmenopausal Osteoporosis. Med. Sci. Monit. 2015, 21, 2428–2432. [Google Scholar] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.V. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Chambel, S.S.; Santos-Gonçalves, A.; Duarte, T.L. The dual role of Nrf2 in nonalcoholic fatty liver disease: Regulation of antioxidant defenses and hepatic lipid metabolism. BioMed Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Ferreira, J.C.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New theraprutic opportunities. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Paniselló-Roselló, A.; Lopez, A.; Folch-Puy, E.; Carbonell, T.; Rolo, A.; Palmeira, C.; Adam, R.; Net, M.; Roselló-Catafau, J. Role of aldehyde dehydrogenase 2 in ischemia reperfusion injury: An update. World J. Gastroenterol. 2018, in press. [Google Scholar]
- Chen, C.H.; Budas, G.R.; Churchill, E.N.; Disatnik, M.H.; Hurley, T.D.; Mochly-Rosen, D. Activation of aldehyde dehydrogenase 2 reduces ischemic damage to heart. Science 2008, 321, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Budas, G.R.; Disatnik, M.H.; Mochly-Rosen, D. Aldehydedehydrogenase 2 in cardiac protection: A new therapeutic target? Trend Cardiovasc. Med. 2009, 19, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.H.; Zhang, H.F.; Yang, Z.B.; Li, T.B.; Liu, B.; Lou, Z.; Ma, Q.L.; Luo, X.J.; Peng, J. Alda 1 reduces cerebral ischemia-reperfusion injury in rat through clearance of reactive aldehydes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 385, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; He, G.; Wang, J.; Wang, Y.; Chen, W. Pretreatment with the ALDH2 agonist Alda-1 reduces intestinal injury induced by ischemia-reperfusion in mice. Clin. Sci. 2017, 131, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Q.; Ye, F.; Huang, C.Y.; Chen, W.M.; Huang, W.Q. Alda-1, an ALDH2 activator, protects against hepatic/ischemia reperfusion in rats injury via inhibition of oxidative stress. Free Radic. Res. 2018, 52, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Kudoh, K.; Uchinami, H.; Yoshioka, M.; Seki, E.; Yamamoto, Y. Nrf2 activation protects the liver from ischemia/reperfusion injury in mice. Ann. Surg. 2014, 260, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Pantazi, E.; Folch-Puy, E.; Baptista, P.M.; Garcia-Gil, A.; Adam, R.; Roselló-Catafau, J. Emerging concepts in liver graft preservation. World J. Gastroenterol. 2015, 21, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, A.; Muller, X.; Dutkowski, P. Hypothermic machine preservation of the liver: State of the art. Curr. Transplant. Rep. 2018, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Lopez, A.; Castro-Benítez, C.; Balloji, S.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Adam, R.; Roselló-Catafau, J. Involvement of ALDH2 in fatty liver graft protection mechanisms against cold ischemic injury. In Proceedings of the American Transplantation Congress, Seattle, WA, USA, 2–6 June 2018. [Google Scholar]
- Ma, H.; Guo, R.; Lu, L.; Zhang, Y.; Ren, J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischemia reperfusion injury: Role of autophagy and toxic aldehyde paradox. Eur. Heart J. 2011, 32, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Roselló-Catafau, J. The future of fatty livers. J. Hepatol. 2004, 41, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Thurman, R.G. Reperfusion injury after liver preservation for transplantation. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Montalvo-Jave, E.E.; Escalante-Tattersfield, T.; Ortega-salgado, J.A.; Pina, E.; Geller, D.A. Factors in the pathophysiology of liver ischemia-reperfusion injury. J. Surg. Res. 2008, 147, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Negre-Salvayre, A.; Coatrieux, C.; Ingueneau, C.; Salvayre, R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br. J. Pharmacol. 2008, 153, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Aberle, N.S.; Picklo, M.J.; Amarnath, V.; Ren, J. Inhibition of cardiac myocyte contraction by 4-hydroxy-trans-2-nonenal. Cardiovasc. Toxicol. 2004, 4, 21–28. [Google Scholar] [CrossRef]
- Perluigi, M.; Coccia, R.; Butterfield, D.A. 4-Hydroxy-2-nonenal, a reactive product of lipid peroxidation, and neurodegenerative diseases: A toxic combination illuminated by redox proteomics studies. Antioxid. Redox Signal. 2012, 17, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.M.; Liu, A.J.; Zang, P.; Dong, W.Z.; Ying, L.; Wang, W.; Xu, P.; Song, X.R.; Cai, J.; Zhang, S.Q.; et al. ALDH2 protects against stroke by clearing 4-HNE. Cell Res. 2013, 23, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Hermes-Lima, M.; Moreira, D.C.; Rivera-Ingraham, G.A.; Giraud-Billoud, M.; Genaro-Mattos, T.C.; Campos, É.G. Preparation for oxidative stress under hypoxia and metabolic depression: Revisiting the proposal two decades later. Free Radic. Biol. Med. 2015, 89, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Luo, Q.; Zhu, H.; Liu, X.; Dong, Z.K.; Wu, J.; Ge, J.; Sun, A. Aldehyde dehydrogenase 2 activation ameliorates CCl4-induced chronic liver fibrosis in mice by up-regulating Nrf2/HO-1 antioxidant pathway. J. Cell. Mol. Med. 2018, 22. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Abdennebi, H.B.; Padrissa-Altés, S.; Alfany-Fernández, I.; Rimola, A.; Roselló-Catafau, J. How Institut Georges Lopez preservation solution protects steatotic and non steatotic liver against ischemia-reperfsuion injury. Transplant. Proc. 2011, 43, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Zaouali, M.A.; Bejaoui, M.; Calvo, M.; Folch-Puy, E.; Pantazi, E.; Pasut, G.; Rimola, A.; Abdennebi, H.B.; Adam, R.; Roselló-Catafau, J. Polyethylene glycol rinse solution: An effective way to prevent ischemia-reperfusion injury. World J. Gastroenterol. 2014, 20, 16203–16214. [Google Scholar] [CrossRef] [PubMed]
- Pasut, G.; Panisello, A.; Folch-Puy, E.; Lopez, A.; Castro-Benítez, C.; Calvo, M.; Carbonell, T.; García-Gil, A.; Adam, R.; Roselló-Catafau, J. Polyethylene glycols: An effective strategy for limiting liver ischemia reperfusion injury. World J. Gastroenterol. 2016, 22, 6501–6508. [Google Scholar] [CrossRef] [PubMed]
- Panisello-Roselló, A.; Verde, E.; Lopez, A.; Flores, M.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Hotter, G.; Carbonell, T.; Adam, R.; et al. Cytoprotective Mechanisms in Fatty Liver Preservation against Cold Ischemia Injury: A Comparison between IGL-1 and HTK. Int. J. Mol. Sci. 2018, 19, 348. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Panisello-Rosello, A.; Castro-Benitez, C.; Adam, R. Glycocalix preservation and NO production in fatty livers-The protective role of high molecular polyethylene glycol in cold ischemia injury. Int. J. Mol. Sci. 2018, 19, 2375. [Google Scholar] [CrossRef] [PubMed]

| Component | IGL-1 | UW | HTK | |
|---|---|---|---|---|
| Colloids (mmol/L) | PEG35 | 0.03 | ||
| HES | 0.25 | |||
| Antioxidants (mmol/L) | Glutathione | 3 | 3 | |
| Precursors (mmol/L) | Adenosine | 5 | 5 | |
| Ketoglutarate | 1 | |||
| Buffers (mmol/L) | Diphosphate | 25 | 25 | |
| Histidine | 198 | |||
| Histidine-HCl | 18 | |||
| Tryptophan | 2 | |||
| Electrolytes (mmol/L) | K+ | 25 | 125 | 10 |
| Na+ | 120 | 27 | 15 | |
| Mg2+ | 5 | 4 | ||
| Cl− | 50 | |||
| SO42− | 5 | 4 | ||
| Ca2+ | 0.5 | 0.015 | ||
| Impermeants (mmol/L) | Raffinose | 30 | 30 | |
| Lactobionic acid | 100 | 105 | ||
| Mannitol | 30 | |||
| Osmolarity (mOs mol/l) | 290 | 320 | 310 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panisello-Roselló, A.; Alva, N.; Flores, M.; Lopez, A.; Castro Benítez, C.; Folch-Puy, E.; Rolo, A.; Palmeira, C.; Adam, R.; Carbonell, T.; et al. Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury. Int. J. Mol. Sci. 2018, 19, 2479. https://doi.org/10.3390/ijms19092479
Panisello-Roselló A, Alva N, Flores M, Lopez A, Castro Benítez C, Folch-Puy E, Rolo A, Palmeira C, Adam R, Carbonell T, et al. Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury. International Journal of Molecular Sciences. 2018; 19(9):2479. https://doi.org/10.3390/ijms19092479
Chicago/Turabian StylePanisello-Roselló, Arnau, Norma Alva, Marta Flores, Alexandre Lopez, Carlos Castro Benítez, Emma Folch-Puy, Anabela Rolo, Carlos Palmeira, René Adam, Teresa Carbonell, and et al. 2018. "Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury" International Journal of Molecular Sciences 19, no. 9: 2479. https://doi.org/10.3390/ijms19092479
APA StylePanisello-Roselló, A., Alva, N., Flores, M., Lopez, A., Castro Benítez, C., Folch-Puy, E., Rolo, A., Palmeira, C., Adam, R., Carbonell, T., & Roselló-Catafau, J. (2018). Aldehyde Dehydrogenase 2 (ALDH2) in Rat Fatty Liver Cold Ischemia Injury. International Journal of Molecular Sciences, 19(9), 2479. https://doi.org/10.3390/ijms19092479