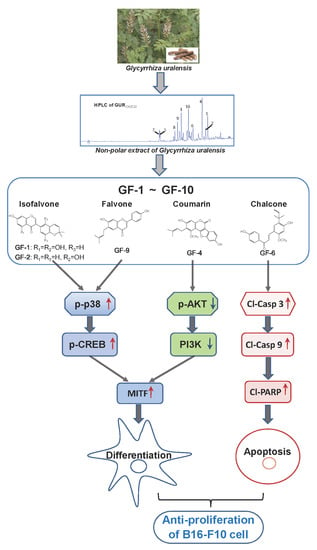
Prenylated Flavonoids from Roots of Glycyrrhiza uralensis Induce Differentiation of B16-F10 Melanoma Cells
Abstract
1. Introduction
2. Results
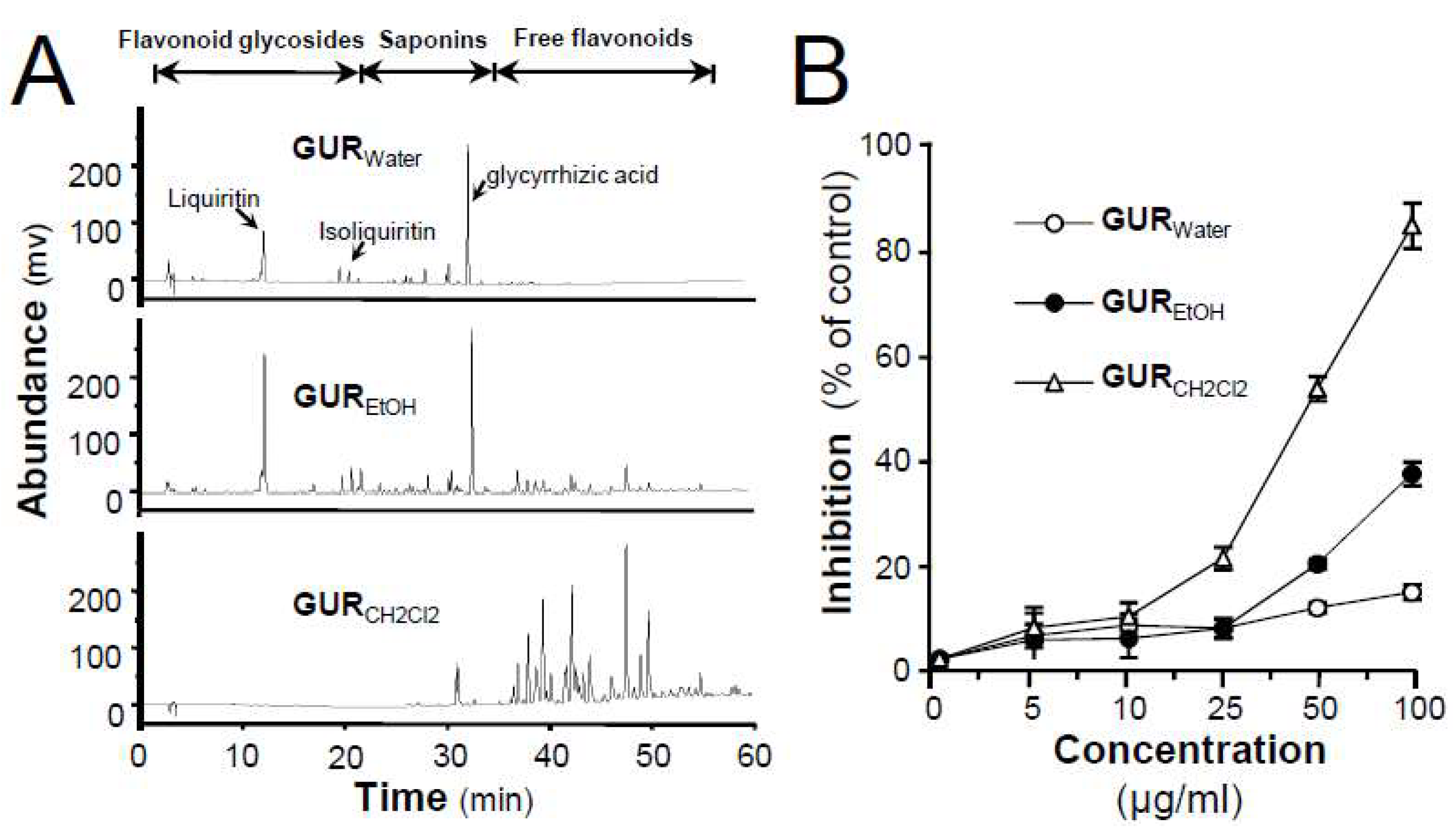
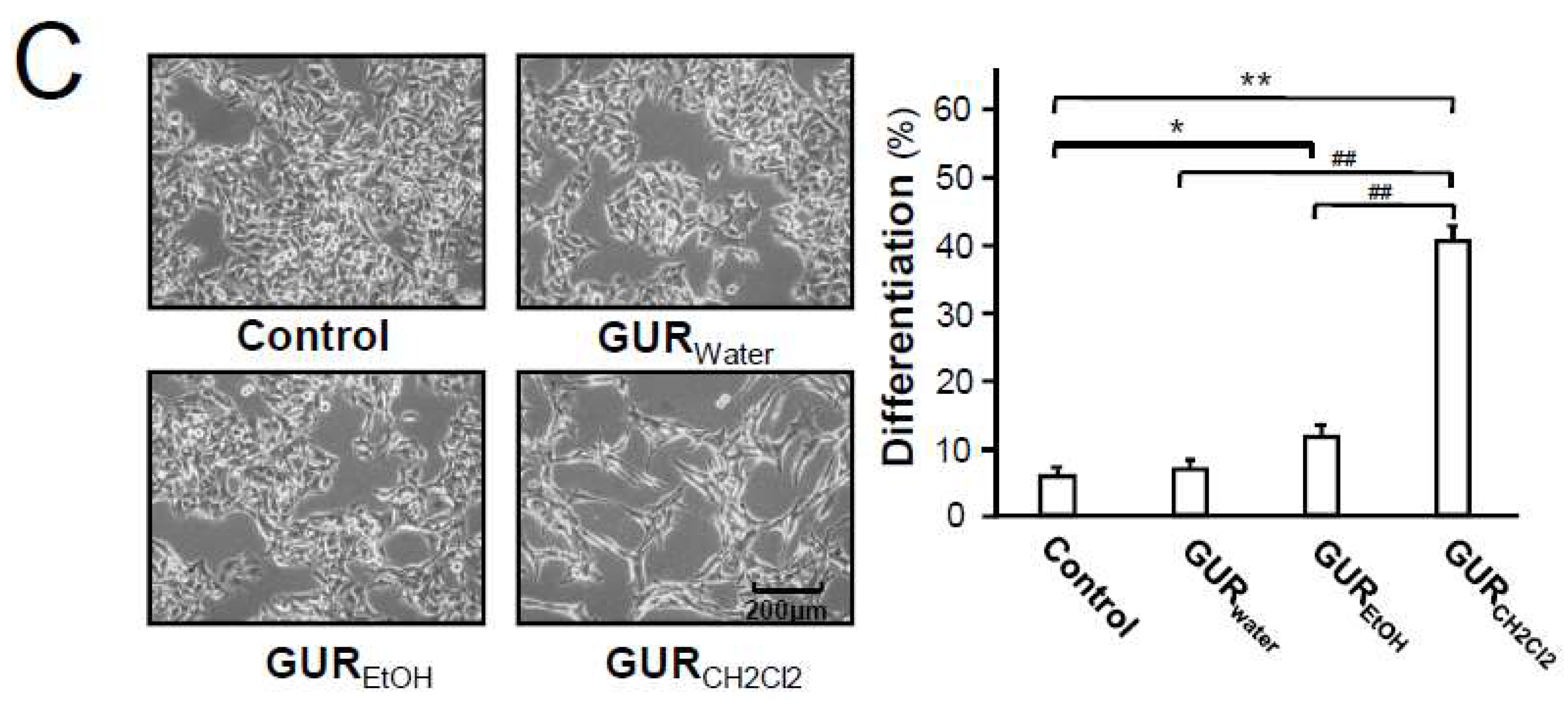
2.1. G. uralensis Extracts in Proliferation and Differentiation of Melanoma Cells
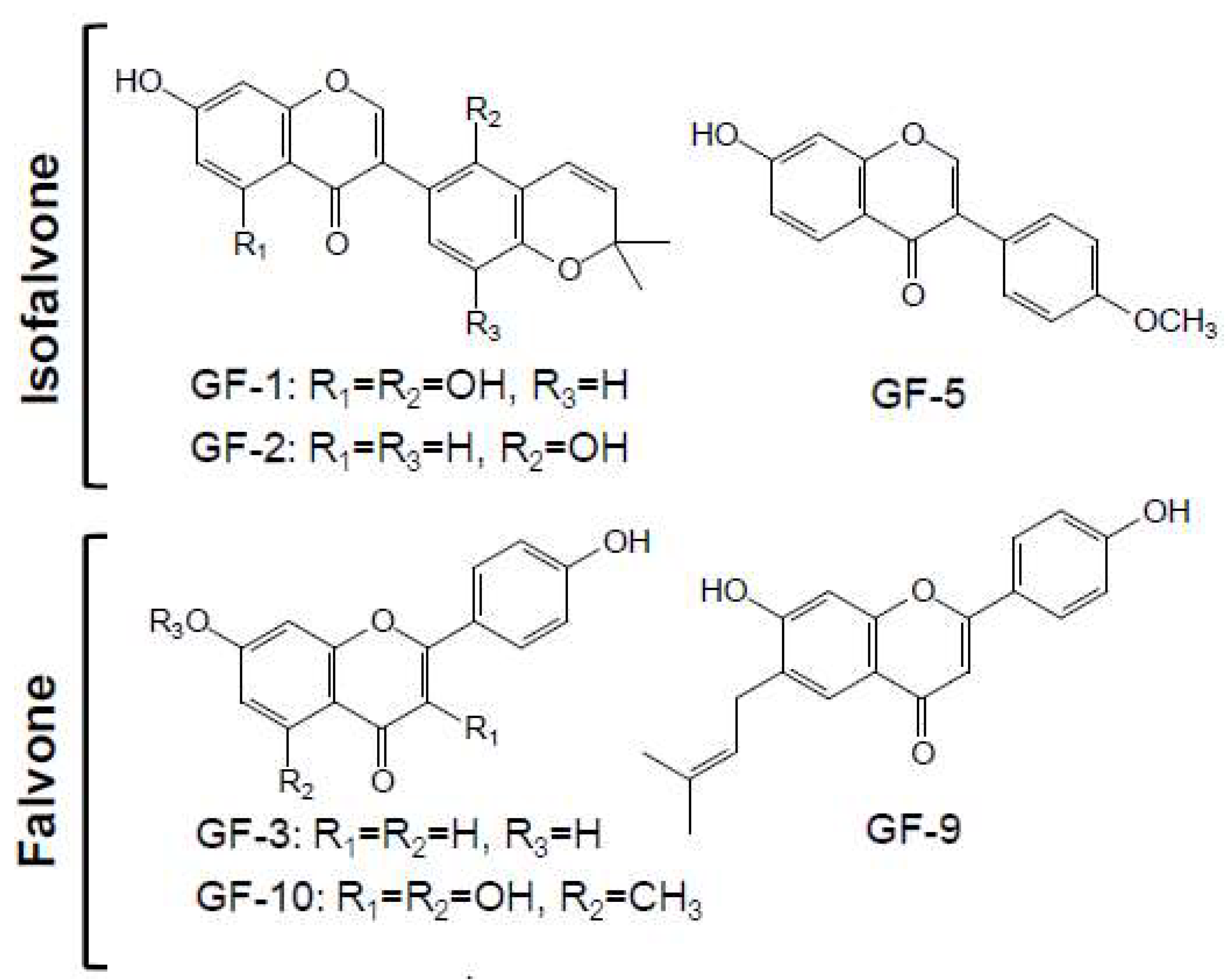
2.2. Structure Identification of Isolated Compounds
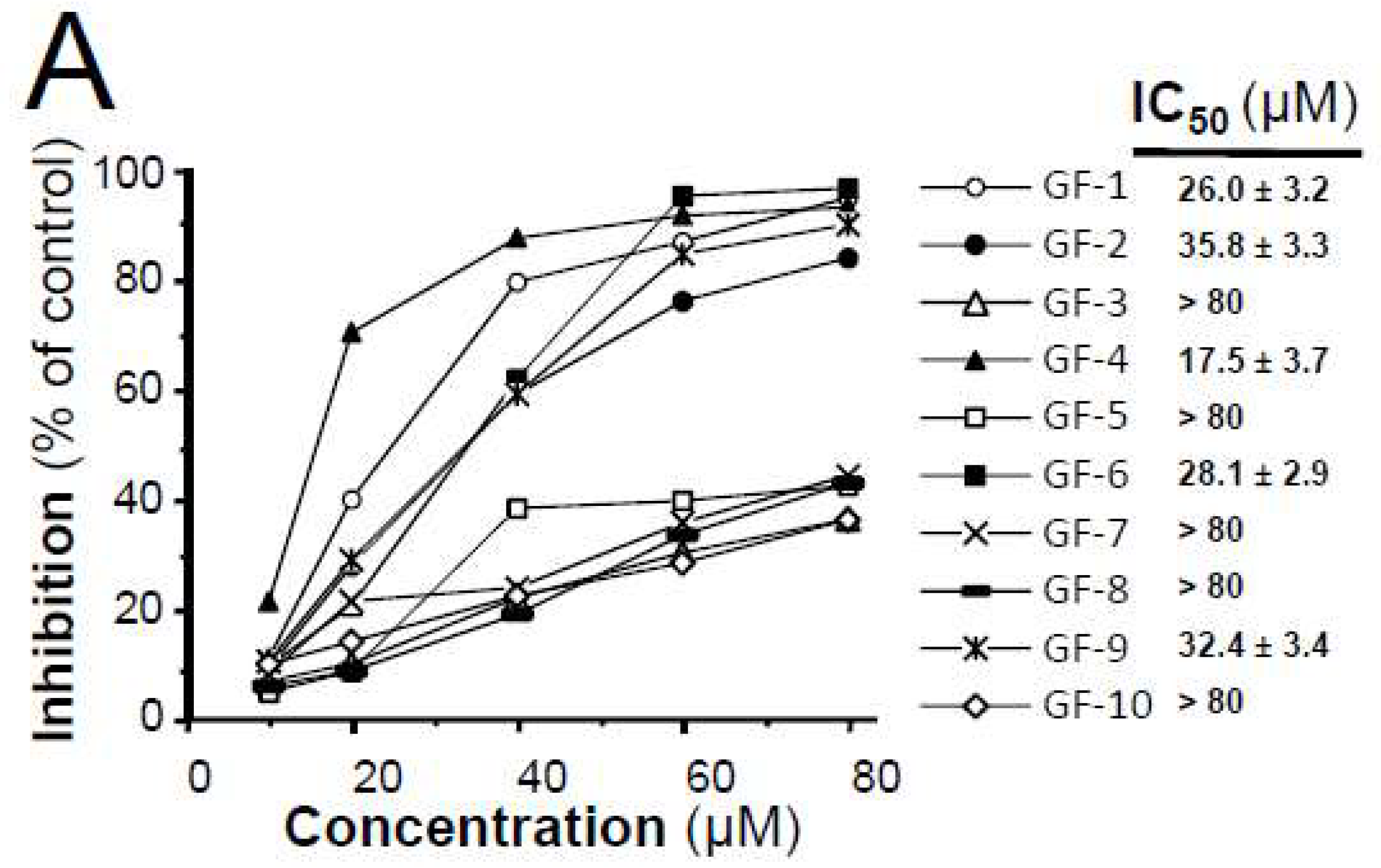
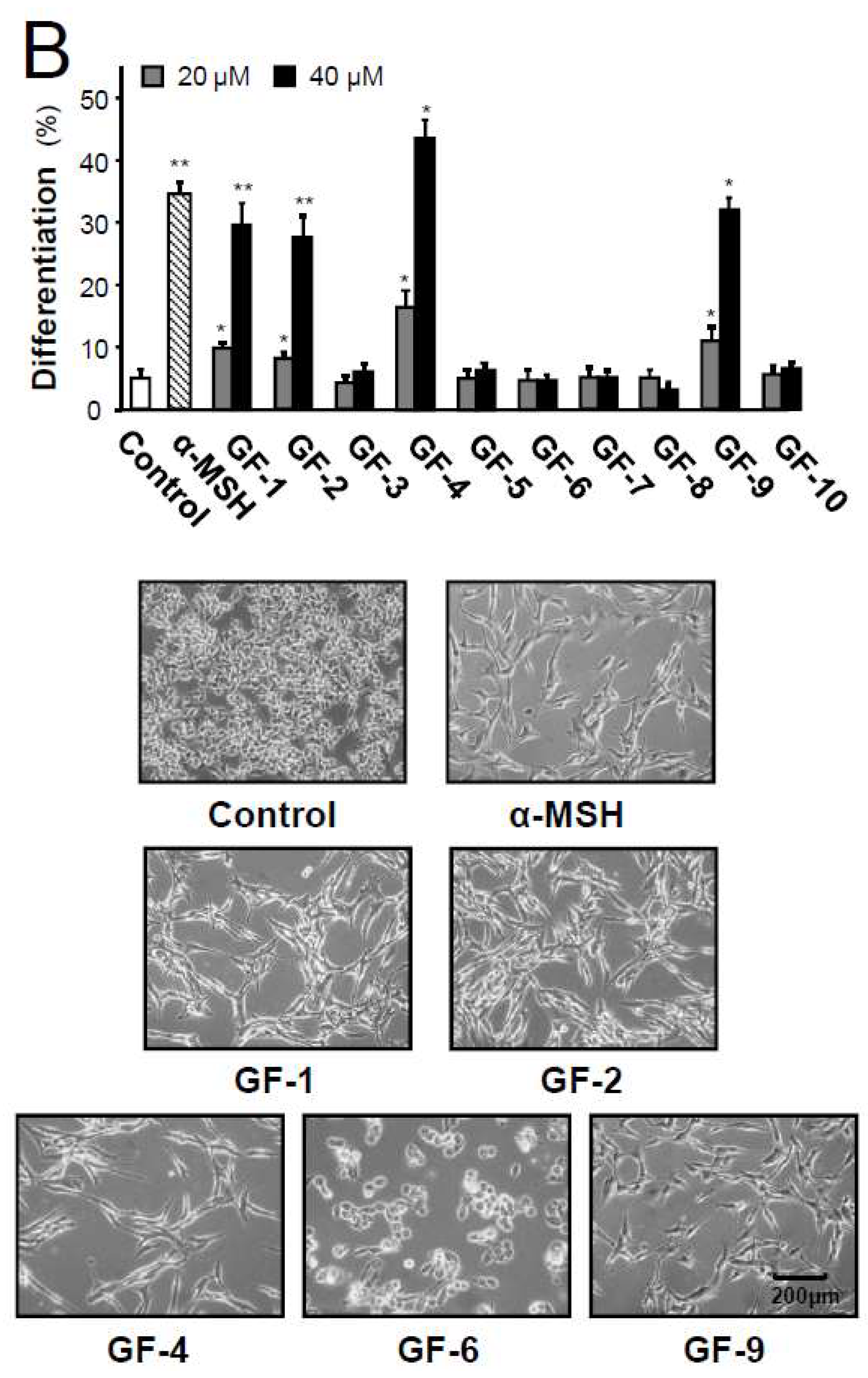
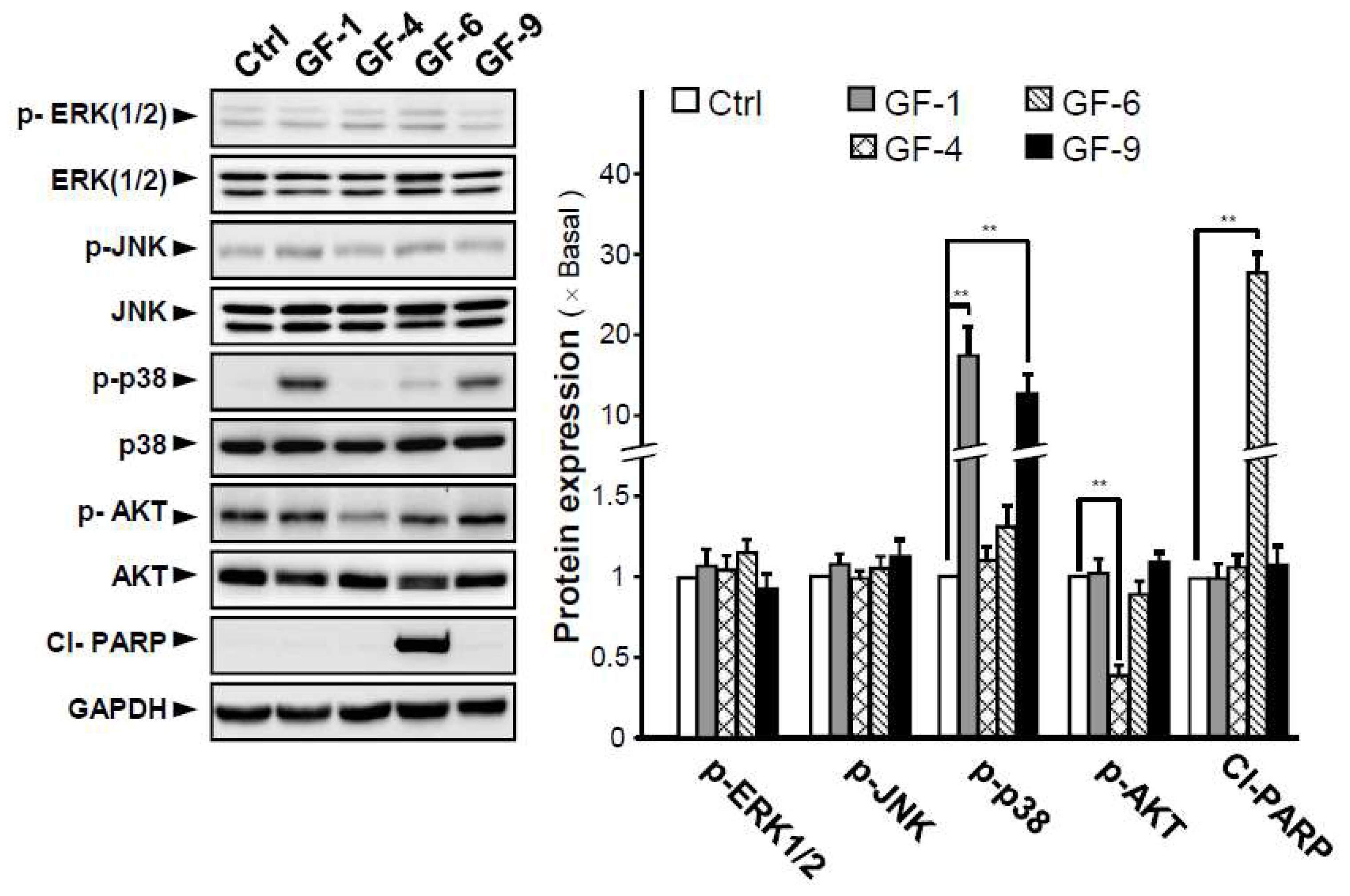
2.3. Isolated Flavonoidsin Proliferation and Differentiation of Melanoma Cells
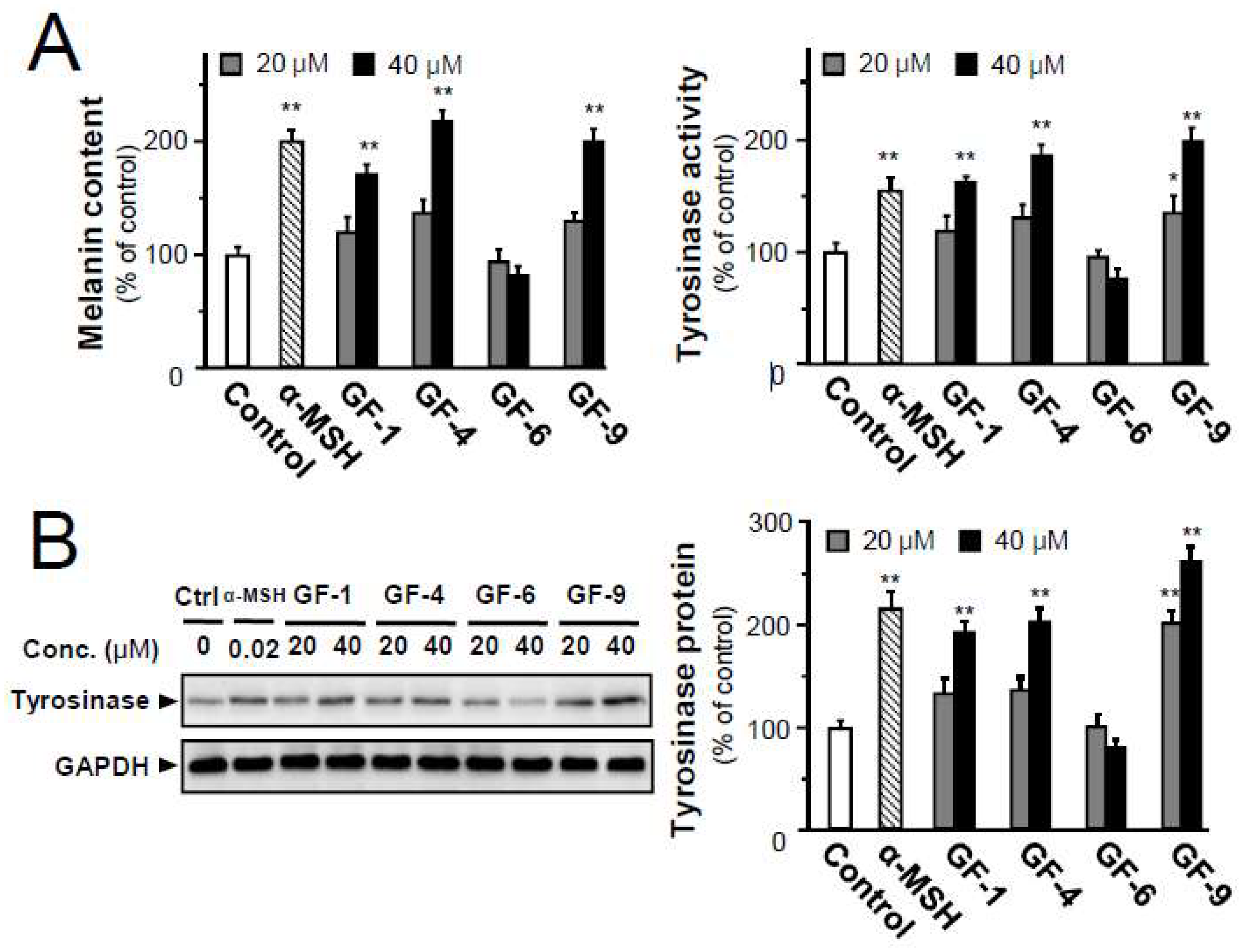
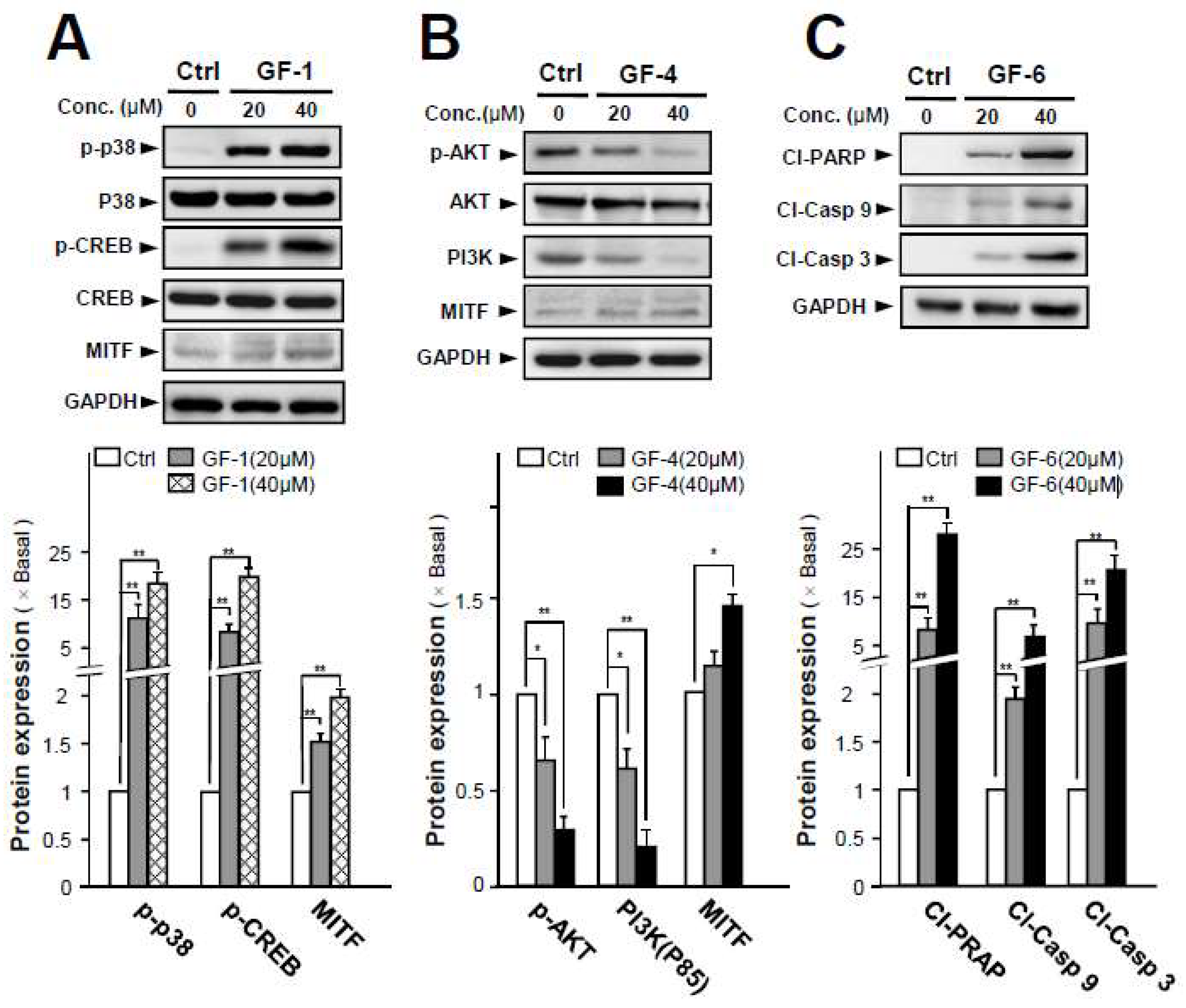
2.4. Isolated Flavonoids in Melanogenesis
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.2.1. Phytochemical Extractions of G. uralensis Roots
4.2.2. Isolation and Identification of Flavonoids
4.3. Cell Culture
4.4. Antiproliferative Activity Assay
4.5. Morphological and Differentiation Analysis
4.6. Measurement of Tyrosinase Activity
4.7. Determination of Melanin Content
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Birari, R.B.; Gupta, S.; Mohan, C.G.; Bhutani, K.K. Antiobesity and lipid lowering effects of Glycyrrhiza chalcones: Experimental and computational studies. Phytomedicine. 2011, 18, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Lin, Y.; Li, K.; Song, W.; Ji, S.; Qiao, X.; Zhang, Q.Y.; Ye, M. Screening of hepatoprotective compounds from licorice against carbon tetrachloride and acetaminophen induced HepG2 cells injury. Phytomedicine. 2017, 34, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.Q.; Qu, Q.Y.; Zheng, Y.F.; Zhong, H.H.; Shan, C.X.; Wang, F.; Li, C.Y.; Guo, P.P. Structural characterization and identification of flavonoid aglycones in three Glycyrrhiza species by liquid chromatography with photodiode array detection and quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2016, 39, 2068–2078. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, J.; Li, Y.J.; Zheng, Y.F.; Li, P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013, 141, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Lim, S.S.; Lee, Y.S.; Kim, J.S.; Lee, C.H.; Kwon, D.Y.; Park, J.H.Y. Hexane/ethanol extract of Glycyrrhiza uralensis licorice exerts potent anti-inflammatory effects in murine macrophages and in mouse skin. Food Chem. 2010, 141, 959–966. [Google Scholar] [CrossRef]
- Huo, X.W.; Liu, D.Y.; Gao, L.; Li, L.Y.; Cao, L. Flavonoids extracted from licorice prevents Colitis-associated carcinogenesis in AOM/DSS mouse model. Int. J. Mol. Sci. 2016, 17, 1343. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.T.; Echenfield, L.F. The rising incidence of melanoma in children and adolescents. Dermatol. Nurs. 2000, 121, 192–193. [Google Scholar]
- Lo, J.A.; Fisher, D.E. Melanoma pathogenesis. Cancer Drug Discov. Develop. 2015, 82, 25–45. [Google Scholar]
- Parka, H.J.; Hana, E.S.; Park, D.K. The ethyl acetate extract of PGP (Phellinuslinteus grown on Panax ginseng) suppresses B16F10 melanoma cell proliferation through inducing cellular differentiation and apoptosis. J. Ethnopharmacol. 2010, 132, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Van den, B.; Verdegaal, E.M.; De Miranda, N.F. Cancer immunotherapy: Broadening the scope of targetable tumours. Open Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.; Rebecca, W.K.; Rafael, C.; Brian, G. Malignant melanoma: Diagnostic and management––Update. Plast. Reconstr. Surg. 2018, 142, 202–216. [Google Scholar]
- Skandrani, P.A.; Simon, A.; Ghedira, K.; Chekir-Ghedira, L. Chloroform extract from Moricandiaarvensis inhibits growth of B16-F0 melanoma cells and promotes differentiation in vitro. Cell Prolif. 2010, 43, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, W.; Chen, X.; Han, J.; Yu, L.; Gao, C.; Hao, W.; Liu, X.; Zheng, Q.; Li, D. Icariin induces cell differentiation and cell cycle arrest in mouse melanoma B16 cells via Erk1/2-p38-JNK-dependent pathway. Oncotarget. 2017, 8, 99504–99513. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mukwaya, E.; Wong, M.; Zhang, Y.A. systematic review on biological activities of isoprenyl flavonoids. Pharm. Biol. 2013, 52, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Keishi, H.; Kyoko, I.; Kazuyuki, H.; Tomokazu, K. Differentiation-inducing activity of lupeol, a lupine-type triterpene from Chinese Dandelion root (Hokouei-kon), on a mouse melanoma cell line. Biol. Pharm. Bull. 2000, 23, 962–967. [Google Scholar]
- Puri, N.; Eller, M.S.; Byers, H.R.; Dykstra, S.; Kubera, J.; Gilchrest, B.A. Telomere-based DNA damage responses: A new approach to melanoma. FASEB J. 2004, 18, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Russo, AE.; Torrisi, E.; Bevelacqua, Y.; Perrotta, R.; Libra, M.; Mccubrey, JA.; Spandidos, DA.; Stivala, F.; Malaponte, G. Melanoma: Molecular pathogenesis and emerging target therapies (Review). Int. J. Oncol. 2009, 34, 1481–1489. [Google Scholar] [PubMed]
- Hata, K.; Hori, K.; Takahashi, S. Role of p38 MAPK in lupeol-induced B16 2F2 mouse melanoma cell differentiation. J. Biochem. 2003, 134, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.L.; Park, S.Y.; Kim, Y.H.; Park, G.; Son, H.J.; Lee, S.J. Suppression of α-MSH and IBMX-induced melanogenesis by cordycepin via inhibition of CREB and MITF, and activation of PI3K/Akt and ERK-dependent mechanisms. Int. J. Mol. Sci. 2012, 29, 119–124. [Google Scholar]
- Takeda, K. Ser298 of MITF, a mutation site in Waardenburg syndrome type 2, is a phosphorylation site with functional significance. Hum. Mol. Genet. 2000, 9, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Neuhouser, M.L. Review: Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr. Cancer. 2004, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.; Stevens, J.; Helmrich, A.; Henderson, M.; Rodriguez, R.; Yang, Y.; Deinzer, M.; Barnes, D.; Buhler, D. Antiproliferative and cytotoxic effects of prenylated flavonoids from hops (Humuluslupulus) in human cancer cell lines. Food Chem. Toxicol. 1999, 37, 271–285. [Google Scholar] [CrossRef]
- Ko, H.; Lu, Y.; Yang, S.; Won, S.; Lin, C. Cytotoxic prenyl flavonoids from Artocarpuselasticus. J. Nat. Prod. 2005, 68, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Leszczyniecka, M.; Roberts, T.; Dent, P.; Grant, S.; Fisher, P.B. Differentiation therapy of human cancer: Basic science and clinical applications. Pharmacol. Ther. 2001, 90, 105–156. [Google Scholar] [CrossRef]
- Serafino, A.; Sinibaldi, V.P.; Lazzarino, G.; Tavazzi, B.; Rasi, G.; Pierimarchi, P.; Andreola, F.; Moroni, G.; Galvano, G.; Galvano, F.; et al. Differentiation of human melanoma cells induced by cyanidin-3-O-β-glucopyranoside. FASEB J. 2004, 18, 1940–1942. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chou, G.X.; Wang, H.; Chu, J.H.; Fong, W.F.; Yu, Z.L. Effects of sesquiterpenes isolated from LargeheadAtractylodes Rhizome on growth, migration, and differentiation of B16 melanoma cells. Integr. Cancer Ther. 2010, 10, 92–100. [Google Scholar]
- Tsai, J.; Lee, C.; Ying, T.; Lin, C.; Lin, C.; Hsueh, J.; Hsieh, Y. Licochalcone A induces autophagy through PI3K/Akt/mTOR inactivation and autophagy suppression enhances Licochalcone A-induced apoptosis of human cervical cancer cells. Oncotarget 2015, 6, 28851–28866. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Gu, G.E.; Jo, A.R.; Bang, J.S.; Yun, H.; Baek, K.J.; Kwon, N.S.; Park, K.C.; Kim, D.S. Baicalin-induced Akt activation decreases melanogenesis through downregulation of microphthalmia-associated transcription factor and tyrosinase. Eur. J. Pharmacol. 2015, 761, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Alesiani, D.; Cicconi, R.; Mattei, M.; Montesano, C.; Bei, R.; Canini, A. Cell cycle arrest and differentiation induction by 5, 7-dimethoxycoumarin in melanoma cell lines. Int. J. Oncol. 2008, 32, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, K.; Naya, Y.; Okamoto, Y.; Hata, K. Differentiation-inducing and antiproliferative activities of lupeol on canine melanoma cells. SpringerPlus. 2014, 3, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Li, D.; Zhao, H.; Jiang, J.; Wang, P.; Ma, X.; Sun, X.; Zheng, Q. Licochalcone A-Induced Human Bladder Cancer T24 Cells apoptosis triggered by mitochondria dysfunction and endoplasmic reticulum stress. BioMed. Res. Int. 2013, 2013, 474272. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.K.; Wang, H.Z.; Xie, L.P.; Chen, D.W.; Liu, X.; Sun, J.; Nie, Y.C.; Zhang, R.Q. The effects of oridonin on cell growth, cell cycle, cell migration and differentiation in melanoma cells. J. Ethnopharmacol. 2006, 103, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Naliwaiko, K.; Luvizon, A.C.; Donatti, L.; Chammas, R.; Mercadante, A.F.; Zanata, S.M.; Nakao, L.S. Guanosine promotes B16F10 melanoma cell differentiation through PKC–ERK 1/2 pathway. Chem. Biol. Interact. 2008, 173, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.S.; Yan, L.; Bi, C.W.; Chan, G.K.; Wu, Q.Y.; Liu, Y.; Wang, H.Y.; Yao, P.; Du, C.Y.; Dong, T.T.; et al. Yu Ping Feng San reverses cisplatin-induced multi-drug resistance in lung cancer cells via regulating drug transporters and p62/TRAF6 signalling. Sci. Rep. 2016, 6, 31926. [Google Scholar] [CrossRef] [PubMed]
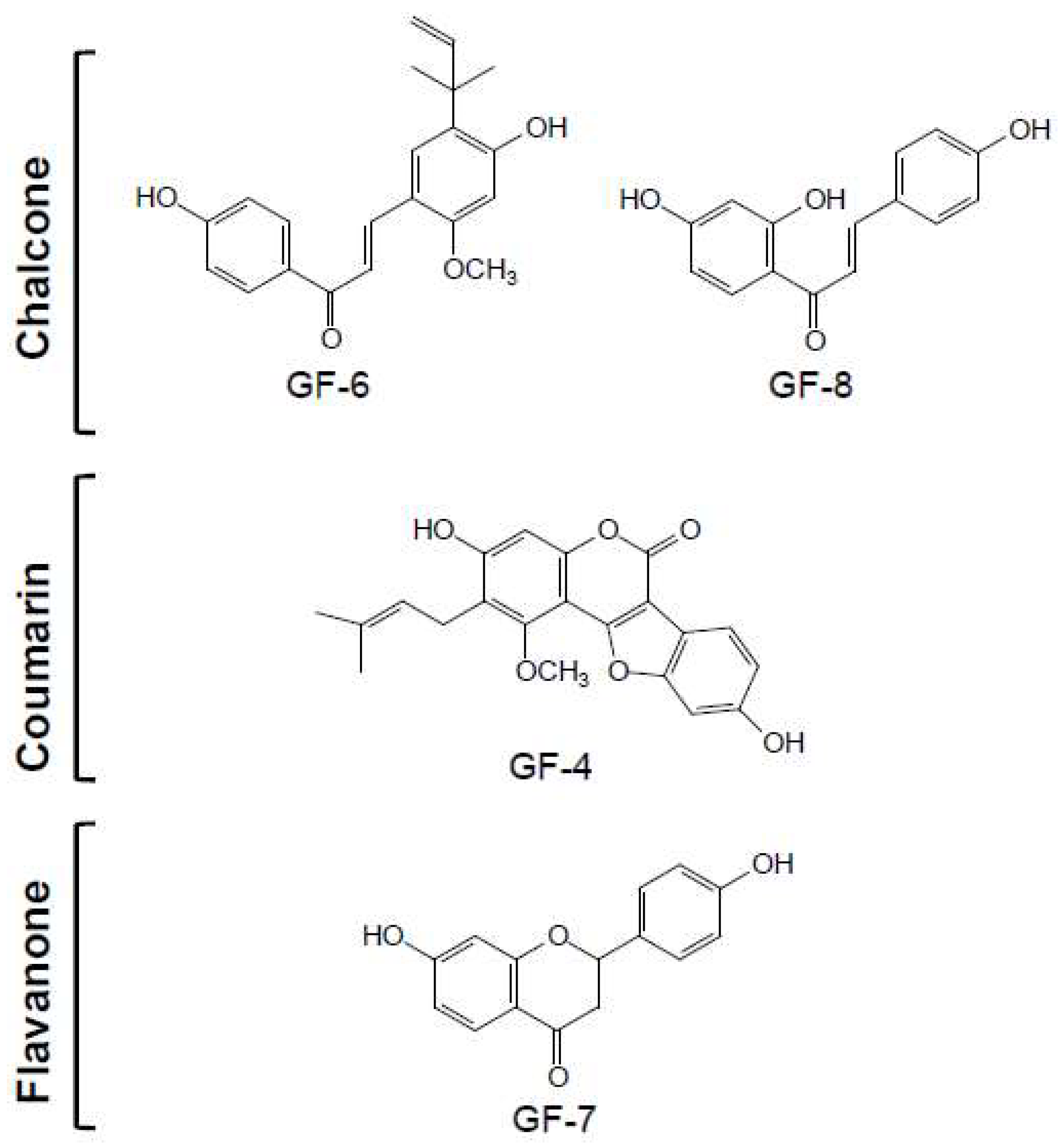
| GF-1 | GF-2 | GF-3 | GF-4 | GF-5 | GF-6 | GF-7 | GF-8 | GF-9 | GF-10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| C-1 | 113.5 | 125.7 | ||||||||
| C-2 | 155.7 | 155.1 | 162.5 | 157.9 | 153.0 | 158.2 | 78.9 | 131.1 | 162.2 | 155.8 |
| C-3 | 120.5 | 122.1 | 104.4 | 102.7 | 124.2 | 99.9 | 43.1 | 115.8 | 104.4 | 137.7 |
| C-4 | 180.6 | 176.0 | 176.2 | 158.6 | 174.5 | 159.6 | 190.0 | 160.3 | 176.2 | 178.0 |
| C-4a | 104.6 | 116.2 | 16.1 | 100.2 | 127.2 | 113.5 | 115.8 | 105.1 | ||
| C-5 | 161.8 | 127.2 | 126.4 | 154.3 | 116.6 | 126.5 | 128.3 | 115.8 | 124.5 | 160.9 |
| C-6 | 98.8 | 115.3 | 114.7 | 120.1 | 115.0 | 127.7 | 110.5 | 131.1 | 127.2 | 97.6 |
| C-7 | 164.1 | 162.8 | 160.6 | 160.0 | 162.5 | 164.6 | 160.3 | 165.0 | ||
| C-8 | 93.6 | 102.0 | 102.4 | 99.7 | 102.0 | 102.5 | 101.7 | 92.2 | ||
| C-8a | 157.7 | 157.5 | 157.3 | 153.4 | 158.9 | 163.1 | 155.6 | 160.2 | ||
| C-α | 117.8 | 144.2 | ||||||||
| C-β | 138.7 | 117.4 | ||||||||
| C-γ | 187.3 | 191.5 | ||||||||
| C-1′ | 111.2 | 112.7 | 121.7 | 114.7 | 123.1 | 129.6 | 129.3 | 112.9 | 121.9 | 120.4 |
| C-2′ | 151.2 | 151.3 | 128.0 | 156.6 | 130.0 | 130.6 | 128.2 | 165.8 | 127.9 | 130.1 |
| C-3′ | 109.7 | 110.1 | 115.8 | 99.0 | 113.5 | 115.2 | 115.1 | 102.5 | 115.8 | 115.6 |
| C-4′ | 153.6 | 153.5 | 162.4 | 157.4 | 157.4 | 161.6 | 157.6 | 165.4 | 160.5 | 156.2 |
| C-5′ | 107.4 | 107.6 | 115.8 | 114.5 | 113.5 | 115.2 | 115.1 | 108.0 | 115.8 | 115.6 |
| C-6′ | 131.3 | 130.9 | 128.0 | 120.9 | 130.0 | 130.6 | 128.2 | 132.7 | 127.9 | 130.1 |
| C-1″ | 22.5 | 39.5 | 27.5 | |||||||
| C-2″ | 75.4 | 75.4 | 122.9 | 147.5 | 121.8 | |||||
| C-3″ | 128.8 | 128.8 | 131.4 | 109.9 | 132.3 | |||||
| C-4″ | 116.9 | 117.0 | 18.2 | 26.9 | 25.5 | |||||
| C-5″ | 27.4 | 27.5 | 25.9 | 26.9 | 17.6 | |||||
| C-6″ | 27.4 | 27.5 | ||||||||
| 2-OCH3 | 55.4 | |||||||||
| 3-OCH3 | 56.0 | |||||||||
| 4′-OCH3 | 55.1 | |||||||||
| 5-OCH3 | 62.8 | |||||||||
| 7-OCH3 | 59.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, H.; Yang, M.; Peng, G.; Dong, T.T.X.; Xu, M.L.; Tsim, K.W.K. Prenylated Flavonoids from Roots of Glycyrrhiza uralensis Induce Differentiation of B16-F10 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 2422. https://doi.org/10.3390/ijms19082422
Zheng Y, Wang H, Yang M, Peng G, Dong TTX, Xu ML, Tsim KWK. Prenylated Flavonoids from Roots of Glycyrrhiza uralensis Induce Differentiation of B16-F10 Melanoma Cells. International Journal of Molecular Sciences. 2018; 19(8):2422. https://doi.org/10.3390/ijms19082422
Chicago/Turabian StyleZheng, Yunfeng, Huaiyou Wang, Min Yang, Guoping Peng, Tina Ting Xia Dong, Miranda Li Xu, and Karl Wah Keung Tsim. 2018. "Prenylated Flavonoids from Roots of Glycyrrhiza uralensis Induce Differentiation of B16-F10 Melanoma Cells" International Journal of Molecular Sciences 19, no. 8: 2422. https://doi.org/10.3390/ijms19082422
APA StyleZheng, Y., Wang, H., Yang, M., Peng, G., Dong, T. T. X., Xu, M. L., & Tsim, K. W. K. (2018). Prenylated Flavonoids from Roots of Glycyrrhiza uralensis Induce Differentiation of B16-F10 Melanoma Cells. International Journal of Molecular Sciences, 19(8), 2422. https://doi.org/10.3390/ijms19082422