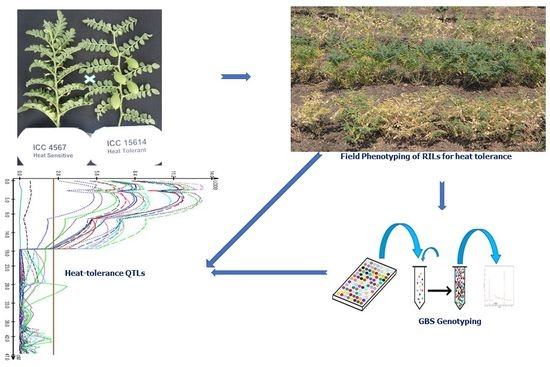
Molecular Mapping of QTLs for Heat Tolerance in Chickpea
Abstract
1. Introduction
2. Results
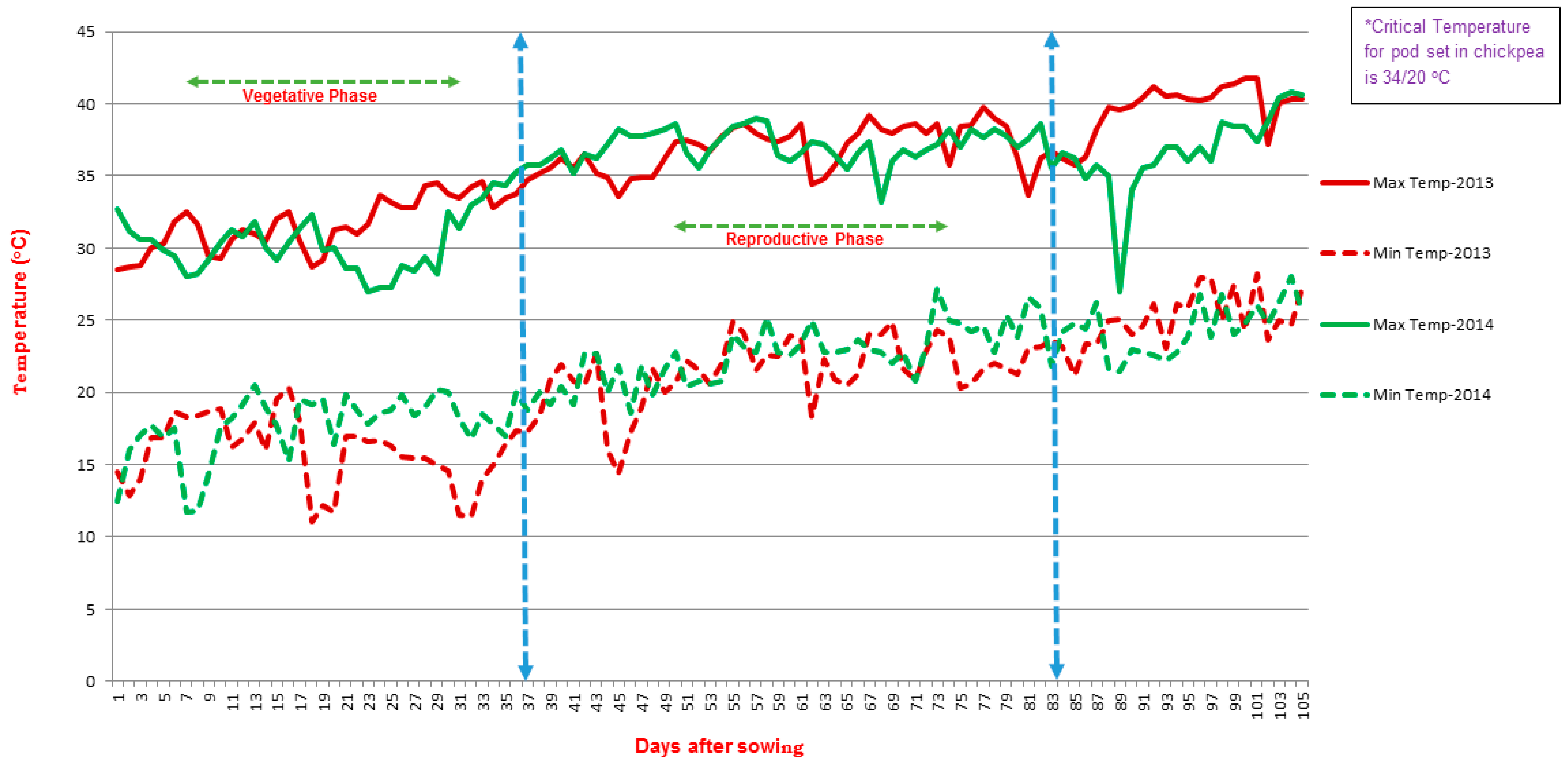
2.1. Response of Parents and Recombinant Inbred Lines (RILs) under Heat-Stress and Non-Stress Environments
2.2. Relationship between Yield and Yield Determining Traits
2.3. Sequencing Data and SNP Discovery
2.4. Genetic Linkage Map and Marker Distribution
2.5. QTL Analysis
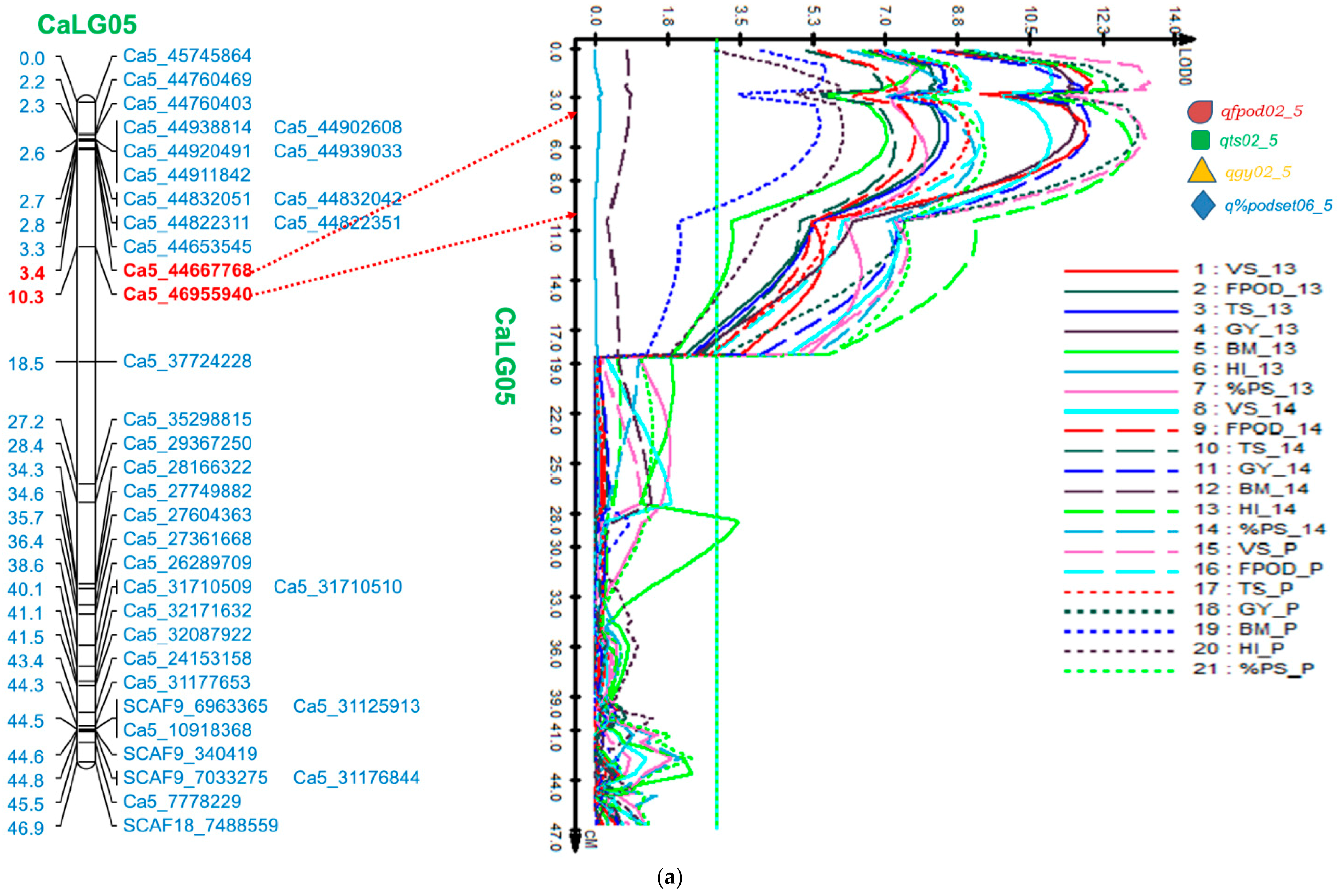
2.5.1. Genomic Region on CaLG05
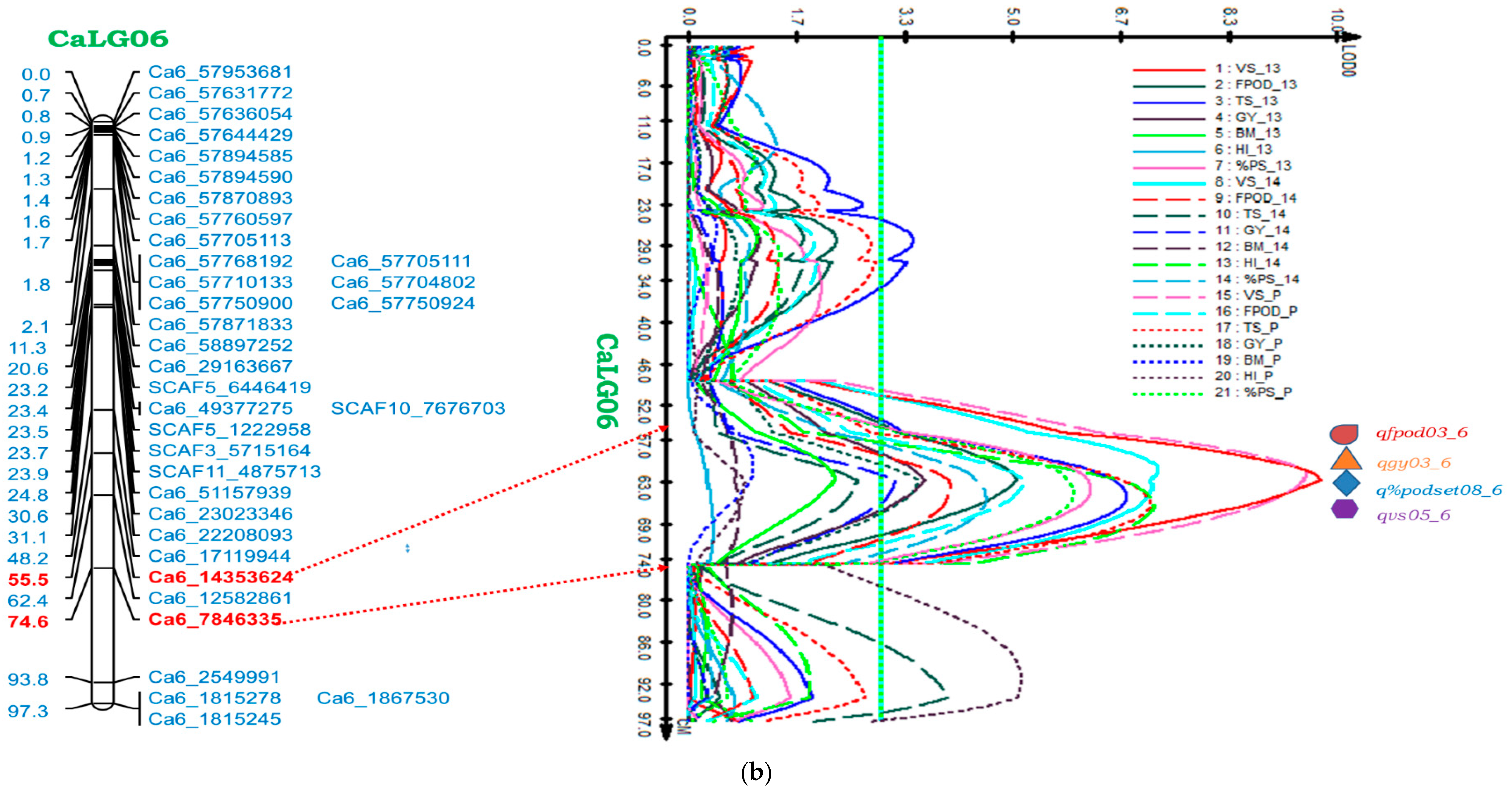
2.5.2. Genomic Region on CaLG06
2.5.3. QTLs Identified on Other LGs
2.5.4. Mapping of Epistatic QTLs (E-QTLs)
3. Discussion
3.1. Phenotypic Evaluation of RILs and Parents in Field Condition
3.2. QTL Mapping for Heat Tolerance
3.3. Epistatic QTLs for Heat Tolerance
3.4. Putative Candidate Genes for Heat Tolerance
4. Materials and Methods
4.1. Plant Material and Treatment Condition
4.2. Variables Measured
4.3. DNA Extraction, Genotyping, and SNP Calling
4.4. Linkage Map Construction, QTL Detection and Mining of Candidate Genes
4.5. Statistical Analyses
Analysis of Variance, Predicted Means (BLUP), Heritability, and Correlations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| %PodSet | Pod Setting Percentage |
| ANOVA | Analysis of Variance |
| BLUP | Best Linear Unbiased Prediction |
| BM | Biomass |
| CaLG | Cicer arietinum Linkage Group |
| CIM | Composite Interval Mapping |
| cM | Centimorgan |
| FPod | Number of Filled Pods Per Plot |
| GY | Grain Yield Per Plot |
| HI | Harvest Index |
| ICRISAT | International Crops Research Institute for the Semi-Arid Tropics |
| LG | Linkage Group |
| QTL | Quantitative Trait Loci |
| ReML | Residual Maximum Likelihood |
| RIL | Recombinant Inbred Line |
| TS | Total Number of Seeds Per Plot |
| VS | Visual Scoring |
References
- Gaur, P.M.; Jukanti, A.K.; Samineni, S.; Chaturvedi, S.K.; Basu, P.S.; Babbar, A.; Jayalakshmi, V.; Nayyar, H.; Devasirvatham, V.; Mallikarjuna, N.; et al. Climate Change and Heat Stress Tolerance in Chickpea. Climate Change and Plant Abiotic Stress Tolerance; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 837–856. [Google Scholar]
- Food and Agriculture Organization (FAO). Food and Agricultural Organization of the United Nation, FAO Statistical Database. 2015. Available online: http://faostat3.fao.org/download/Q/QC/E (accessed on 8 February 2018).
- Krishnamurthy, L.; Gaur, P.M.; Basu, P.S.; Chaturvedi, S.K.; Tripathi, S.; Vadez, V.; Rathore, A.; Varshney, R.K.; Gowda, C.L.L. Large genetic variation for heat tolerance in the reference collection of chickpea (Cicer arietinum L.) germplasm. Plant Genet. Resour. 2011, 9, 59–69. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.M.; Mallikarjuna, N.; Tokachichu, R.N.; Trethowan, R.M.; Tan, D.K.Y. Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant. Biol. 2012, 39, 1009–1018. [Google Scholar] [CrossRef]
- Wang, J.; Gan, Y.T.; Clarke, F.; McDonald, C.L. Response of chickpea yield to high temperature stress during reproductive development. Crop Sci. 2006, 46, 2171–2178. [Google Scholar] [CrossRef]
- Dehghani, H.; Sabaghpour, S.H.; Ebadi, A. Study of genotype × environment interaction for chickpea yieldin Iran. Agron. J. 2010, 102, 1–8. [Google Scholar] [CrossRef]
- Gaur, P.M.; Thudi, M.; Samineni, S.; Varshney, R.K. Advances in chickpea genomics. In Legumes in the Omic Era; Springer: New York, NY, USA, 2014; pp. 73–94. [Google Scholar]
- Sabbavarapu, M.M.; Sharma, M.; Chamarthi, S.K.; Swapna, N.; Rathore, A.; Thudi, M.; Gaur, P.M.; Pande, S.; Singh, S.; Kaur, L.; et al. Molecular mapping of QTLs for resistance to Fusarium wilt (race 1) and Ascochyta blight in chickpea (Cicer arietinum L.). Euphytica 2013, 193, 121–133. [Google Scholar] [CrossRef]
- Varshney, R.K.; Thudi, M.; Nayak, S.N.; Gaur, P.M.; Kashiwagi, J.; Krishnamurthy, L.; Jaganathan, D.; Koppolu, J.; Bohra, A.; Tripathi, S.; et al. Genetic dissection of drought tolerance in chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2014, 127, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Pushpavalli, R.; Krishnamurthy, L.; Thudi, M.; Gaur, P.M.; Rao, M.V.; Siddique, K.H.; Colmer, T.D.; Turner, N.C.; Varshney, R.K.; Vadez, V. Two key genomic regions harbour QTLs for salinity tolerance in ICCV 2× JG 11 derived chickpea (Cicer arietinum L.) recombinant inbred lines. BMC Plant Biol. 2015, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Krishnamurthy, L.; Thudi, M.; Anuradha, C.; Colmer, T.D.; Turner, N.C.; Siddique, K.H.; Gaur, P.M.; Varshney, R.K. Assessment of ICCV 2 × JG 62 chickpea progenies shows sensitivity of reproduction to salt stress and reveals QTL for seed yield and yield components. Mol. Breed. 2012, 30, 9–21. [Google Scholar] [CrossRef]
- Samineni, S. Physiology, Genetics and QTL Mapping of Salt Tolerance in Chickpea (Cicer arietinum L.). Ph.D. Thesis, The University of Western Australia, Perth, Australia, 2011. [Google Scholar]
- Jha, U.C.; Bohra, A.; Singh, N.P. Heat stress in crop plants: Its nature, impacts and integrated breeding strategies to improve heat tolerance. Plant Breed. 2014, 133, 679–701. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Craufurd, P.Q.; Wheeler, T.R. Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Sci. 2008, 48, 1140–1146. [Google Scholar] [CrossRef]
- Ye, C.; Argayoso, M.A.; Redoña, E.D.; Sierra, S.N.; Laza, M.A.; Dilla, C.J.; Mo, Y.; Thomson, M.J.; Chin, J.; Delaviña, C.B.; et al. Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed. 2012, 131, 33–41. [Google Scholar] [CrossRef]
- Xiao, Y.; Pan, Y.; Luo, L.; Zhang, G.; Deng, H.; Dai, L.; Liu, X.; Tang, W.; Chen, L.; Wang, G.L. Quantitative trait loci associated with seed set under high temperature stress at the flowering stage in rice (Oryza sativa L.). Euphytica 2011, 178, 331–338. [Google Scholar] [CrossRef]
- Pinto, R.S.; Reynolds, M.P.; Mathews, K.L.; McIntyre, C.L.; Olivares-Villegas, J.J.; Chapman, S.C. Heat and drought adaptive QTL in a wheat population designed to minimize confounding agronomic effects. Theor. Appl. Genet. 2010, 121, 1001–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Chen, L.Y.; Xiao, G.Y.; Xiao, Y.H.; Chen, X.B.; Zhang, S.T. Bulked segregant analysis to detect QTL related to heat tolerance in rice (Oryza sativa L.) using SSR markers. Agric. Sci. China 2009, 8, 482–487. [Google Scholar] [CrossRef]
- Devasirvatham, V.; Gaur, P.M.; Mallikarjuna, N.; Raju, T.N.; Trethowan, R.M.; Tan, D.K.Y. Reproductive biology of chickpea response to heat stress in the field is associated with the performance in controlled environments. Field Crop Res. 2013, 142, 9–19. [Google Scholar] [CrossRef]
- Varshney, R.K.; Song, C.; Saxena, R.K.; Azam, S.; Yu, S.; Sharpe, A.G.; Cannon, S.; Baek, J.; Rosen, B.D.; Tar’an, B.; et al. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotechnol. 2013, 31, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.M.; Srinivasan, S.; Gowda, C.L.L.; Rao, B.V. Rapid generation advancement in chickpea. J. SAT Agric. Res. 2007, 3, 3. [Google Scholar]
- Berger, J.D.; Milroy, S.P.; Turner, N.C.; Siddique, K.H.M.; Imtiaz, M.; Malhotra, R. Chickpea evolution has selected for contrasting phenological mechanisms among different habitats. Euphytica 2011, 180, 1–15. [Google Scholar] [CrossRef]
- Paliwal, R.; Röder, M.S.; Kumar, U.; Srivastava, J.P.; Joshi, A.K. QTL mapping of terminal heat tolerance in hexaploid wheat (T. aestivum L.). Theor. Appl. Genet. 2012, 125, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Buu, B.C.; Ha, P.T.T.; Tam, B.P.; Nhien, T.T.; Van Hieu, N.; Phuoc, N.T.; Giang, L.H.; Lang, N.T. Quantitative trait loci associated with heat tolerance in rice (Oryza sativa L.). Plant Breed. Biotechnol. 2014, 2, 14–24. [Google Scholar] [CrossRef]
- Jaganathan, D.; Thudi, M.; Kale, S.; Azam, S.; Roorkiwal, M.; Gaur, P.M.; Kishor, P.K.; Nguyen, H.; Sutton, T.; Varshney, R.K. Genotyping-by-sequencing based intra-specific genetic map refines a “QTL-hotspot” region for drought tolerance in chickpea. Mol. Genet. Genom. 2015, 290, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Bocianowski, J. Epistasis interaction of QTL effects as a genetic parameter influencing estimation of the genetic additive effect. Genet. Mol. Biol. 2013, 36, 093–100. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.J.M.; Radhika, P.; Mhase, L.B.; Jamadagni, B.M.; Gupta, V.S.; Kadoo, N.Y. Mapping of QTLs governing agronomic and yield traits in chickpea. J. Appl. Genet. 2011, 52, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Mao, L.; Sun, C.; Pu, Y.; Fu, T.; Ma, C.; Shen, J.; Tu, J.; Yi, B.; Wen, J. Interpreting the genetic basis of silique traits in Brassica napus using a joint QTL network. Plant Breed. 2014, 133, 52–60. [Google Scholar] [CrossRef]
- Urano, K.; Kurihara, Y.; Seki, M.; Shinozaki, K. ‘Omics’ analyses of regulatory networks in plant abiotic stress responses. Curr. Opin. Plant Biol. 2010, 13, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Sopory, S.K.; Kishor, P.K. Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene 2007, 388, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Vij, S.; Tyagi, A.K. Emerging trends in the functional genomics of the abiotic stress response in crop plants. Plant Biotechnol. J. 2007, 5, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Pottorff, M.; Roberts, P.A.; Close, T.J.; Lonardi, S.; Wanamaker, S.; Ehlers, J.D. Identification of candidate genes and molecular markers for heat-induced brown discoloration of seed coats in (Vigna unguiculata (L.) Walp). BMC Genom. 2014, 15, 328. [Google Scholar] [CrossRef] [PubMed]
- Maestri, E.; Klueva, N.; Perrotta, C.; Gulli, M.; Nguyen, H.T.; Marmiroli, N. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol. Biol. 2002, 48, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, G.; Garg, V.; Kudapa, H.; Doddamani, D.; Pazhamala, L.T.; Khan, A.W.; Thudi, M.; Lee, S.H.; Varshney, R.K. Genome-wide dissection of AP2/ERF and HSP90 gene families in five legumes and expression profiles in chickpea and pigeonpea. Plant Biotechnol. J. 2016, 14, 1563–1577. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Larkindale, J.; Huang, B. Effects of abscisic acid, salicylic acid, ethylene and hydrogen peroxide in thermotolerance and recovery for creeping bentgrass. Plant Growth Regul. 2005, 47, 17–28. [Google Scholar] [CrossRef]
- Cuc, L.M.; Mace, E.S.; Crouch, J.H.; Quang, V.D.; Long, T.D.; Varshney, R.K. Isolation and characterization of novel microsatellite markers and their application for diversity assessment in cultivated groundnut (Arachis hypogaea). BMC Plant Biol. 2008, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, C.; Li, Y.; Lam, T.W.; Yiu, S.M.; Kristiansen, K.; Wang, J. SOAP2: An improved ultrafast tool for short read alignment. Bioinformatics 2009, 25, 1966–1967. [Google Scholar] [CrossRef] [PubMed]
- Van Ooijen, J.J. JoinMap® 4.1, Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Kyazma BV: Wageningen, The Netherlands, 2006. [Google Scholar]
- Wang, S.; Basten, C.J.; Zeng, Z.B. Windows QTL Cartographer 2.5; Department of Statistics, North Carolina State University: Raleigh, NC, USA, 2012. [Google Scholar]
- Searle, S. Linear Models; John Wiley & Sons, Inc.: New York, NY, USA, 1971. [Google Scholar]
- Falconer, D.S.; Mackay, T.F.; Frankham, R. Introduction to Quantitative Genetics. Trends in Genetics; Longman Frankel: Harlow, UK, 1996; Volume 12, p. 280. [Google Scholar]
- Hill, J.; Becker, H.C.; Tigerstedt, P.M. Quantitative and Ecological Aspects of Plant Breeding; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
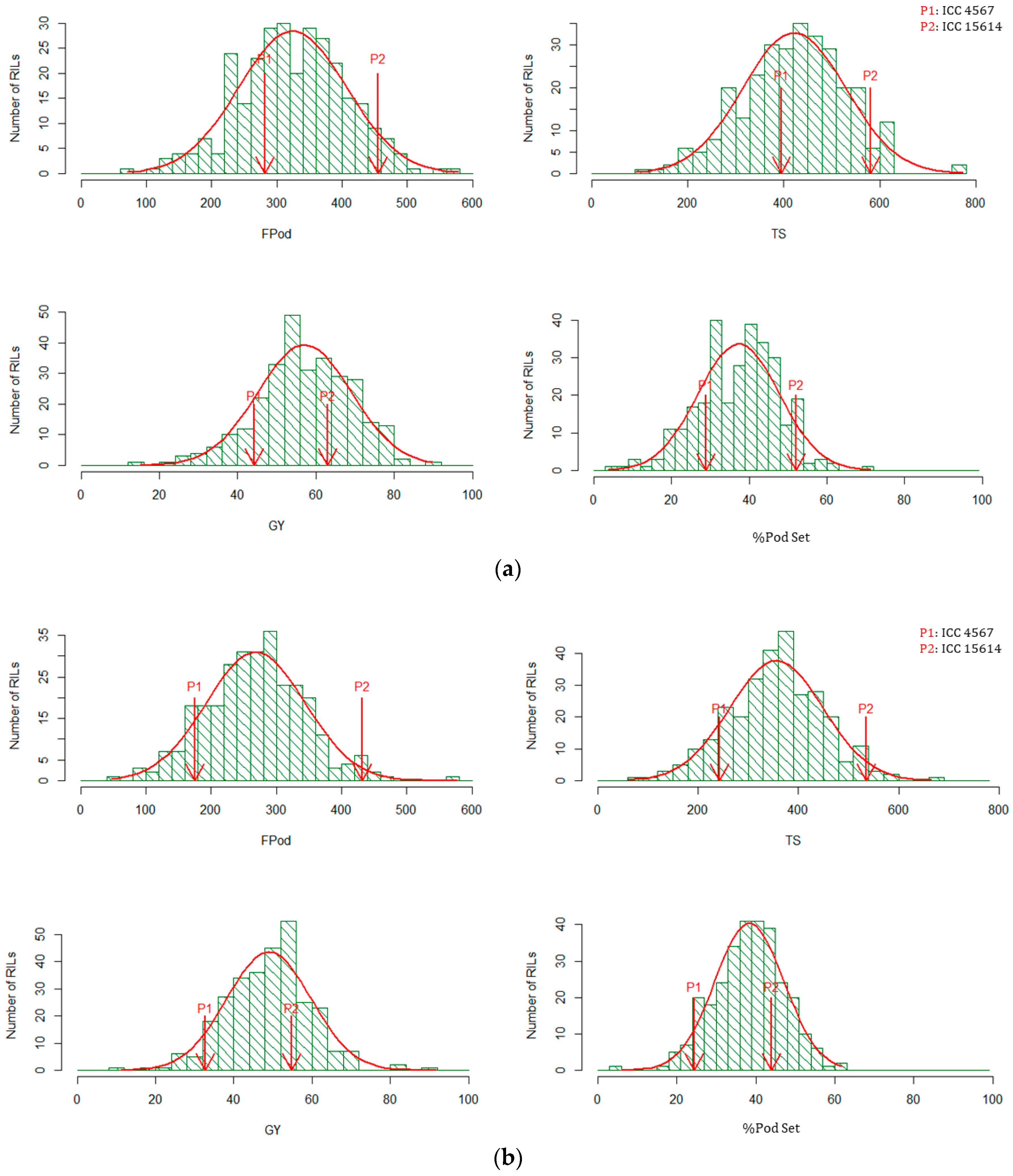
| Trait | Visual Score | Filled Pods Plot−1 | Total No. of Seeds Plot−1 | Grain Yield Plot−1 (g) | Biomass Plot−1 (g) | Harvest Index | Percent PodSet (%) |
|---|---|---|---|---|---|---|---|
| Non-stress Environment, 2013 | |||||||
| ICC 4567 (heat sensitive) | - | 406.8 | 429.2 | 76.0 | 144.8 | 52.1 | 67.7 |
| ICC 15614 (heat tolerant) | - | 538.7 | 553.0 | 70.2 | 132.3 | 53.9 | 75.6 |
| Contrast analysis between parents | - | −131.9 * | −123.9 * | 5.8 ns | 12.5 ns | −1.9 ns | −7.9 ns |
| Mean of RILs | - | 459.0 | 486.3 | 73.5 | 139.7 | 53.0 | 68.8 |
| Range of RILs | - | 360.8–580.1 | 378.3–604.7 | 57.6–93.3 | 118.1–165.2 | 45.5–59.2 | 48.1–84.2 |
| Heritability (%) | - | 62.1 | 60.5 | 57.6 | 47.6 | 63.4 | 66.0 |
| Heat-stress environment, 2013 | |||||||
| ICC 4567 (heat sensitive) | 2 | 281.3 | 395.1 | 44.3 | 147.6 | 34.2 | 28.8 |
| ICC 15614 (heat tolerant) | 5 | 455.6 | 580.7 | 62.9 | 125.9 | 50.6 | 52.0 |
| Contrast analysis between parents | −0.5 * | −174.3 * | −185.6 * | −18.6 * | 21.7 ns | −16.3 * | −23.1 * |
| Mean of RILs | 3.0 | 323.9 | 421.3 | 57.1 | 114.6 | 50.6 | 37.3 |
| Range of RILs | (1–5) | 70.5–578.3 | 91.9–772.4 | 14.9–89.8 | 32.9–185.6 | 34.5–69.1 | 3.7–71.3 |
| Heritability (%) | 79.8 | 86.9 | 86.3 | 82.2 | 83.2 | 72.0 | 90.7 |
| Heat-stress environment, 2014 | |||||||
| ICC 4567 (heat sensitive) | 2 | 175.3 | 242.0 | 32.6 | 123.2 | 23.9 | 24.4 |
| ICC 15614 (heat tolerant) | 5 | 431.2 | 534.9 | 54.8 | 111.6 | 52.0 | 43.9 |
| Contrast analysis between parents | −0.6 * | −255.9 * | −292.9 * | −22.1 * | 11.7 ns | −28.2 * | −19.6 * |
| Mean of RILs | 3.0 | 268.0 | 355.7 | 49.0 | 119.7 | 40.9 | 38.4 |
| Range of RILs | (1–5) | 46.9–576.8 | 61.8–665.8 | 11.0–91.6 | 65.4–142.4 | 12.8–63.4 | 5.8–61.6 |
| Heritability (%) | 86.5 | 86.8 | 86.6 | 80.9 | 49.8 | 91.3 | 84.7 |
| Pooled environments (Heat-stress environments, 2013 and 2014) | |||||||
| ICC 4567 (heat sensitive) | 2 | 201.6 | 278.1 | 37.5 | 134.8 | 28.6 | 26.1 |
| ICC 15614 (heat tolerant) | 5 | 453.6 | 570.3 | 59.6 | 116.4 | 51.2 | 48.7 |
| Contrast analysis between parents | −0.6 * | −252 * | −292.2 * | −22 * | 18.4 ns | −22.6 * | −22.6 * |
| Mean of RILs | 3.0 | 296.0 | 388.5 | 53.0 | 117.2 | 45.8 | 37.9 |
| Range of RILs | (1–5) | 42.2–516 | 54.9–672.5 | 9.01–82.3 | 37.14–157.5 | 24.13–58.8 | 2.61–63.9 |
| Heritability (%) | 72.2 | 81.6 | 82.3 | 73.1 | 19.2 | NA | 81.6 |
| Environments | Traits | VS | FPod | TS | BM | HI | %PodSet | GY |
|---|---|---|---|---|---|---|---|---|
| HSE-2013 | VS | 1 | ||||||
| HSE-2014 | VS | 1 | ||||||
| Pooled years | VS | 1 | ||||||
| HSE-2013 | FPod | 0.68 ** | 1 | |||||
| HSE-2014 | FPod | 0.78 ** | 1 | |||||
| Pooled years | FPod | 0.80 ** | 1 | |||||
| HSE-2013 | TS | 0.67 ** | 0.97 ** | 1 | ||||
| HSE-2014 | TS | 0.78 ** | 0.96 ** | 1 | ||||
| Pooled years | TS | 0.79 ** | 0.97 ** | 1 | ||||
| HSE-2013 | BM | 0.69 ** | 0.70 ** | 0.68 ** | 1 | |||
| HSE-2014 | BM | 0.15 ** | 0.40 ** | 0.38 ** | 1 | |||
| Pooled years | BM | 0.61 ** | 0.67 ** | 0.65 ** | 1 | |||
| HSE-2013 | HI | −0.04 ns | 0.22 ** | 0.25 ** | −0.35 ** | 1 | ||
| HSE-2014 | HI | 0.83 ** | 0.84 ** | 0.84 ** | 0.08 ns | 1 | ||
| Pooled years | HI | 0.62 ** | 0.70 ** | 0.72 ** | 0.24 ** | 1 | ||
| HSE-2013 | %PodSet | 0.63 ** | 0.72 ** | 0.73 ** | 0.62 ** | 0.00 | 1 | |
| HSE-2014 | %PodSet | 0.61 ** | 0.59 ** | 0.60 ** | 0.05 ** | 0.62 ** | 1 | |
| Pooled years | %PodSet | 0.71 ** | 0.77 ** | 0.78 ** | 0.50 ** | 0.59 ** | 1 | |
| HSE-2013 | GY | 0.66 ** | 0.88 ** | 0.89 ** | 0.74 ** | 0.32 ** | 0.63 ** | 1 |
| HSE-2014 | GY | 0.73 ** | 0.90 ** | 0.89 ** | 0.57 ** | 0.84 ** | 0.50 ** | 1 |
| Pooled years | GY | 0.79 ** | 0.89 ** | 0.88 ** | 0.78 ** | 0.76 ** | 0.69 ** | 1 |
| Traits | FPod | TS | BM | HI | %PodSet | GY | ||
| NSE-2013 | FPod | 1 | ||||||
| NSE-2013 | TS | 0.94 ** | 1 | |||||
| NSE-2013 | BM | 0.60 ** | 0.63 ** | 1 | ||||
| NSE-2013 | HI | 0.15 ** | 0.22 ** | −0.07 ns | 1 | |||
| NSE-2013 | %PodSet | 0.23 ** | 0.27 ** | 0.17 ** | 0.05 ns | 1 | ||
| NSE-2013 | GY | 0.63 ** | 0.69 ** | 0.91 ** | 0.33 ** | 0.17 ** | 1 |
| LG | Marker Interval | Trait | QTL Name | Heat-Stress Environment, 2013 | Heat-Stress Environment, 2014 | Pooled Environments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position (cM) | %PVE | LOD | Add | Position (cM) | %PVE | LOD | Add | Position (cM) | %PVE | LOD | Add | ||||
| CaLG05 | Ca5_44667768-Ca5_46955940 | FPod | qfpod02_5 | 4.41 | 11.57 | 8.37 | 27.93 | 5.41 | 12.03 | 7.79 | 27.31 | 5.41 | 12.03 | 9.41 | 28.83 |
| TS | qts02_5 | 5.41 | 12.00 | 8.54 | 36.14 | 5.41 | 10.00 | 7.30 | 31.27 | 5.41 | 10.00 | 9.07 | 35.27 | ||
| GY | qgy02_5 | 4.41 | 16.04 | 11.69 | 4.72 | 4.41 | 16.56 | 12.00 | 4.61 | 4.41 | 16.56 | 13.17 | 4.64 | ||
| %PodSet | q%podset06_5 | 6.41 | 11.51 | 8.04 | 3.47 | 6.41 | 13.30 | 9.20 | 3.40 | 6.41 | 13.30 | 9.48 | 3.47 | ||
| CaLG06 | Ca6_7846335-Ca6_14353624 | VS | qvs05_6 | 62.41 | 11.07 | 9.79 | 0.05 | 61.51 | 9.04 | 7.26 | 0.06 | 61.51 | 9.04 | 9.54 | 0.06 |
| FPod | qfpod03_6 | 62.41 | 6.56 | 5.10 | 20.88 | 63.40 | 5.92 | 4.10 | 19.01 | 62.41 | 5.92 | 5.22 | 19.91 | ||
| GY | qgy03_6 | 62.41 | 4.43 | 3.68 | 2.48 | 62.41 | 3.92 | 3.21 | 2.24 | 62.41 | 3.92 | 3.58 | 2.24 | ||
| %PodSet | q%podset08_6 | 63.41 | 8.44 | 6.22 | 3.00 | 65.41 | 6.96 | 4.61 | 2.46 | 64.41 | 6.96 | 5.97 | 2.77 | ||
| SL. No. | Trait | QTL_i | LG | Marker Interval (QTL i) | Position (QTL_i) | QTL_j | LG | Marker Interval (QTL j) | Position (QTL_j) | AA | h2 (%) (AA) | h2 (%) (AAE) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | VS | eqvs1_1 | 1 | Ca1_1732919Ca1_4429044 | 48.5 | eqvs4_7 | 7 | Ca7_3634430-Ca7_6584610 | 4.6 | −0.02 *** | 1.02 | 0.12 |
| 2 | VS | neqvs2_4 | 4 | Ca4_48498166-Ca4_48498181 | 2.6 | neqvs3_5 | 5 | Ca5_29367250-Ca5_28166322 | 30.4 | 0.03 *** | 2.41 | 0.17 |
| 3 | FPod | eqfpod1_2 | 2 | Ca2_24709295-Ca2_30876552 | 30.7 | eqfpod2_2 | 2 | Ca2_34481663-Ca2_35860429 | 64.8 | −8.85 *** | 0.73 | 0.01 |
| 4 | FPod | neqfpod3_4 | 4 | Ca4_48497765-Ca4_48458381 | 2.2 | neqfpod4_5/neqts9_5 | 5 | SCAF9_6963365-Ca5_31125913 | 44.5 | 13.10 *** | 2.21 | 0.01 |
| 5 | TS | eqts1_1 | 1 | Ca1_11321839-Ca1_11411540 | 10.8 | eqts11_6 | 6 | Ca6_51157939-Ca6_23023346 | 27.8 | 13.15 *** | 0.42 | 0.01 |
| 6 | TS | eqts2_1/eqpodset2_1 | 1 | Ca1_39746426-Ca1_34727065 | 26.4 | eqts14_8 | 8 | Ca8_14753681-Ca8_14587797 | 5.6 | 9.78 *** | 0.46 | 0.02 |
| 7 | TS | eqts4_2 | 2 | Ca2_34481663-Ca2_35860429 | 65.8 | eqts12_6 | 6 | Ca6_12582861-Ca6_7846335 | 62.4 | −9.79 *** | 0.38 | 0.05 |
| 8 | TS | eqts4_2 | 2 | Ca2_34481663-Ca2_35860429 | 65.8 | eqts14_8 | 8 | Ca8_14753681-Ca8_14587797 | 5.6 | 16.97 *** | 0.96 | 0.01 |
| 9 | TS | eqts7_5 | 5 | Ca5_45745864-Ca5_44760469 | 2 | eqts13_6 | 6 | Ca6_2549991-Ca6_1815278 | 93.8 | −8.86 *** | 0.6 | 0.00 |
| 10 | TS | eqts2_1/eqpodset2_1 | 1 | Ca1_39746426-Ca1_34727065 | 26.4 | neqts10_6 | 6 | Ca6_58897252-Ca6_29163667 | 14.4 | 17.68 *** | 2.22 | 0.03 |
| 11 | TS | neqts3_2 | 2 | Ca2_32483185-Ca2_32979328 | 47.7 | neqts6_4 | 4 | Ca4_47243660-Ca4_44753224 | 22.3 | 13.47 *** | 2.12 | 0.01 |
| 12 | TS | neqts5_4 | 4 | Ca4_48458381-Ca4_48475589 | 2.2 | neqts8_5 | 5 | Ca5_27604363-Ca5_27361668 | 35.7 | 10.76 *** | 2.52 | 0.03 |
| 13 | TS | neqts5_4 | 4 | Ca4_48458381-Ca4_48475589 | 2.2 | neqts9_5/neqfpod4_5 | 5 | SCAF9_6963365-Ca5_31125913 | 44.5 | 12.02 *** | 2.7 | 0.00 |
| 14 | GY | eqgy1_1 | 1 | Ca1_1732919-Ca1_4429044 | 45.5 | eqgy2_2 | 2 | Ca2_34481663-Ca2_35860429 | 63.8 | 1.41 *** | 0.83 | 0.01 |
| 15 | BM | aaeqbm1_1 | 1 | Ca1_11685790-Ca1_11372972 | 9.1 | neqbm2_3 | 3 | Ca3_24194574-Ca3_22539683 | 52.9 | −2.09 *** | 1.22 | 0.21 |
| 16 | %PodSet | eqpodset1_1 | 1 | Ca1_11685790-Ca1_11372972 | 10.1 | eqpodset6_4 | 4 | Ca4_13699195-Ca4_7818876 | 75.6 | −1.33 *** | 0.83 | 0.01 |
| 17 | %PodSet | eqpodset2_1/eqts2_1 | 1 | Ca1_39746426-Ca1_34727065 | 26.4 | eqpodset6_4 | 4 | Ca4_13699195-Ca4_7818876 | 75.6 | 1.89 *** | 0.99 | 0.03 |
| 18 | %PodSet | eqpodset1_1 | 1 | Ca1_11685790-Ca1_11372972 | 10.1 | neqpodset4_4 | 4 | Ca4_48478303-Ca4_48475461 | 2.5 | −1.38 *** | 2.13 | 0.02 |
| 19 | %PodSet | neqpodset3_3 | 3 | Ca3_9400875-SCAF14_6484051 | 63.2 | neqpodset5_4 | 4 | Ca4_48269138-Ca4_47243656 | 11 | −1.44 *** | 1.84 | 0.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, P.J.; Samineni, S.; Thudi, M.; Sajja, S.B.; Rathore, A.; Das, R.R.; Khan, A.W.; Chaturvedi, S.K.; Lavanya, G.R.; Varshney, R.K.; et al. Molecular Mapping of QTLs for Heat Tolerance in Chickpea. Int. J. Mol. Sci. 2018, 19, 2166. https://doi.org/10.3390/ijms19082166
Paul PJ, Samineni S, Thudi M, Sajja SB, Rathore A, Das RR, Khan AW, Chaturvedi SK, Lavanya GR, Varshney RK, et al. Molecular Mapping of QTLs for Heat Tolerance in Chickpea. International Journal of Molecular Sciences. 2018; 19(8):2166. https://doi.org/10.3390/ijms19082166
Chicago/Turabian StylePaul, Pronob J., Srinivasan Samineni, Mahendar Thudi, Sobhan B. Sajja, Abhishek Rathore, Roma R. Das, Aamir W. Khan, Sushil K. Chaturvedi, Gera Roopa Lavanya, Rajeev. K. Varshney, and et al. 2018. "Molecular Mapping of QTLs for Heat Tolerance in Chickpea" International Journal of Molecular Sciences 19, no. 8: 2166. https://doi.org/10.3390/ijms19082166
APA StylePaul, P. J., Samineni, S., Thudi, M., Sajja, S. B., Rathore, A., Das, R. R., Khan, A. W., Chaturvedi, S. K., Lavanya, G. R., Varshney, R. K., & Gaur, P. M. (2018). Molecular Mapping of QTLs for Heat Tolerance in Chickpea. International Journal of Molecular Sciences, 19(8), 2166. https://doi.org/10.3390/ijms19082166