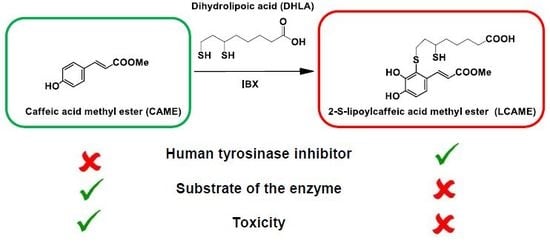
Conjugation with Dihydrolipoic Acid Imparts Caffeic Acid Ester Potent Inhibitory Effect on Dopa Oxidase Activity of Human Tyrosinase
Abstract
1. Introduction
2. Results and Discussion
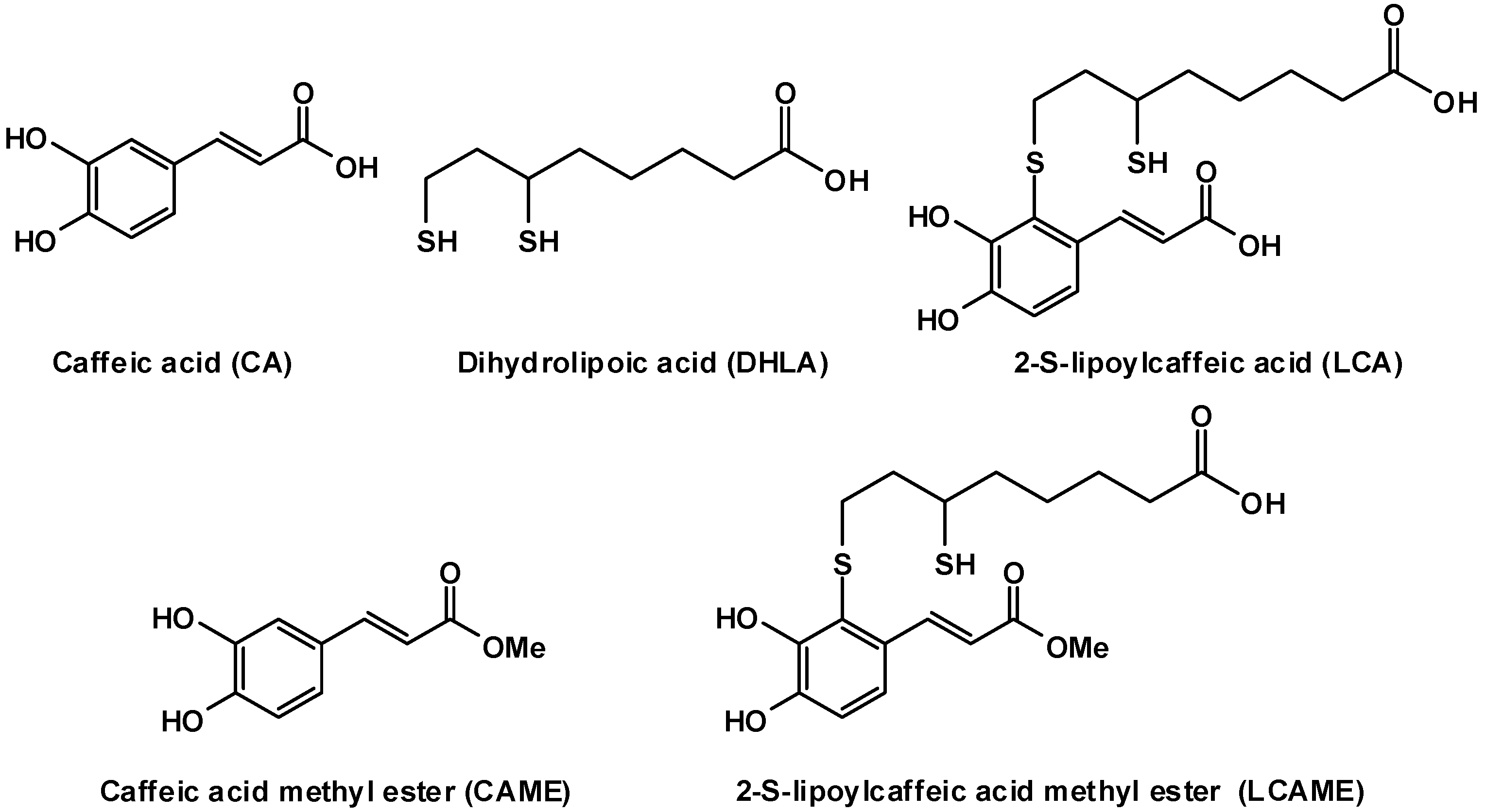
2.1. Preparation of LCAME
2.2. Inhibition of Mushroom Tyrosinase Activities by LCAME
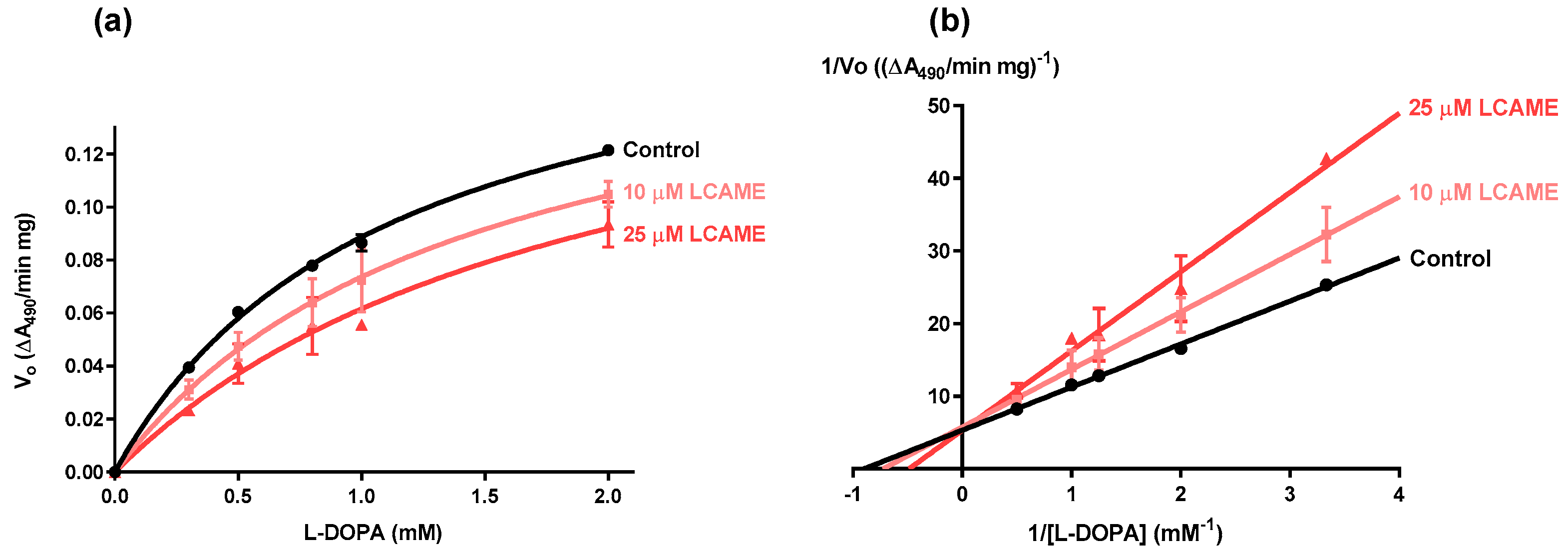
2.3. Effect of Lipoylconjugates on Human Tyrosinase Activity
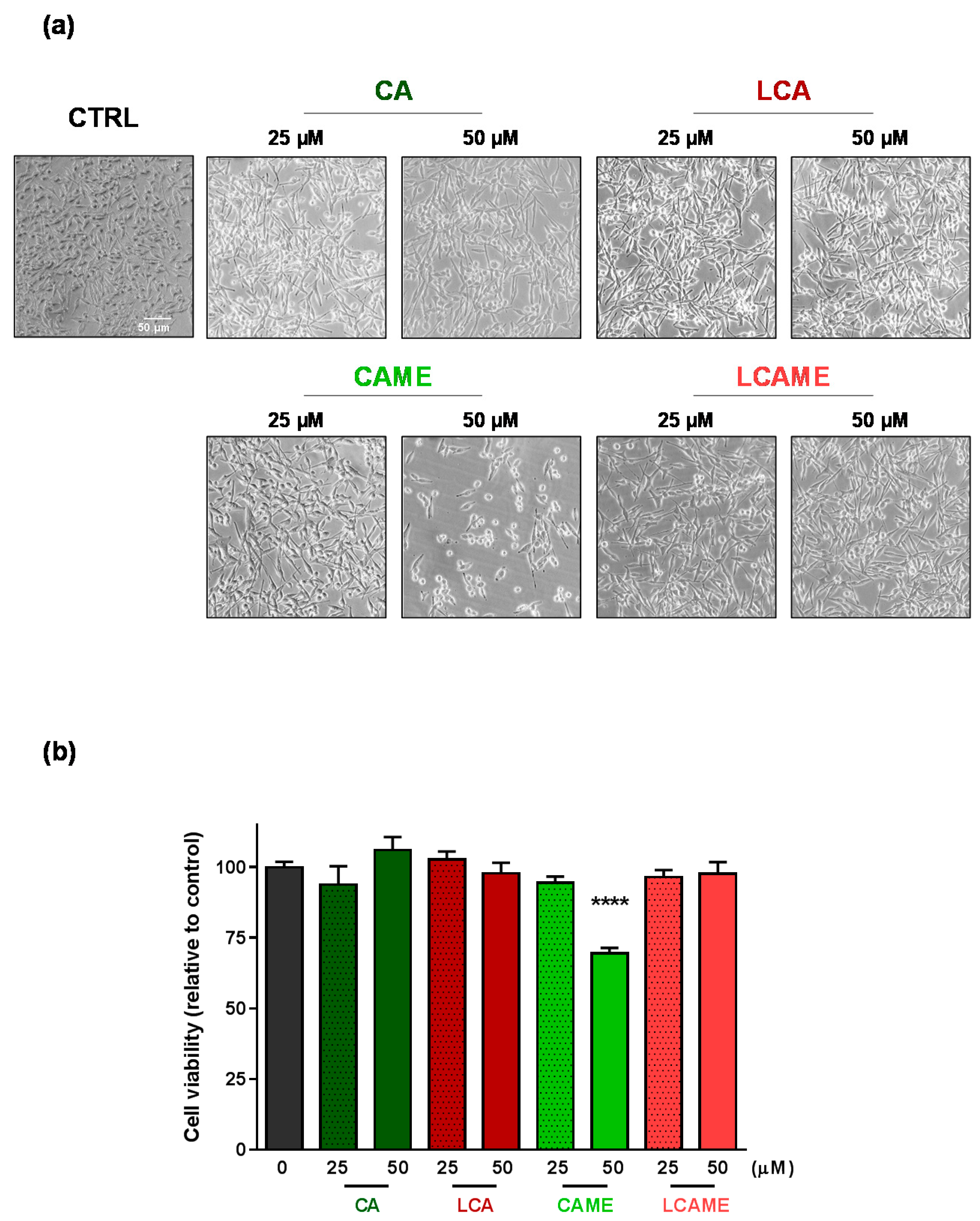
2.4. Effects on HBL Cell Viability
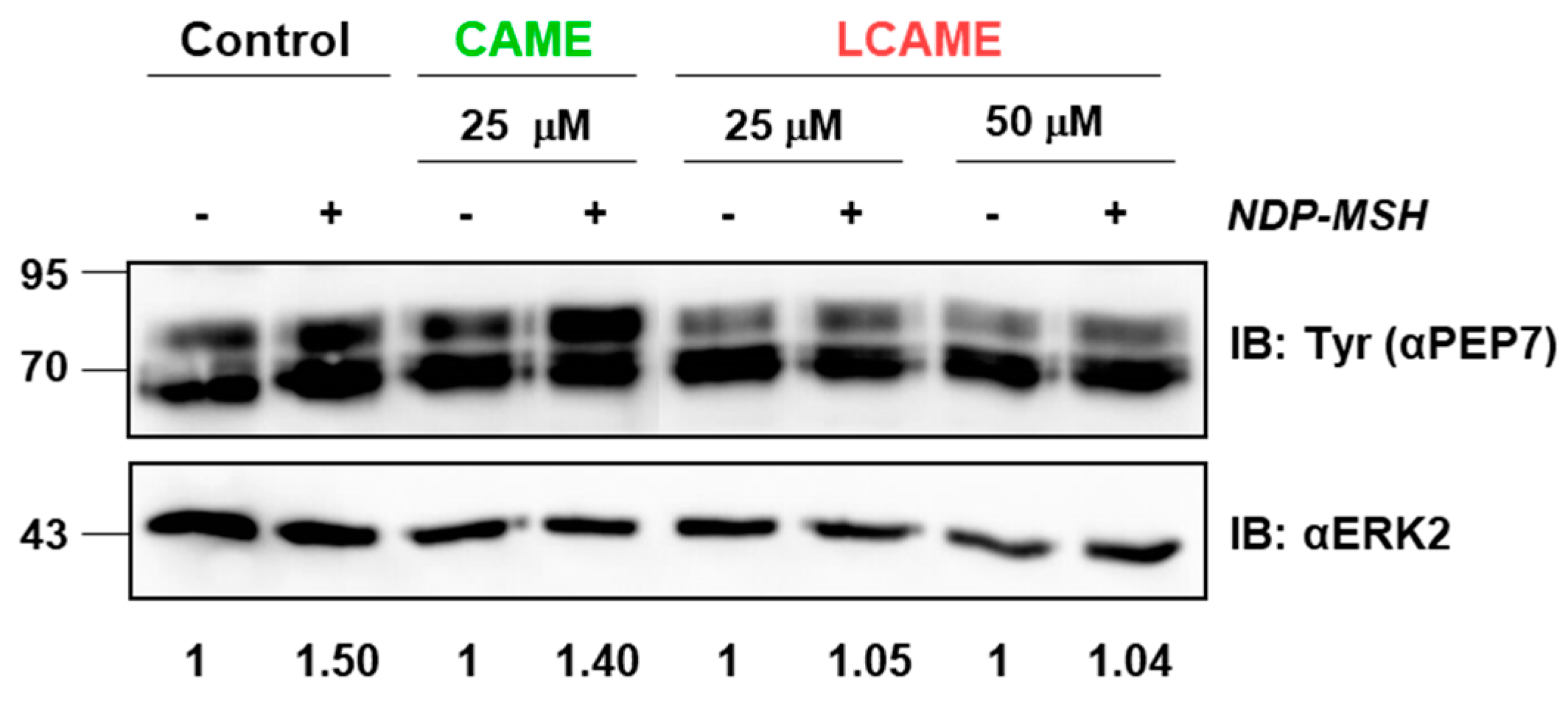
2.5. Effect of Inhibitors on Human Tyrosinase Expression
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Synthesis of p-Coumaric Acid Methyl Ester
3.4. Synthesis of 2-S-Lipoylcaffeic Acid Methyl Ester (LCAME)
3.5. Mushroom Tyrosinase Activity Inhibition Assay
3.6. Cell Culture
3.7. Enzyme Activity Determination
3.8. Immunochemical Techniques
3.9. MTT Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| α-MSH | Melanocyte Stimulating Hormone |
| CA | Caffeic acid |
| CAME | Caffeic acid methylester |
| LCA | 2-S-lipoylcaffeic acid |
| TH | Tyrosine hydroxylase |
| DHLA | Dihydrolipoic acid |
| DO | Dopa oxidase |
| DOPA | l-3,4-Dihydroxyohenylalanine |
| IBX | 2-iodoxybenzoic acid |
| IC50 | Half maximum inhibitory concentration |
| KA | Kojic acid |
| LA | Lipoic acid |
| LCAME | 2-S-lipoylcaffeic acid methylester |
| MBTH | 3-methyl-2-benzothiazolinone hydrazone |
| Mitf | Microphthalmia-associated transcription |
| MTT | 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| PMSF | Phenylmethylsulfonylfluorid |
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A. Melanin Pigmentation in Mammalian Skin and Its Hormonal Regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hearing, V.J. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev. Dermatol. 2011, 6, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Hearing, V.J. Melanocytes and their diseases. Cold Spring Harb. Perspect. Med. 2014, 4, a017046. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, G.; Kovacs, D.; Picardo, M. Mechanisms underlying post-inflammatory hyperpigmentation: Lessons from solar lentigo. Ann. Dermatol. Venereol. 2012, 139, S148–S152. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The Hunt for Natural Skin Whitening Agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef] [PubMed]
- Mann, T.; Scherner, C.; Röhm, K.-H.; Kolbe, L. Structure-Activity Relationships of Thiazolyl Resorcinols, Potent and Selective Inhibitors of Human Tyrosinase. Int. J. Mol. Sci. 2018, 19, 690. [Google Scholar] [CrossRef] [PubMed]
- Gunia-Krzyżak, A.; Popiół, J.; Marona, H. Melanogenesis inhibitors: Strategies for searching for and evaluation of active compounds. Curr. Med. Chem. 2016, 23, 3548–3574. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, H.R.; Kim, D.S.; Park, K.C. Topical hypopigmenting agents for pigmentary disorders and their mechanisms of action. Ann. Dermatol. 2012, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Namasivayam, V.; Manickam, M.; Jung, S.-H. Inhibitors of Melanogenesis: An Updated Review. J. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms regulating skin pigmentation: The rise and fall of complexion coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zmijewski, M.A.; Pawelek, J. L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 2012, 25, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. Tyrosinase Kinetics: A Semi-quantitative Model of the Mechanism of Oxidation of Monohydric and Dihydric Phenolic Substrates. J. Theor. Biol. 2000, 203, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Chemistry of mixed melanogenesis—Pivotal roles of dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. On the metal cofactor in the tyrosinase family. Int. J. Mol. Sci. 2018, 19, 633. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M. Reactivities of quinone methides versus o-Quinones in catecholamine metabolism and eumelanin biosynthesis. Int. J. Mol. Sci. 2016, 17, 1576. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, M.; Barek, H. Critical analysis of the melanogenic pathway in insects and higher animals. Int. J. Mol. Sci. 2016, 17, 1753. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Hughes, J.; Hong, M.; Jia, Q.; Orndorff, S. Modulation of Melanogenesis by Aloesin: A Competitive Inhibitor of Tyrosinase. Pigment Cell Res. 2002, 15, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. A convenient screening method to differentiate phenolic skin whitening tyrosinase inhibitors from leukoderma-inducing phenols. J. Dermatol. Sci. 2015, 80, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J.; Ekel, T.M.; Montague, P.M.; Nicholson, J.M. Mammalin tyrosinase. Stoichiometry and measurement of reaction products. Biochim. Biophys. Acta 1980, 611, 251–268. [Google Scholar] [CrossRef]
- D’Ischia, M.; Ruiz-Molina, D. Bioinspired Catechol-Based Systems: Chemistry and Applications. Biomimetics 2017, 2, 25. [Google Scholar] [CrossRef]
- Micillo, R.; Pistorio, V.; Pizzo, E.; Panzella, L.; Napolitano, A.; D’Ischia, M. 2-S-Lipoylcaffeic Acid, a Natural Product-Based Entry to Tyrosinase Inhibition via Catechol Manipulation. Biomimetics 2017, 2, 15. [Google Scholar] [CrossRef]
- Bilska, A.; Włodek, L. Biologic properties of lipoic acid. Postep. Hig. Med. Dosw. 2002, 56, 201–219. [Google Scholar]
- Packer, L.; Witt, E.H.; Tritschler, H.J. α-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995, 19, 227–250. [Google Scholar] [CrossRef]
- Lin, C.B.; Babiarz, L.; Liebel, F.; Kizoulis, M.; Gendimenico, G.J.; Seiberg, M.; Roydon Price, E.; Fisher, D.E. Modulation of Microphthalmia-associated Transcription Factor Gene Expression Alters Skin Pigmentation. J. Investig. Dermatol. 2002, 119, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Cheli, Y.; Ohanna, M.; Ballotti, R.; Bertolotto, C. Fifteen-year quest for microphthalmia-associated transcription factor target genes. Pigment Cell Melanoma Res. 2010, 23, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Tsuji-Naito, K.; Hatani, T.; Okada, T.; Tehara, T. Evidence for covalent lipoyl adduction with dopaquinone following tyrosinase-catalyzed oxidation. Biochem. Biophys. Res. Commun. 2006, 343, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Joo, Y.H.; Baek, H.S.; Park, M.; Kim, J.H.; Shin, H.J.; Park, N.H.; Lee, J.H.; Park, Y.H.; Shin, S.S.; et al. Different effects of five depigmentary compounds, rhododendrol, raspberry ketone, monobenzone, rucinol and AP736 on melanogenesis and viability of human epidermal melanocytes. Exp. Dermatol. 2016, 25, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hinoshita, M.; Suzuki, E.; Ojika, M.; Wakamatsu, K. Tyrosinase-Catalyzed Oxidation of the Leukoderma-Inducing Agent Raspberry Ketone Produces (E)-4-(3-Oxo-1-butenyl)-1,2-benzoquinone: Implications for Melanocyte Toxicity. Chem. Res. Toxicol. 2017, 30, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Yamashita, T.; Ishii-Osai, Y.; Yoshikawa, M.; Sumikawa, Y.; Wakamatsu, K.; Ito, S. Effects of rhododendrol and its metabolic products on melanocytic cell growth. J. Dermatol. Sci. 2015, 80, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ojika, M.; Yamashita, T.; Wakamatsu, K. Tyrosinase-catalyzed oxidation of rhododendrol produces 2-methylchromane-6,7-dione, the putative ultimate toxic metabolite: Implications for melanocyte toxicity. Pigment Cell Melanoma Res. 2014, 27, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Kondo, M.; Sato, K.; Umeda, M.; Kawabata, K.; Takahashi, Y.; Suzuki, T.; Matsunaga, K.; Inoue, S. Rhododendrol, a depigmentation-inducing phenolic compound, exerts melanocyte cytotoxicity via a tyrosinase-dependent mechanism. Pigment Cell Melanoma Res. 2014, 27, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Muñoz, J.L.; Berna, J.; Garcia-Molina, F.; Garcia-Ruiz, P.A.; Tudela, J.; Rodriguez-Lopez, J.N.; Garcia-Canovas, F. Unravelling the suicide inactivation of tyrosinase: A discrimination between mechanisms. J. Mol. Catal. B Enzym. 2012, 75, 11–19. [Google Scholar] [CrossRef]
- Kwak, S.-Y.Y.; Lee, S.; Choi, H.-R.R.; Park, K.-C.C.; Lee, Y.-S.S. Dual effects of caffeoyl-amino acidyl-hydroxamic acid as an antioxidant and depigmenting agent. Bioorgan. Med. Chem. Lett. 2011, 21, 5155–5158. [Google Scholar] [CrossRef] [PubMed]
- Schurink, M.; van Berkel, W.J.H.; Wichers, H.J.; Boeriu, C.G. Novel peptides with tyrosinase inhibitory activity. Peptides 2007, 28, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Ohnishi, K.; Komiya, T.; Imai, K. Synthetic Search for Cosmetic Ingredients: Preparations, Tyrosinase Inhibitory and Antioxidant Activities of Caffeic Amides. J. Oleo Sci. 2002, 51, 19–27. [Google Scholar] [CrossRef]
- Olivares, C.; García-Borrón, J.C.; Solano, F. Identification of active site residues involved in metal cofactor binding and stereospecific substrate recognition in mammalian tyrosinase. Implications to the catalytic cycle. Biochemistry 2002, 41, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A.; Cooksey, C.J.; Johnson, C.I.; Land, E.J.; Latter, A.M.; Ramsden, C.A. Melanogenesis-targeted anti-melanoma pro-drug development: Effect of side-chain variations on the cytotoxicity of tyrosinase-generated ortho-quinones in a model screening system. Eur. J. Cancer 1997, 33, 135–143. [Google Scholar] [CrossRef]
- Cooksey, C.J.; Land, E.J.; Ramsden, C.A.; Riley, P.A. Tyrosinase-mediated cytotoxicity of 4-substituted phenols: Prediction of thiol reactivity of the derived o-quinones60. Melanoma Res. 1995, 5, 35. [Google Scholar] [CrossRef]
- Winder, A.J.; Harris, H. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. Eur. J. Biochem. 1991, 198, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, C.; Garcia-Borron, J.C.; Jiménez-Cervantes, C.; Olivares, C. MC1R signaling. Intracellular partners and pathophysiological implications. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2448–2461. [Google Scholar] [CrossRef] [PubMed]
- Flori, E.; Rosati, E.; Cardinali, G.; Kovacs, D.; Bellei, B.; Picardo, M.; Maresca, V. The α-melanocyte stimulating hormone/peroxisome proliferator activated receptor-γ pathway down-regulates proliferation in melanoma cell lines. J. Exp. Clin. Cancer Res. 2017, 36, 1–13. [Google Scholar] [CrossRef] [PubMed]
- García-Borrón, J.C.; Abdel-Malek, Z.; Jimenez-Cervantes, C.; García-Borrón, J.C.; Abdel-Malek, Z.; Jiménez-Cervantes, C. MC1R, the cAMP pathway, and the response to solar UV: Extending the horizon beyond pigmentation. Pigment Cell Melanoma Res. 2014, 27, 699–720. [Google Scholar] [CrossRef] [PubMed]
- Abrisqueta, M.; Herraiz, C.; Perez Oliva, A.B.; Sanchez-Laorden, B.L.; Olivares, C.; Jimenez-Cervantes, C.; Garcia-Borron, J.C. Differential and competitive regulation of human melanocortin 1 receptor signaling by -arrestin isoforms. J. Cell Sci. 2013, 126, 3724–3737. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, C.; Journé, F.; Abdel-Malek, Z.; Ghanem, G.; Jiménez-Cervantes, C.; García-Borrón, J.C. Signaling from the human melanocortin 1 receptor to ERK1 and ERK2 mitogen-activated protein kinases involves transactivation of cKIT. Mol. Endocrinol. 2011, 25, 138–156. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Oliva, A.B.; Olivares, C.; Jiménez-Cervantes, C.; García-Borrón, J.C. Mahogunin Ring Finger-1 (MGRN1) E3 Ubiquitin Ligase Inhibits Signaling from Melanocortin Receptor by Competition with Gαs. J. Biol. Chem. 2009, 284, 31714–31725. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.; Solano, F.; García-Borrón, J.C. Conformation-dependent post-translational glycosylation of tyrosinase: Requirement of a specific interaction involving the CuB metal binding site. J. Biol. Chem. 2003, 278, 15735–15743. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M.; Santagostino, M.; Sputore, S. A user-friendly entry to 2-iodoxybenzoic acid (IBX). J. Org. Chem. 1999, 64, 4537–4538. [Google Scholar] [CrossRef]
- Gunsalus, I.C.; Barton, L.S.; Gruber, W. Biosynthesis and Structure of Lipoic Acid Derivatives. J. Am. Chem. Soc. 1956, 78, 1763–1766. [Google Scholar] [CrossRef]
- Jiménez-Cervantes, C.; Solano, F.; Kobayashi, T.; Urabe, K.; Hearing, V.J.; Lozano, J.A.; García-Borrón, J.C. A new enzymatic function in the melanogenic pathway: The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J. Biol. Chem. 1994, 269, 17993–18000. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
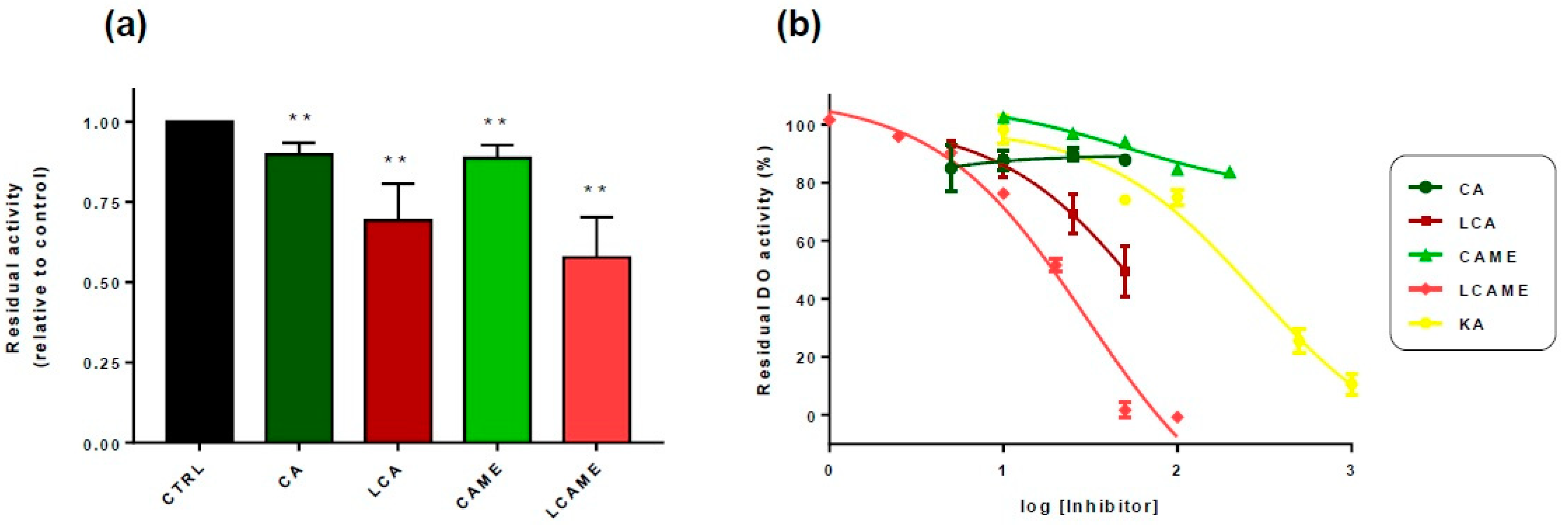
| Compound | IC50 (DO Activity) | IC50 (TH Activity) |
|---|---|---|
| CAME | 155 ± 7 μM | 79 ± 7 μM |
| LCAME | 0.05 + 0.01 μM | 0.83 ± 0.09 μM |
| LCA [25] | 3.22 ± 0.02 μM | 2.0 ± 0.1 μM |
| Compound | IC50 (Mean ± SEM, μM) |
|---|---|
| CA | n.d. |
| LCA | 76.2 ± 6.0 |
| CAME | n.d. |
| LCAME | 30.1 ± 1.5 |
| Kojic acid | 282.2 ± 1.8 |
| [LCAME] (μM) | Vmax (∆A490/min mg) | Km (mM) |
|---|---|---|
| 0 | 0.19 ± 0.01 | 1.12 ± 0.06 |
| 10 | 0.18 ± 0.02 | 1.43 ± 0.30 |
| 25 | 0.19 ± 0.03 | 1.95 ± 0.50 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micillo, R.; Sirés-Campos, J.; García-Borrón, J.C.; Panzella, L.; Napolitano, A.; Olivares, C. Conjugation with Dihydrolipoic Acid Imparts Caffeic Acid Ester Potent Inhibitory Effect on Dopa Oxidase Activity of Human Tyrosinase. Int. J. Mol. Sci. 2018, 19, 2156. https://doi.org/10.3390/ijms19082156
Micillo R, Sirés-Campos J, García-Borrón JC, Panzella L, Napolitano A, Olivares C. Conjugation with Dihydrolipoic Acid Imparts Caffeic Acid Ester Potent Inhibitory Effect on Dopa Oxidase Activity of Human Tyrosinase. International Journal of Molecular Sciences. 2018; 19(8):2156. https://doi.org/10.3390/ijms19082156
Chicago/Turabian StyleMicillo, Raffaella, Julia Sirés-Campos, José Carlos García-Borrón, Lucia Panzella, Alessandra Napolitano, and Conchi Olivares. 2018. "Conjugation with Dihydrolipoic Acid Imparts Caffeic Acid Ester Potent Inhibitory Effect on Dopa Oxidase Activity of Human Tyrosinase" International Journal of Molecular Sciences 19, no. 8: 2156. https://doi.org/10.3390/ijms19082156
APA StyleMicillo, R., Sirés-Campos, J., García-Borrón, J. C., Panzella, L., Napolitano, A., & Olivares, C. (2018). Conjugation with Dihydrolipoic Acid Imparts Caffeic Acid Ester Potent Inhibitory Effect on Dopa Oxidase Activity of Human Tyrosinase. International Journal of Molecular Sciences, 19(8), 2156. https://doi.org/10.3390/ijms19082156