The Air Sac Primordium of Drosophila: A Model for Invasive Development
Abstract
1. Introduction
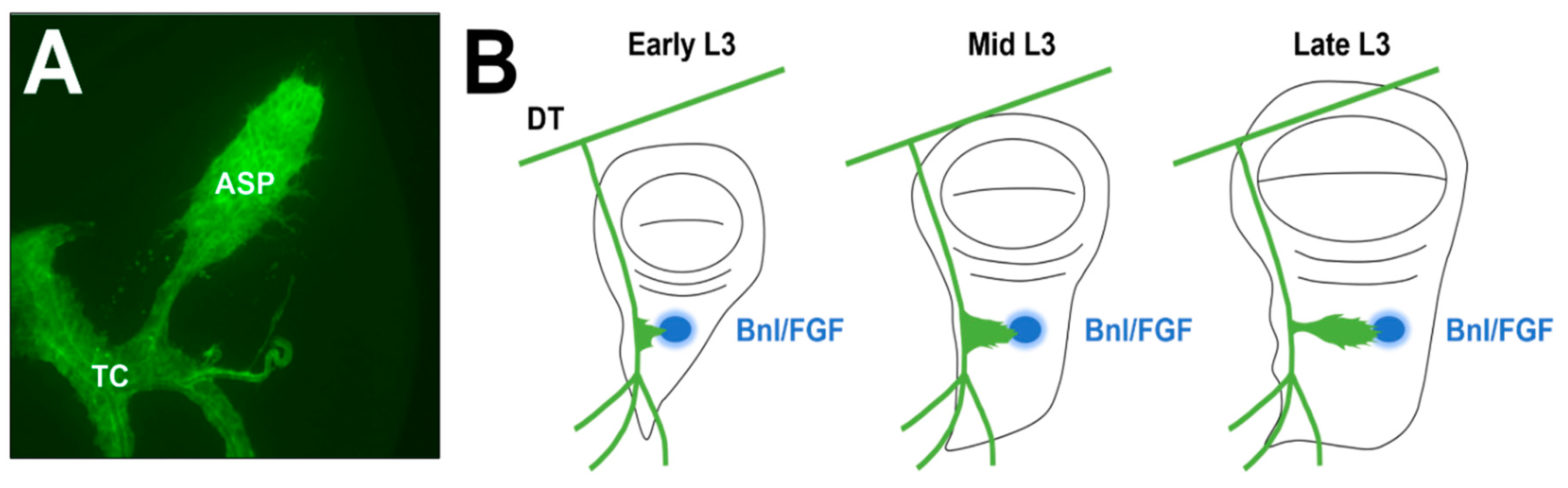
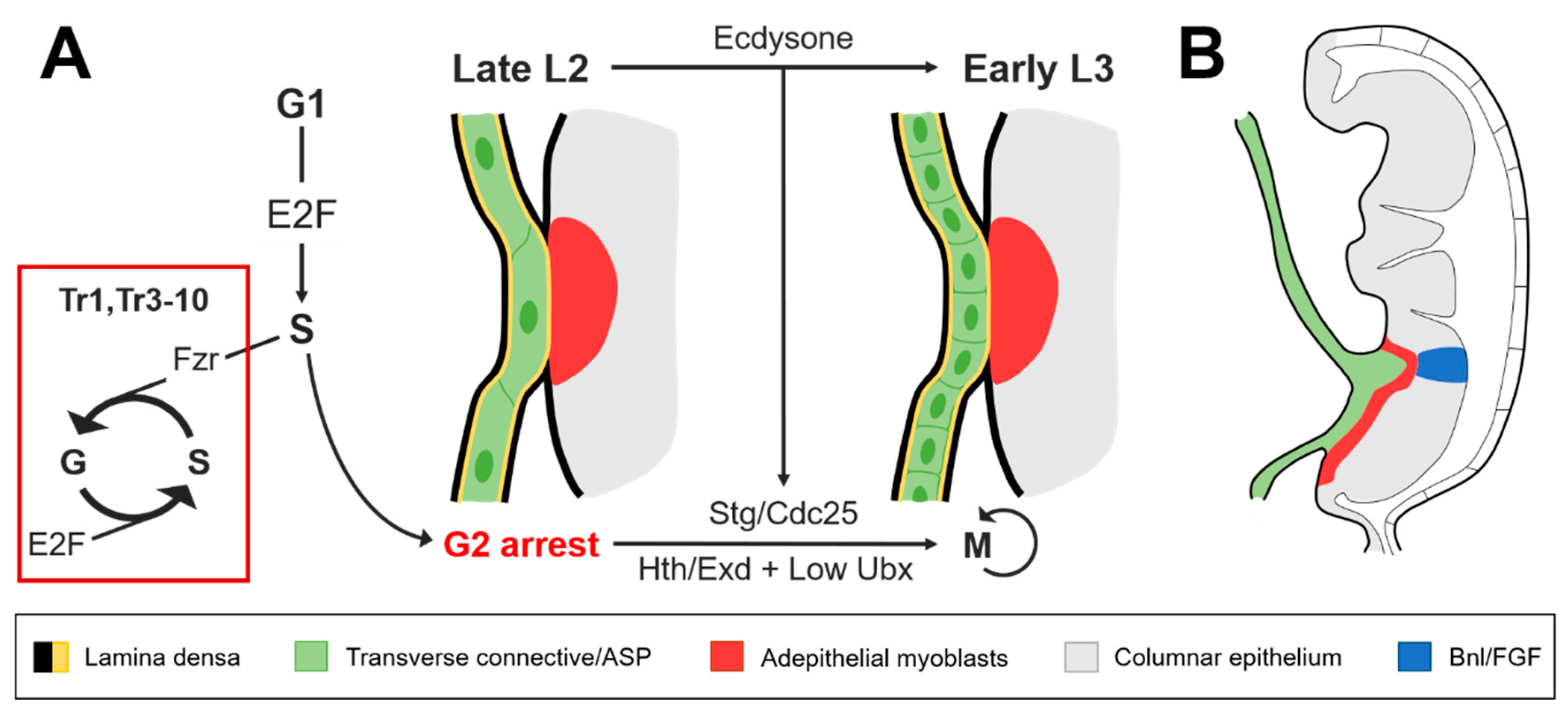
2. ASP Function and Development
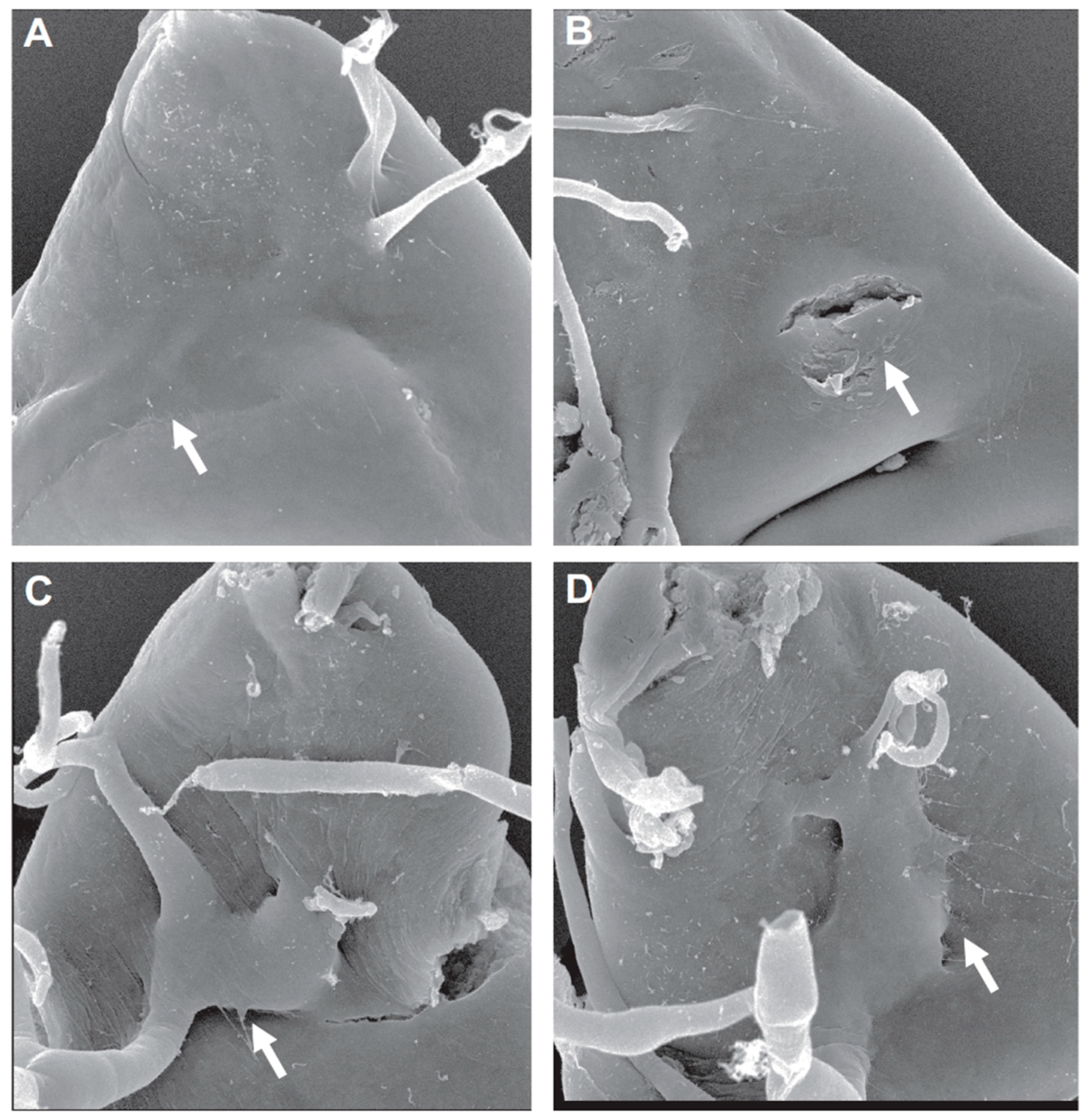
3. ECM Remodeling and ASP Invasion
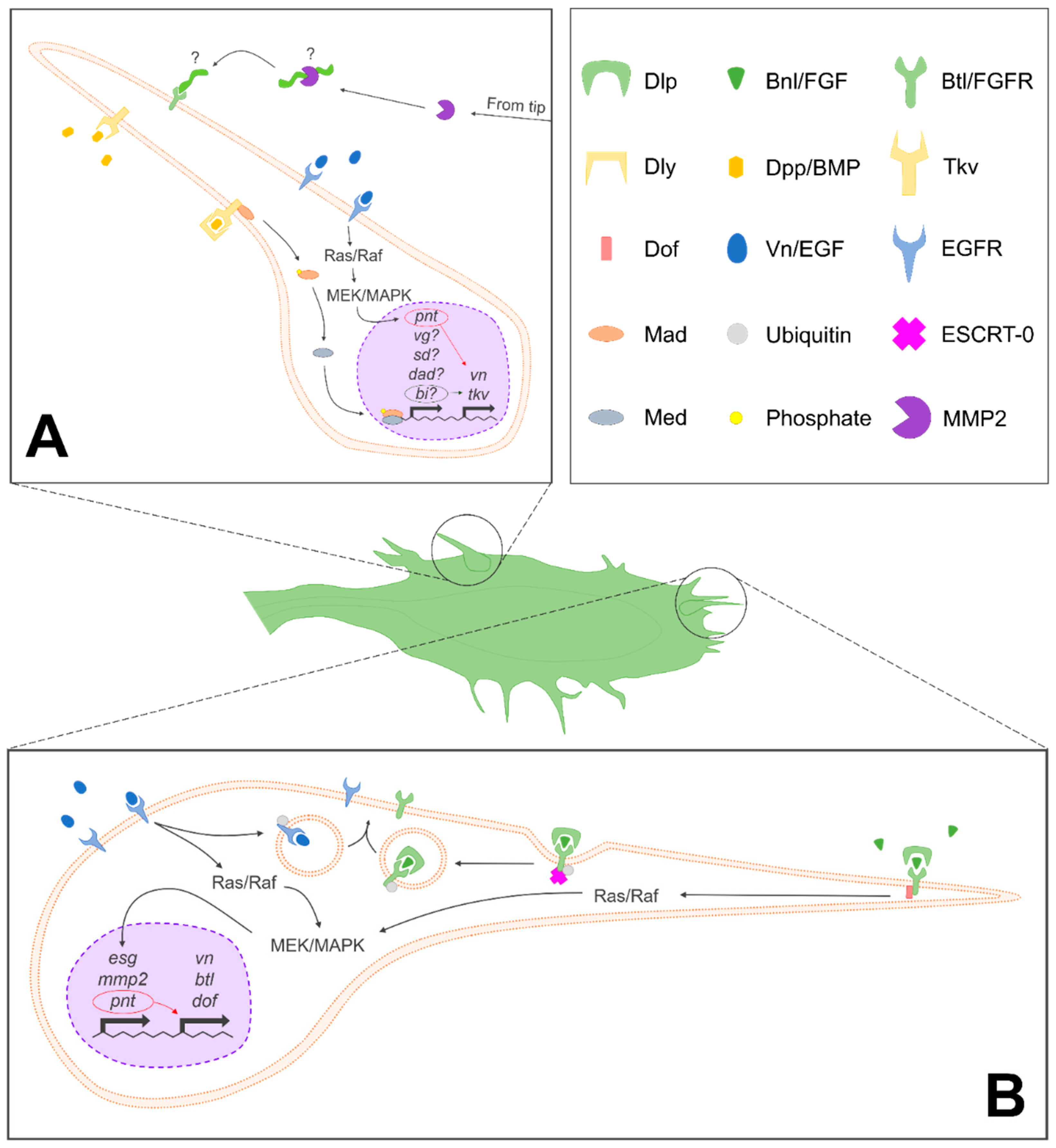
4. Additional Regulators of ASP Development
5. Conclusions and Future Directions
Funding
Conflicts of Interest
References
- Terry, S.; Buart, S.; Chouaib, S. Hypoxic Stress-Induced Tumor and Immune Plasticity, Suppression, and Impact on Tumor Heterogeneity. Front. Immunol. 2017, 8, 1625. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Pastor-Pareja, J.C.; Igaki, T.; Pagliarini, R.; Xu, T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc. Natl. Acad. Sci. USA 2007, 104, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Beccari, S.; Teixeira, L.; Rørth, P. The JAK/STAT pathway is required for border cell migration during Drosophila oogenesis. Mech. Dev. 2002, 111, 115–123. [Google Scholar] [CrossRef]
- Khanna, P.; Chua, P.J.; Bay, B.H.; Baeg, G.H. The JAK/STAT signaling cascade in gastric carcinoma (Review). Int. J. Oncol. 2015, 47, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, S.N.; Girardot, M.; Pecquet, C. Mining for JAK-STAT mutations in cancer. Trends Biochem. Sci. 2008, 33, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Amoyel, M.; Anderson, A.M.; Bach, E.A. JAK/STAT pathway dysregulation in tumors: A Drosophila perspective. Semin. Cell Dev. Biol. 2014, 28, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Siddik, Z.H. Platelet-derived growth factor (PDGF) signalling in cancer: Rapidly emerging signalling landscape. Cell Biochem. Funct. 2015, 33, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Alevizakos, M.; Kaltsas, S.; Syrigos, K.N. The VEGF pathway in lung cancer. Cancer Chemother. Pharmacol. 2013, 72, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Costache, M.I.; Ioana, M.; Iordache, S.; Ene, D.; Costache, C.A.; Săftoiu, A. VEGF expression in pancreatic cancer and other malignancies: A review of the literature. Rom. J. Intern. Med. 2015, 53, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Aman, A.; Piotrowski, T. Cell migration during morphogenesis. Dev. Biol. 2010, 341, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Poukkula, M.; Cliffe, A.; Changede, R.; Rørth, P. Cell behaviors regulated by guidance cues in collective migration of border cells. J. Cell Biol. 2011, 192, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Tarin, D. Comparisons of metastases in different organs: Biological and clinical implications. Clin. Cancer Res. 2008, 14, 1923–1925. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Rudrapatna, V.A.; Cagan, R.L.; Das, T.K. Drosophila cancer models. Dev. Dyn. 2012, 241, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H. Transition from non-motile behaviour to directed migration during early PGC development in zebrafish. J. Cell Sci. 2005, 118, 4027–4038. [Google Scholar] [CrossRef] [PubMed]
- De Graeve, F.M.; Van de Bor, V.; Ghiglione, C.; Cerezo, D.; Jouandin, P.; Ueda, R.; Shashidhara, L.S.; Noselli, S. Drosophila apc regulates delamination of invasive epithelial clusters. Dev. Biol. 2012, 368, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Kulesa, P.M.; Morrison, J.A.; Bailey, C.M. The neural crest and cancer: A developmental spin on melanoma. Cells Tissues Organs 2013, 198, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, V.E.; Varshney, G.K.; Lee, M.; Bupp, S.; Xu, L.; Shinn, P.; Crawford, N.P.; Inglese, J.; Burgess, S.M. Phenotype-driven chemical screening in zebrafish for compounds that inhibit collective cell migration identifies multiple pathways potentially involved in metastatic invasion. Dis. Model. Mech. 2015, 8, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kornberg, T.B. FGF is an essential mitogen and chemoattractant for the air sacs of the Drosophila tracheal system. Dev. Cell 2002, 3, 195–207. [Google Scholar] [CrossRef]
- Cabernard, C.; Affolter, M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev. Cell 2005, 9, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kitada, Y.; Tabata, T. Larval cells become imaginal cells under the control of homothorax prior to metamorphosis in the Drosophila tracheal system. Dev. Biol. 2008, 318, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Brenneman, B.; Fields, C.; Srivastava, A. A Cathepsin-L is required for invasive behavior during Air Sac Primordium development in Drosophila melanogaster. FEBS Lett. 2015, 589, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.; Lin, L.; Kornberg, T.B. Regulation of Drosophila matrix metalloprotease Mmp2 is essential for wing imaginal disc:trachea association and air sac tubulogenesis. Dev. Biol. 2009, 335, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Uhlirova, M.; Bohmann, D. Spatial Restriction of FGF Signaling by a Matrix Metalloprotease Controls Branching Morphogenesis. Dev. Cell 2010, 18, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Švácha, P. What are and what are not imaginal discs: Reevaluation of some basic concepts (insecta, holometabola). Dev. Biol. 1992, 154, 101–117. [Google Scholar] [CrossRef]
- Tanaka, K.; Truman, J.W. Development of the adult leg epidermis in Manduca sexta: Contribution of different larval cell populations. Dev. Genes Evol. 2005, 215, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.; Kornberg, T.B. Tracheal branch repopulation precedes induction of the Drosophila dorsal air sac primordium. Dev. Biol. 2005, 287, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Djabrayan, N.J.V.; Cruz, J.; de Miguel, C.; Franch-Marro, X.; Casanova, J. Specification of differentiated adult progenitors via inhibition of endocycle entry in the Drosophila trachea. Cell Rep. 2014, 9, 859–865. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.E.; Michael, A.; Pandha, H.; Morgan, R. Engrailed homeobox transcription factors as potential markers and targets in cancer. FEBS Lett. 2013, 587, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.K.; Park, J.J.; Chun, H.J. Impact of homeobox genes in gastrointestinal cancer. World J. Gastroenterol. 2016, 22, 8247–8256. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Maqbool, S.B.; Kolpakas, A.; Murnen, K.; Calvi, B.R. Endocycling cells do not apoptose in response to DNA rereplication genotoxic stress. Genes Dev. 2008, 22, 3158–3171. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Fisk, B.C.; Wadehra, M.; Su, H.; Braun, J. Overexpression of murine fizzy-related (fzr) increases natural killer cell-mediated cell death and suppresses tumor growth. Blood 2000, 96, 259–263. [Google Scholar] [PubMed]
- Crawford, L.J.; Anderson, G.; Johnston, C.K.; Irvine, A.E.; Crawford, L.J.; Anderson, G.; Johnston, C.K.; Irvine, A.E. Identification of the APC/C co-factor FZR1 as a novel therapeutic target for multiple myeloma. Oncotarget 2016, 7, 70481–70493. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, J.; Sugihara, E.; Kuninaka, S.; Mogushi, K.; Kojima, K.; Benton, C.B.; Zhao, R.; Chachad, D.; Hashimoto, N.; Jacamo, R.O.; et al. FZR1 loss increases sensitivity to DNA damage and consequently promotes murine and human B-cell acute leukemia. Blood 2017, 129, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Huang, H.; Liu, S.; Kornberg, T.B. Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein. Science 2014, 343, 1244624. [Google Scholar] [CrossRef] [PubMed]
- Bower, D.V.; Lansdale, N.; Navarro, S.; Truong, T.V.; Bower, D.J.; Featherstone, N.C.; Connell, M.G.; Al Alam, D.; Frey, M.R.; Trinh, L.A.; et al. SERCA directs cell migration and branching across species and germ layers. Biol. Open 2017, 6, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Papp, B.; Brouland, J.-P.; Arbabian, A.; Gélébart, P.; Kovács, T.; Bobe, R.; Enouf, J.; Varin-Blank, N.; Apáti, Á. Endoplasmic Reticulum Calcium Pumps and Cancer Cell Differentiation. Biomolecules 2012, 2, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Arbabian, A.; Brouland, J.P.; Gélébart, P.; Kovàcs, T.; Bobe, R.; Enouf, J.; Papp, B. Endoplasmic reticulum calcium pumps and cancer. BioFactors 2011, 37, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.D.; Cagan, R.L. Drosophila Lung Cancer Models Identify Trametinib plus Statin as Candidate Therapeutic. Cell Rep. 2016, 14, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Thomssen, C.; Schmitt, M.; Goretzki, L.; Oppelt, P.; Pache, L.; Dettmar, P.; Jänicke, F.; Graeff, H. Prognostic value of the cysteine proteases cathepsins B and cathepsin L in human breast cancer. Clin. Cancer Res. 1995, 1, 741–746. [Google Scholar] [PubMed]
- Sivula, A.; Talvensaari-Mattila, A.; Lundin, J.; Joensuu, H.; Haglund, C.; Ristimäki, A.; Turpeenniemi-Hujanen, T. Association of cyclooxygenase-2 and matrix metalloproteinase-2 expression in human breast cancer. Breast Cancer Res. Treat. 2005, 89, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.A.; Xiao, H.; Ji, H.X.; Shen, W.H.; Zhou, Z.S.; Song, B.; Chen, Z.W.; Li, W.B. Cathepsin L is associated with proliferation and clinical outcome of urothelial carcinoma of the bladder. J. Int. Med. Res. 2010, 38, 1913–1922. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liang, H.; Li, T.; Li, M.; Li, R.; Qin, X.; Li, S. The MMP-1, MMP-2, and MMP-9 gene polymorphisms and susceptibility to bladder cancer: A meta-analysis. Tumor Biol. 2014, 35, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Xu, S.; Xu, Y.; Ma, J.; Li, J.; Xu, P. The expression of tumor-derived and stromal-derived matrix metalloproteinase 2 predicted prognosis of ovarian cancer. Int. J. Gynecol. Cancer 2015, 25, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Shi, C.; Yan, Z.; Wu, M. Overexpression of Cathepsin L is associated with chemoresistance and invasion of epithelial ovarian cancer. Oncotarget 2016, 7, 45995–46001. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Kaur, P.; Sheehan, C.E.; Fisher, H.A.G.; Kaufman, R.A.; Kallakury, B.V.S. Prognostic significance of matrix metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 expression in prostate cancer. Mod. Pathol. 2003, 16, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sudhan, D.R.; Siemann, D.W. Cathepsin L inhibition by the small molecule KGP94 suppresses tumor microenvironment enhanced metastasis associated cell functions of prostate and breast cancer cells. Clin. Exp. Metastasis 2013, 30, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Takenaka, K.; Yanagihara, K. Matrix Metalloproteinase-2 Status in Stromal Fibroblasts, Not in Tumor Cells, Is a Significant Prognostic Factor in Non-Small-Cell Lung Cancer. Clin. Cancer Res. 2004, 10, 6579–6585. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Wang, W.; Wu, D.; He, X.; Wu, J.; Wang, M. Overexpression of Cathepsin L is associated with gefitinib resistance in non-small cell lung cancer. Clin. Transl. Oncol. 2016, 18, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Das, P.; Datta Gupta, S.; Sahni, P.; Pandey, R.M.; Gupta, S.; Chauhan, S.S.; Saraya, A. Prognostic significance of extracellular matrix degrading enzymes-cathepsin L and matrix metalloproteases-2 [MMP-2] in human pancreatic cancer. Cancer Investig. 2013, 31, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Das, P.; Gupta, S.; Sachdev, V.; Srivasatava, S.; Datta Gupta, S.; Pandey, R.M.; Sahni, P.; Chauhan, S.S.; Saraya, A. Plasma cathepsin L: A prognostic marker for pancreatic cancer. World J. Gastroenterol. 2014, 20, 17532–17540. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.; Bota-Rabassedas, N.; Franch-Marro, X. FGF coordinates air sac development by activation of the EGF ligand Vein through the transcription factor PntP2. Sci. Rep. 2015, 5, 17806. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Danilenko, D.M.; Scully, S.A.; Bolon, B.; Ring, B.D.; Tarpley, J.E.; DeRose, M.; Simonet, W.S. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998, 12, 3156–3161. [Google Scholar] [CrossRef] [PubMed]
- Park, W.Y.; Miranda, B.; Lebeche, D.; Hashimoto, G.; Cardoso, W.V. FGF-10 Is a Chemotactic Factor for Distal Epithelial Buds during Lung Development. Dev. Biol. 1998, 201, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Abler, L.L.; Mansour, S.L.; Sun, X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev. Dyn. 2008, 238, 1999–2013. [Google Scholar] [CrossRef] [PubMed]
- Volckaert, T.; Campbell, A.; Dill, E.; Li, C.; Minoo, P.; De Langhe, S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development 2013, 140, 3731–3742. [Google Scholar] [CrossRef] [PubMed]
- Chanut-Delalande, H.; Jung, A.C.; Baer, M.M.; Lin, L.; Payre, F.; Affolter, M. The Hrs/Stam Complex Acts as a Positive and Negative Regulator of RTK Signaling during Drosophila Development. PLoS ONE 2010, 5, e10245. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Matakatsu, M.; Uemura, T.; Oda, H.; Takeichi, M.; Hayashi, S. Cadherin-mediated cell adhesion and cell motility in Drosophila trachea regulated by the transcription factor Escargot. Development 1996, 122, 3697–3705. [Google Scholar] [PubMed]
- Morishita, K.; Suong, D.N.A.; Yoshida, H.; Yamaguchi, M. The Drosophila DOCK family protein Sponge is required for development of the air sac primordium. Exp. Cell Res. 2017, 354, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Miaczynska, M. Effects of membrane trafficking on signaling by receptor tyrosine kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a009035. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hsiung, F.; Kornberg, T.B. Specificity of Drosophila Cytonemes for Distinct Signaling Pathways. Science 2011, 332, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kornberg, T.B. Cells must express components of the planar cell polarity system and extracellular matrix to support cytonemes. eLife 2016, 5, e18979. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Lin, X. Drosophila glypican Dally-like acts in FGF-receiving cells to modulate FGF signaling during tracheal morphogenesis. Dev. Biol. 2007, 312, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Dejima, K.; Kanai, M.I.; Akiyama, T.; Levings, D.C.; Nakato, H. Novel contact-dependent bone morphogenetic protein (BMP) signaling mediated by heparan sulfate proteoglycans. J. Biol. Chem. 2011, 286, 17103–17111. [Google Scholar] [CrossRef] [PubMed]
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Arjonen, A.; Kaukonen, R.; Mattila, E.; Rouhi, P.; Hognas, G.; Sihto, H.; Miller, B.W.; Morton, J.P.; Bucher, E.; Taimen, P.; et al. Mutant p53-associated myosin-X upregulation promotes breast cancer invasion and metastasis. J. Clin. Investig. 2014, 124, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.A.A.; Hughes, R.E.E.; White, K.J.J.; Wells, C.M.M.; Peckham, M. Specific Myosins Control Actin Organization, Cell Morphology, and Migration in Prostate Cancer Cells. Cell Rep. 2015, 13, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Chen, J.; Zhang, X.; Zhai, Y.; Qing, X.; Xing, W.; Zhang, L.; Malik, Y.S.; Yu, H.; Zhu, X. Elevated expression of myosin X in tumours contributes to breast cancer aggressiveness and metastasis. Br. J. Cancer 2014, 111, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Friedmann-Morvinski, D.; Bushong, E.A.; Ke, E.; Soda, Y.; Marumoto, T.; Singer, O.; Ellisman, M.H.; Verma, I.M. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 2012, 338, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, S.; Fingerle, A.A.; Cammareri, P.; Nebelsiek, T.; Göktuna, S.I.; Ziegler, P.K.; Canli, O.; Heijmans, J.; Huels, D.J.; Moreaux, G.; et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 2013, 152, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Saijo, H.; Hirohashi, Y.; Torigoe, T.; Horibe, R.; Takaya, A.; Murai, A.; Kubo, T.; Kajiwara, T.; Tanaka, T.; Shionoya, Y.; et al. Plasticity of lung cancer stem-like cells is regulated by the transcription factor HOXA5 that is induced by oxidative stress. Oncotarget 2016, 7, 50043–50056. [Google Scholar] [CrossRef] [PubMed]
- Jilkine, A.; Gutenkunst, R.N. Effect of Dedifferentiation on Time to Mutation Acquisition in Stem Cell-Driven Cancers. PLoS Comput. Biol. 2014, 10, e1003481. [Google Scholar] [CrossRef] [PubMed]
- Sher, N.; Von Stetina, J.R.; Bell, G.W.; Matsuura, S.; Ravid, K.; Orr-Weaver, T.L. Fundamental differences in endoreplication in mammals and Drosophila revealed by analysis of endocycling and endomitotic cells. Proc. Natl. Acad. Sci. USA 2013, 110, 9368–9373. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Zhang, D.; Yang, Z.; Wang, S.; Wang, X.; Wu, Z.; Wu, Q.; Zhang, S. The number of polyploid giant cancer cells and epithelial-mesenchymal transition-related proteins are associated with invasion and metastasis in human breast cancer. J. Exp. Clin. Cancer Res. 2015, 34, 158. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sánchez, L.M.; Jimenez, C.; Valverde, A.; Hernandez, V.; Peñarando, J.; Martinez, A.; Lopez-Pedrera, C.; Muñoz-Castañeda, J.R.; De La Haba-Rodríguez, J.R.; Aranda, E.; et al. CoCl2, a mimic of hypoxia, induces formation of polyploid giant cells with stem characteristics in colon cancer. PLoS ONE 2014, 9, e99143. [Google Scholar] [CrossRef] [PubMed]
- Bhatlekar, S.; Fields, J.Z.; Boman, B.M. HOX genes and their role in the development of human cancers. J. Mol. Med. 2014, 92, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.L.; Lippman, S.; El-Naggar, A. Targeting the Mitogen-Activated Protein Kinase RAS-RAF Signaling Pathway in Cancer Therapy. Expert Opin. Ther. Targets 2012, 16, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Huntington, J.T.; Shields, J.M.; Der, C.J.; Wyatt, C.A.; Benbow, U.; Slingluff, C.L.; Brinckerhoff, C.E. Overexpression of collagenase 1 (MMP-1) is mediated by the ERK pathway in invasive melanoma cells. Role of BRAF mutation and fibroblast growth factor signaling. J. Biol. Chem. 2004, 279, 33168–33176. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.R.; Lin, L.; Huang, H.; Guha, A.; Roy, S.; Kornberg, T.B. Developmental compartments in the larval trachea of Drosophila. eLife 2015, 4, e08666. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kornberg, T.B. Myoblast cytonemes mediate Wg signaling from the wing imaginal disc and Delta-Notch signaling to the air sac primordium. eLife 2015, 4, e06114. [Google Scholar] [CrossRef] [PubMed]
- Speicher, S.A.; Thomas, U.; Hinz, U.; Knust, E. The Serrate locus of Drosophila and its role in morphogenesis of the wing imaginal discs: Control of cell proliferation. Development 1994, 120, 535–544. [Google Scholar] [PubMed]
- Zhai, Z.; Ha, N.; Papagiannouli, F.; Hamacher-Brady, A.; Brady, N.; Sorge, S.; Bezdan, D.; Lohmann, I. Antagonistic regulation of apoptosis and differentiation by the cut transcription factor represents a tumor-suppressing mechanism in drosophila. PLoS Genet. 2012, 8, e1002582. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Kühnlein, R.P.; Eulenberg, K.G.; Vincent, S.; Affolter, M.; Schuh, R. The transcription factors KNIRPS and KNIRPS RELATED control cell migration and branch morphogenesis during Drosophila tracheal development. Development 1998, 125, 4959–4968. [Google Scholar] [PubMed]
- Gilkes, D.M.; Bajpai, S.; Chaturvedi, P.; Wirtz, D.; Semenza, G.L. Hypoxia-inducible factor 1 (HIF-1) promotes extracellular matrix remodeling under hypoxic conditions by inducing P4HA1, P4HA2, and PLOD2 expression in fibroblasts. J. Biol. Chem. 2013, 288, 10819–10829. [Google Scholar] [CrossRef] [PubMed]
- Centanin, L.; Dekanty, A.; Romero, N.; Irisarri, M.; Gorr, T.A.; Wappner, P. Cell Autonomy of HIF Effects in Drosophila: Tracheal Cells Sense Hypoxia and Induce Terminal Branch Sprouting. Dev. Cell 2008, 14, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Moreno, V.; Gadea, G.; Ahn, J.; Paterson, H.; Marra, P.; Pinner, S.; Sahai, E.; Marshall, C.J. Rac Activation and Inactivation Control Plasticity of Tumor Cell Movement. Cell 2008, 135, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Harada, K.; Negishi, M.; Katoh, H. Dock4 forms a complex with SH3YL1 and regulates cancer cell migration. Cell. Signal. 2014, 26, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Disanza, A.; Steffen, A.; Hertzog, M.; Frittoli, E.; Rottner, K.; Scita, G. Actin polymerization machinery: The finish line of signaling networks, the starting point of cellular movement. Cell. Mol. Life Sci. 2005, 62, 955–970. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Powers, N.; Srivastava, A. The Air Sac Primordium of Drosophila: A Model for Invasive Development. Int. J. Mol. Sci. 2018, 19, 2074. https://doi.org/10.3390/ijms19072074
Powers N, Srivastava A. The Air Sac Primordium of Drosophila: A Model for Invasive Development. International Journal of Molecular Sciences. 2018; 19(7):2074. https://doi.org/10.3390/ijms19072074
Chicago/Turabian StylePowers, Nathan, and Ajay Srivastava. 2018. "The Air Sac Primordium of Drosophila: A Model for Invasive Development" International Journal of Molecular Sciences 19, no. 7: 2074. https://doi.org/10.3390/ijms19072074
APA StylePowers, N., & Srivastava, A. (2018). The Air Sac Primordium of Drosophila: A Model for Invasive Development. International Journal of Molecular Sciences, 19(7), 2074. https://doi.org/10.3390/ijms19072074



