The Dead Can Nurture: Novel Insights into the Function of Dead Organs Enclosing Embryos
Abstract
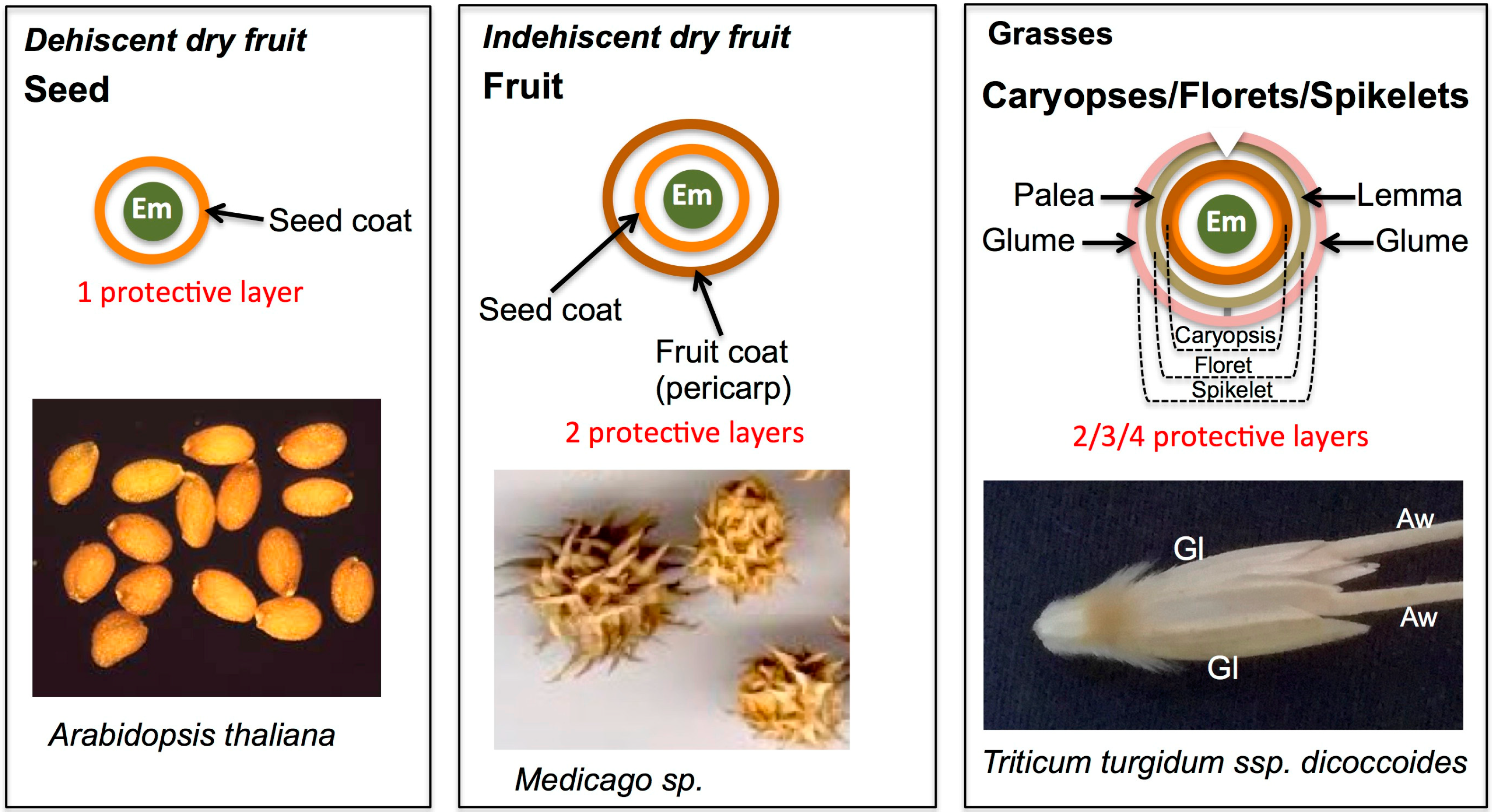
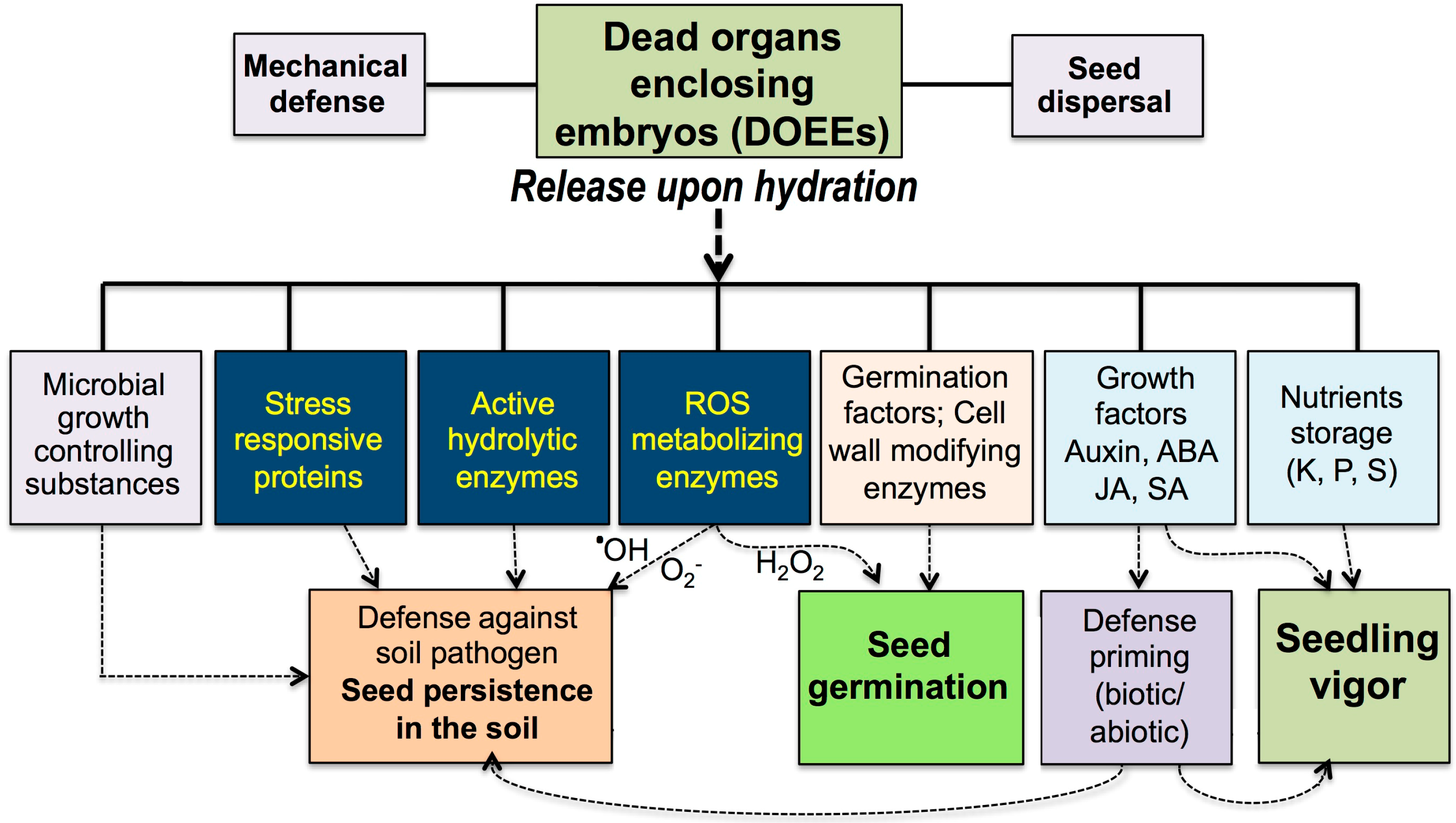
1. Introduction
2. DOEEs Release Hundreds of Proteins upon Hydration
3. ROS Detoxifying Enzymes
4. Cell Wall Modification Enzymes
5. DOEEs as a Rich Storage for Nutrients and Growth Factors
6. Control of Microbial Growth by DOEEs
7. Concluding Remarks
- Do substances released from DOEEs (e.g., JA, SA) have the capability of inducing plant defense priming against biotic and abiotic stresses?
- Can we use substances released from DOEEs as a substitute for the hazardous chemical coating of seeds?
- Does storage of seeds in gene banks with their associated dead organs better preserve and maintain seed viability?
- How do mother plant growth conditions affect the composition of substances stored in DOEEs and consequently seed longevity, germination and seedling establishment?
- Can we modify the composition of proteins and of other substances within DOEEs to build up a superior natural coating?
Funding
Conflicts of Interest
References
- Howe, H.F.; Smallwood, J. Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 1982, 13, 201–228. [Google Scholar] [CrossRef]
- Eriksson, O. Evolution of Seed Size and Biotic Seed Dispersal in Angiosperms: Paleoecological and Neoecological Evidence. Int. J. Plant Sci. 2008, 169, 863–870. [Google Scholar] [CrossRef]
- Booth, D.T. Plant diaspore functions. J. Seed Technol. 1990, 14, 61–73. [Google Scholar]
- Janzen, D.H. Seed predation by animals. Annu. Rev. Ecol. Syst. 1971, 2, 465–492. [Google Scholar] [CrossRef]
- Berenbaum, M.R.; Zangerl, A.R.; Nitao, J.K. Constraints on Chemical Coevolution: Wild Parsnips and the Parsnip Webworm. Evolution 1986, 40, 1215. [Google Scholar] [CrossRef] [PubMed]
- Sroelov, R. On germination inhibitors. IV. Germination inhibitors of Sinapis alba and other seeds when enclosed in their fruit. Palestine J. Bot. 1940, 2, 33–45. [Google Scholar]
- Miyamoto, T.; Tolbert, N.E.; Everson, E.H. Germination inhibitors related to dormancy in wheat seeds. Plant Physiol. 1961, 36, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Wurzburger, J.; Leshem, Y. Physiological action of the germination inhibitor in the husk of Aegilops kotschyi Boiss. New Phytol. 1969, 68, 337–341. [Google Scholar] [CrossRef]
- Gatford, K.T.; Eastwood, R.F.; Halloran, G.M. Germination inhibitors in bracts surrounding the grain of Triticum tauschii. Funct. Plant Biol. 2002, 29, 881–890. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Li, Q.; Liu, Y.; Hou, L.; Li, G. Influence of Pericarp, Cotyledon and Inhibitory Substances on Sharp Tooth Oak (Quercus aliena var. acuteserrata) Germination. PLoS ONE 2012, 7, e47682. [Google Scholar] [CrossRef] [PubMed]
- Adkins, S.W.; Bellairs, S.M.; Loch, D.S. Seed dormancy mechanisms in warm season grass species. Euphytica 2002, 126, 13–20. [Google Scholar] [CrossRef]
- Jain, A.; Singh, A.; Chaudhary, A.; Singh, S.; Singh, H.B. Modulation of nutritional and antioxidant potential of seeds and pericarp of pea pods treated with microbial consortium. Food Res. Int. 2014, 64, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Booth, D.T.; Schuman, G.E. Seedbed Ecology of Winterfat: Fruits versus Threshed Seeds. J. Range Manag. 1983, 36, 387. [Google Scholar] [CrossRef]
- Ohadi, S.; Mashhadi, H.R.; Tavakol-Afshari, R. Effects of Storage and Burial on Germination Responses of Encapsulated and Naked Seeds of Turnipweed (Rapistrum rugosum) to Light. Weed Sci. 2011, 59, 483–488. [Google Scholar] [CrossRef]
- Lu, J.J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Role of indehiscent pericarp in formation of soil seed bank in five cold desert Brassicaceae species. Plant Ecol. 2017, 218, 1187–1200. [Google Scholar] [CrossRef]
- Raviv, B.; Granot, G.; Chalifa-Caspi, V.; Grafi, G. The dead, hardened floral bracts of dispersal units of wild wheat function as storage for active hydrolases and in enhancing seedling vigor. PLoS ONE 2017, 12, e0177537. [Google Scholar] [CrossRef] [PubMed]
- Buchanan-Wollaston, V.; Earl, S.; Harrison, E.; Mathas, E.; Navabpour, S.; Page, T.; Pink, D. The molecular analysis of leaf senescence—A genomics approach. Plant Biotechnol. J. 2002, 1, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Nam, H.G. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Y.H.; Yang, J.J.; Liu, Y.D.; Shen, F.F. Protein degradation and nitrogen remobilization during leaf senescence. J. Plant Biol. 2008, 51, 11–19. [Google Scholar] [CrossRef]
- Raviv, B.; Aghajanyan, L.; Granot, G.; Makover, V.; Frenkel, O.; Gutterman, Y.; Grafi, G. The dead seed coat functions as a long-term storage for active hydrolytic enzymes. PLoS ONE 2017, 12, e0181102. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.; Raviv, B.; Grafi, G. Dead Pericarps of Dry Fruits Function as Long-Term Storage for Active Hydrolytic Enzymes and Other Substances That Affect Germination and Microbial Growth. Plants 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Terras, F.; Cammue, B.; Osborn, R.W. Plant Defensins: Novel Antimicrobial Peptides as Components of the Host Defense System. Plant Physiol. 1995, 108, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.O.; Gomes, V.M. Plant Defensins and Defensin-Like Peptides—Biological Activities and Biotechnological Applications. Curr. Pharm. Des. 2011, 17, 4270–4293. [Google Scholar] [CrossRef]
- De Coninck, B.; Cammue, B.P.; Thevissen, K. Modes of antifungal action and in planta functions of plant defensins and defensin-like peptides. Fungal Biol. Rev. 2013, 26, 109–120. [Google Scholar] [CrossRef]
- Vriens, K.; Cammue, B.; Thevissen, K. Antifungal plant defensins: Mechanisms of action and production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef] [PubMed]
- Terras, F.R.G.; Eggermont, K.; Kovaleva, V.; Raikhel, N.V.; Osborn, R.W.; Kester, A.; Rees, S.B.; Torrekens, S.; Leuven, F.V.; Vanderleyden, J.; et al. Small cysteine-rich antifungal proteins from radish: Their role in host defense. Plant Cell 1995, 7, 573. [Google Scholar] [CrossRef] [PubMed]
- Coca, M.; Bortolotti, C.; Rufat, M.; Peñas, G.; Eritja, R.; Tharreau, D.; Pozo, A.M.D.; Messeguer, J.; Segundo, B.S. Transgenic rice plants expressing the antifungal AFP protein from aspergillus giganteus show enhanced resistance to the rice blast fungus Magnaporthe grisea. Plant Mol. Biol. 2004, 54, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, K.; Gaur, R.; Gupta, V. Role of chitinase in plant defense. Asian J. Biochem. 2011, 6, 29–37. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Ignacimuthu, S. Genetic engineering of crop plants for fungal resistance: Role of antifungal genes. Biotechnol. Lett. 2012, 34, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, L.; Bambara, R.A. Flap endonuclease 1. Annu. Rev. Biochem. 2013, 82, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Ito, J.; Aoyagi, S.; Fukuda, H. Endonucleases. Plant Mol. Biol. 2000, 44, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Granot, G.; Morgenstern, Y.; Khan, A.; Rapp, Y.G.; Pesok, A.; Nevo, E.; Grafi, G. Internucleosomal DNA fragmentation in wild emmer wheat is catalyzed by S1-type endonucleases translocated to the nucleus upon induction of cell death. BBA Gene Regul. Mech. 2015, 1849, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Givaty-Rapp, Y.; Yadav, N.S.; Khan, A.; Grafi, G. S1-Type Endonuclease 2 in Dedifferentiating Arabidopsis Protoplasts: Translocation to the Nucleus in Senescing Protoplasts Is Associated with De-Glycosylation. PLoS ONE 2017, 12, e0170067. [Google Scholar] [CrossRef] [PubMed]
- Gartemann, K.-H.; Kirchner, O.; Engemann, J.; Gräfen, I.; Eichenlaub, R.; Burger, A. Clavibacter michiganensis subsp. michiganensis: First steps in the understanding of virulence of a Gram-positive phytopathogenic bacterium. J. Biotechnol. 2003, 106, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, E.A.; Sapotsky, M.V.; Komarova, M.L.; Scherban, A.B.; Shumny, V.K.; Polyakova, A.M.; Lapshina, L.A.; Kochetov, A.V.; Malinovsky, V.I. Protection of transgenic tobacco plants expressing bovine pancreatic ribonuclease against tobacco mosaic virus. Plant Cell Rep. 2007, 26, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Trifonova, E.A.; Kochetov, A.V.; Kanayama, Y. Expression of an extracellular ribonuclease gene increases resistance to Cucumber mosaic virus in tobacco. BMC Plant Biol. 2016, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Galiana, E.; Bonnet, P.; Conrod, S.; Keller, H.; Panabieres, F.; Ponchet, M.; Poupet, A.; Ricci, P. RNase Activity Prevents the Growth of a Fungal Pathogen in Tobacco Leaves and Increases upon Induction of Systemic Acquired Resistance with Elicitin. Plant Physiol. 1997, 115, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- Hugot, K.; Ponchet, M.; Marais, A.; Ricci, P.; Galiana, E. A tobacco S-like RNase inhibits hyphal elongation of plant pathogens. Mol. Plant Microbe Interact. 2002, 15, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bertini, L.; Caporale, C.; Testa, M.; Proietti, S.; Caruso, C. Structural basis of the antifungal activity of wheat PR4 proteins. FEBS Lett. 2009, 583, 2865–2871. [Google Scholar] [CrossRef] [PubMed]
- Jerkovic, A.; Kriegel, A.M.; Bradner, J.R.; Atwell, B.J.; Roberts, T.H.; Willows, R.D. Strategic distribution of protective proteins within bran layers of wheat protects the nutrient-rich endosperm. Plant Physiol. 2010, 152, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, E.P.; Okubara, P.A.; Anderson, J.V.; Morris, C.F. Polyphenol oxidase as a biochemical seed defense mechanism. Front. Plant Sci. 2014, 5, 689. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 2004, 14, 93–107. [Google Scholar] [CrossRef]
- Richards, S.L.; Wilkins, K.A.; Swarbreck, S.M.; Anderson, A.A.; Habib, N.; Smith, A.G.; Mcainsh, M.; Davies, J.M. The hydroxyl radical in plants: From seed to seed. J. Exp. Bot. 2014, 66, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Havaux, M. Singlet oxygen in plants: Production, detoxification and signaling. Trends Plant Sci. 2009, 14, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Laloi, C.; Havaux, M. Key players of singlet oxygen-induced cell death in plants. Front. Plant Sci. 2015, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [PubMed]
- Puntarulo, S.; Galleano, M.; Sanchez, R.A.; Boveris, A. Superoxide anion and hydrogen peroxide metabolism in soybean embryonic axes during germination. Biochim. Biophys. Acta 1991, 1074, 277–283. [Google Scholar] [CrossRef]
- Schopfer, P.; Plachy, C.; Frahry, G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001, 125, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Tawaratsumida, T.; Kondo, K.; Kasa, S.; Sakamoto, M.; Aoki, N.; Zheng, S.H.; Yuasa, T.; Iwaya-Inoue, M. Reactive oxygen species are involved in gibberellin/abscisic acid signaling in barley aleurone cells. Plant Physiol. 2012, 158, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Wojtyla, L.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different modes of hydrogen peroxide action during seed germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- El-Maarouf-Bouteau, H.; Bailly, C. Oxidative signaling in seed germination and dormancy. Plant Signal. Behav. 2008, 3, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.P.; Baek, K.H.; Lee, H.S.; Kwak, S.S.; Bang, J.W.; Kwon, S.Y. Tobacco seeds simultaneously over-expressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. J. Exp. Bot. 2010, 61, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Sénéchal, F.; Wattier, C.; Rustérucci, C.; Pelloux, J. Homogalacturonan-modifying enzymes: Structure, expression, and roles in plants. J. Exp. Bot. 2014, 65, 5125–5160. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Gupta, R. Alkaline pectinases: A review. Biocatal. Agric. Biotechnol. 2015, 4, 279–285. [Google Scholar] [CrossRef]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan methyl-esterification and plant development. Mol. Plant 2009, 2, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Peaucelle, A.; Braybrook, S.A.; Höfte, H. Cell wall mechanics and growth control in plants: The role of pectins revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Paynel, F.; Leroux, C.; Surcouf, O.; Schaumann, A.; Pelloux, J.; Driouich, A.; Mollet, J.C.; Lerouge, P.; Lehner, A.; Mareck, A. Kiwi fruit PMEI inhibits PME activity, modulates root elongation and induces pollen tube burst in Arabidopsis thaliana. Plant Growth Regul. 2014, 74, 285–297. [Google Scholar] [CrossRef]
- Nighojkar, A.; Srivastava, S.; Kumar, A. Pectin methylesterase from germinating Vigna sinensis seeds. Plant Sci. 1994, 103, 115–120. [Google Scholar] [CrossRef]
- Ren, C.; Kermode, A.R. An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol. 2000, 124, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Scheler, C.; Weitbrecht, K.; Pearce, S.P.; Hampstead, A.; Büttner-Mainik, A.; Lee, K.J.; Voegele, A.; Oracz, K.; Dekkers, B.J.; Wang, X.; et al. Promotion of testa rupture during garden cress germination involves seed compartment-specific expression and activity of pectin methylesterases. Plant Physiol. 2015, 167, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Levesque-Tremblay, G.; Bartels, S.; Weitbrecht, K.; Wormit, A.; Usadel, B.; Haughn, G.; Kermode, A.R. Demethylesterification of cell wall pectins in Arabidopsis plays a role in seed germination. Plant Physiol. 2013, 161, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Sitrit, Y.; Hadfield, K.A.; Bennett, A.B.; Bradford, K.J.; Downie, A.B. Expression of a polygalacturonase associated with tomato seed germination. Plant Physiol. 1999, 121, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Peretto, R.; Favaron, F.; Bettini, V.; De Lorenzo, G.; Marini, S.; Alghisi, P.; Cervone, F.; Bonfante, P. Expression and localization of polygalacturonase during the outgrowth of lateral roots in Allium porrum L. Planta 1992, 188, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, I.; Beeckman, T.; Graham, N.; Bhalerao, R.; Zhang, H.; Casero, P.; Sandberg, G.; Bennett, M.J. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 2003, 8, 165–171. [Google Scholar] [CrossRef]
- Fukaki, H.; Tasaka, M. Hormone interactions during lateral root formation. Plant Mol. Biol. 2008, 69, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Péret, B.; Rybel, B.D.; Casimiro, I.; Benková, E.; Swarup, R.; Laplaze, L.; Beeckman, T.; Bennett, M.J. Arabidopsis lateral root development: An emerging story. Trends Plant Sci. 2009, 14, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.C. Comparison Of The Effects of A Localised Supply of Phosphate, Nitrate, Ammonium and Potassium on the Growth of the Seminal Root System, and the Shoot, in Barley. New Phytol. 1975, 75, 479–490. [Google Scholar] [CrossRef]
- Zhang, H.; Forde, B.G. Regulation of Arabidopsis root development by nitrate availability. J. Exp. Bot. 2000, 51, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liu, S.; Meng, L.; Xue, R.; Wang, C.; Liu, G.; Dong, C.; Wang, S.; Dong, J.; Zhang, Y. Potassium deficiency inhibits lateral root development in tobacco seedlings by changing auxin distribution. Plant Soil 2015, 396, 163–173. [Google Scholar] [CrossRef]
- Remans, T.; Nacry, P.; Pervent, M.; Filleur, S.; Diatloff, E.; Mounier, E.; Tillard, P.; Forde, B.G.; Gojon, A. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitraterich patches. Proc. Natl. Acad. Sci. USA 2006, 103, 19206–19211. [Google Scholar] [CrossRef] [PubMed]
- Raviv, B.; Grafi, G. Dead floral bracts of the wheat dispersal unit store and release upon hydration multiple phytohormones: Implications for seedling vigor. 2018; in press. [Google Scholar]
- Leigh, R.A.; Wynn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Tisdale, S.L.; Nelson, W.L.; Beaton, J.D.; Havlin, J.L. Soil and fertilizer nitrogen. Soil Fertil. Fertil. 1993, 4, 112–183. [Google Scholar]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Bakker, J.P.; Bekker, R.M. Soil Seed Banks of North West Europe: Methodology, Density and Longevity; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Hendry, G.A.F.; Thompson, K.; Moss, C.J.; Edwards, E.; Thorpe, P.C. Seed persistence: A correlation between seed longevity in the soil and ortho-dihydroxyphenol concentration. Funct. Ecol. 1994, 8, 658–664. [Google Scholar] [CrossRef]
- De Bolle, M.F.; Eggermont, K.; Duncan, R.E.; Osborn, R.W.; Terras, F.R.; Broekaert, W.F. Cloning and characterization of two cDNA clones encoding seed-specific antimicrobial peptides from Mirabilis jalapa L. Plant Mol. Biol. 1995, 28, 713–721. [Google Scholar] [CrossRef]
- Rose, T.L.; Conceicao, A.D.S.; Jose, X.F.; Okorokov, L.A.; Fernandes, K.V.S.; Marty, F.; Marty-Mazars, D.; Carvalho, A.O.; Gomes, V.M. Defense proteins from Vigna ungaiculata seed exudates: Characterization and inhibitory activity against Fusarium oxysporum. Plant Soil 2006, 286, 181–191. [Google Scholar] [CrossRef]
- Lepiniec, L.; Debeaujon, I.; Routaboul, J.M.; Baudry, A.; Pourcel, L.; Nesi, N.; Caboche, M. Genetics and biochemistry of seed flavonoids. Annu. Rev. Plant Biol. 2006, 57, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Yasseen, Y.; Barringer, S.A.; Splittstoesser, W.E.; Costanza, S. The role of seed coats in seed viability. Bot. Rev. 1994, 60, 426–439. [Google Scholar] [CrossRef]
- Smykal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [PubMed]
- Gijzen, M.; Kuflu, K.; Qutob, D.; Chernys, J.T. A class I chitinase from soybean seed coat. J. Exp. Bot. 2001, 52, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Schlumbaum, A.; Mauch, F.; Vogeli, U.; Boller, T. Plant chitinases are potent inhibitors of fungal growth. Nature 1986, 324, 365–367. [Google Scholar] [CrossRef]
- Brogue, K.; Chet, I.; Holliday, M.; Cressman, R.; Biddle, P.; Knowlton, S.; Mauvais, C.J.; Broglie, R. Transgenic plants with enhanced resistance to the pathogen Rhizoctonia solani. Science 1991, 254, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Verbon, E.H.; Liberman, L.M. Beneficial Microbes Affect Endogenous Mechanisms Controlling Root Development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Zamioudis, C.; Pieterse, C.M. Modulation of host immunity by beneficial microbes. Mol. Plant Microbe Interact. 2012, 25, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.d.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raviv, B.; Godwin, J.; Granot, G.; Grafi, G. The Dead Can Nurture: Novel Insights into the Function of Dead Organs Enclosing Embryos. Int. J. Mol. Sci. 2018, 19, 2455. https://doi.org/10.3390/ijms19082455
Raviv B, Godwin J, Granot G, Grafi G. The Dead Can Nurture: Novel Insights into the Function of Dead Organs Enclosing Embryos. International Journal of Molecular Sciences. 2018; 19(8):2455. https://doi.org/10.3390/ijms19082455
Chicago/Turabian StyleRaviv, Buzi, James Godwin, Gila Granot, and Gideon Grafi. 2018. "The Dead Can Nurture: Novel Insights into the Function of Dead Organs Enclosing Embryos" International Journal of Molecular Sciences 19, no. 8: 2455. https://doi.org/10.3390/ijms19082455
APA StyleRaviv, B., Godwin, J., Granot, G., & Grafi, G. (2018). The Dead Can Nurture: Novel Insights into the Function of Dead Organs Enclosing Embryos. International Journal of Molecular Sciences, 19(8), 2455. https://doi.org/10.3390/ijms19082455






