Comparative Proteomic Profiling between Each of Two Consecutive Developmental Stages of the Solanum Fruit Fly, Bactrocera latifrons (Hendel)
Abstract
1. Introduction
2. Results and Discussion
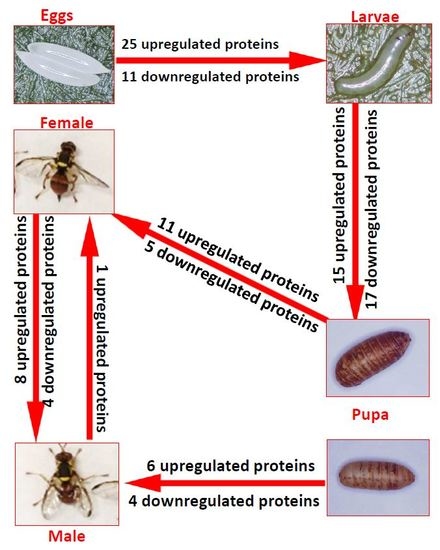
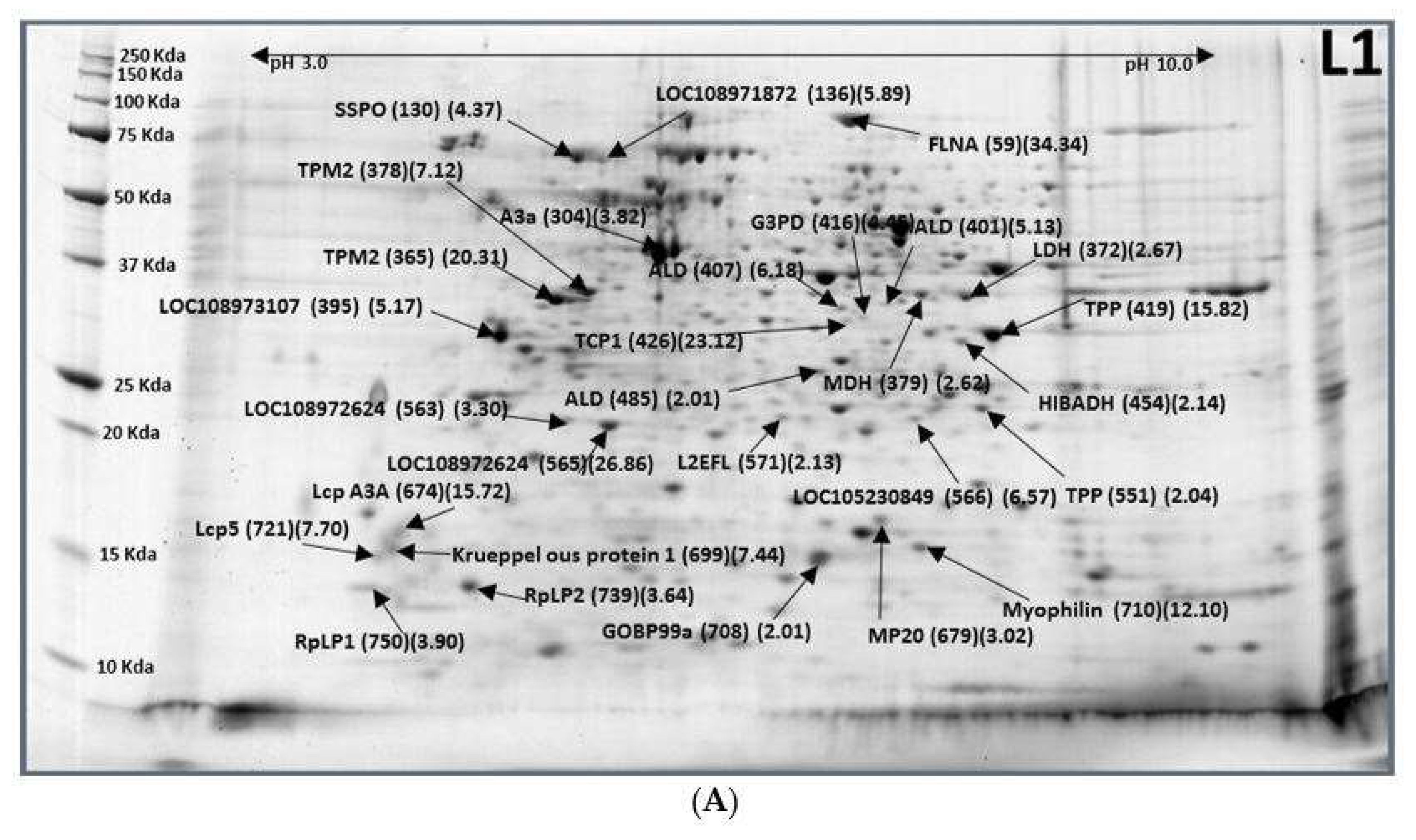
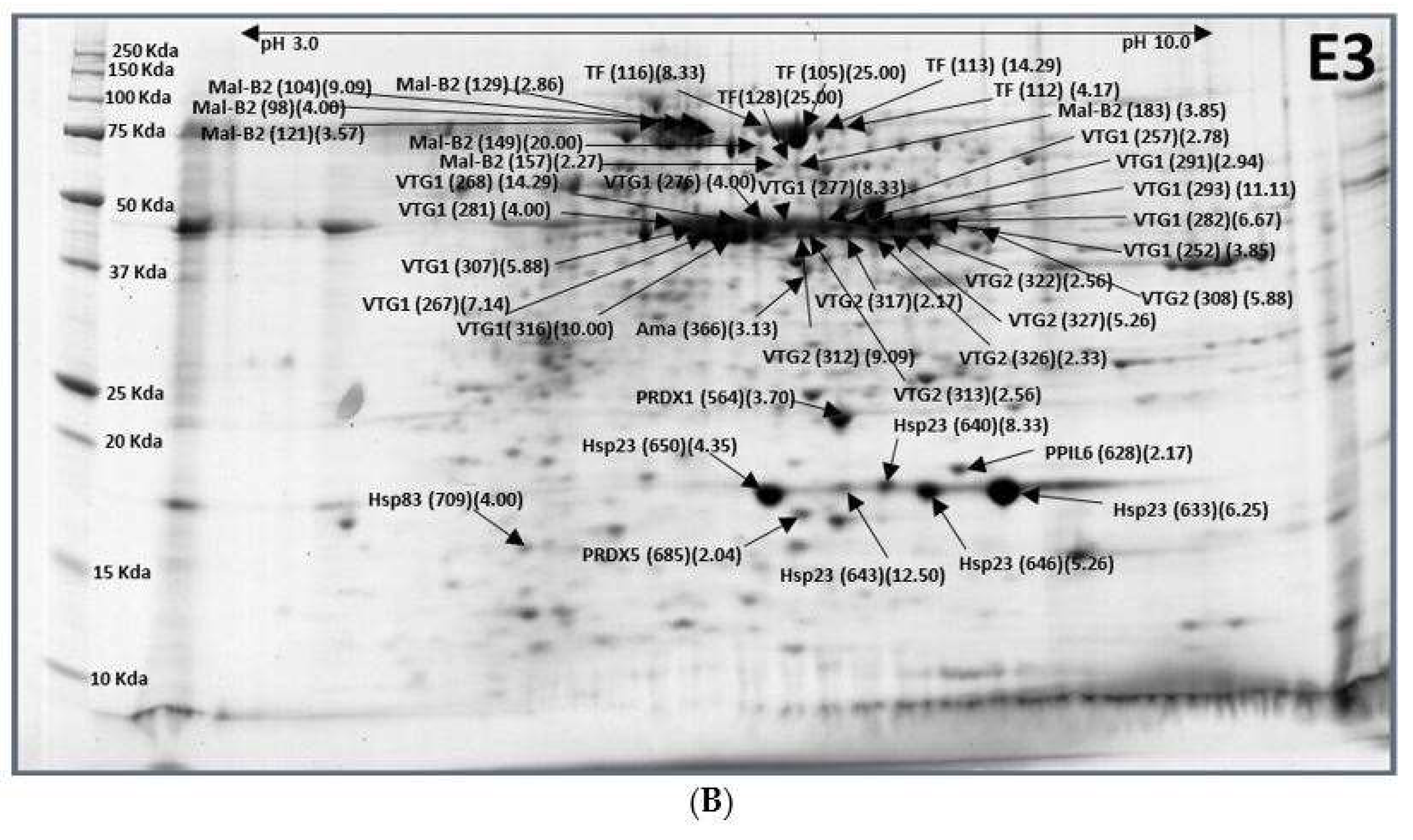
2.1. Eggs (E3)–Larvae (L1)
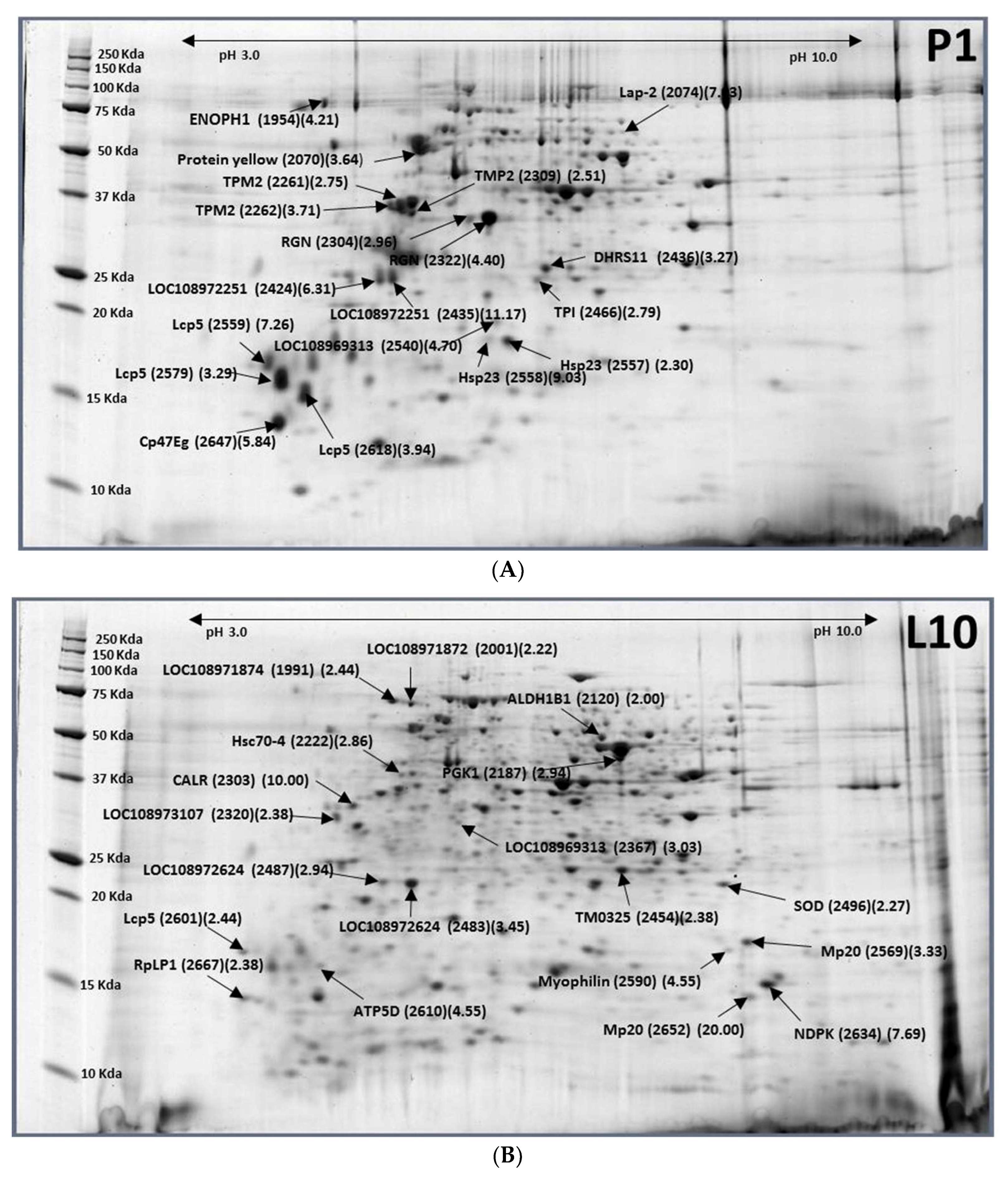
2.2. Larvae (10-Day-Old, L10)–Pupae (1-Day-Old, P1)
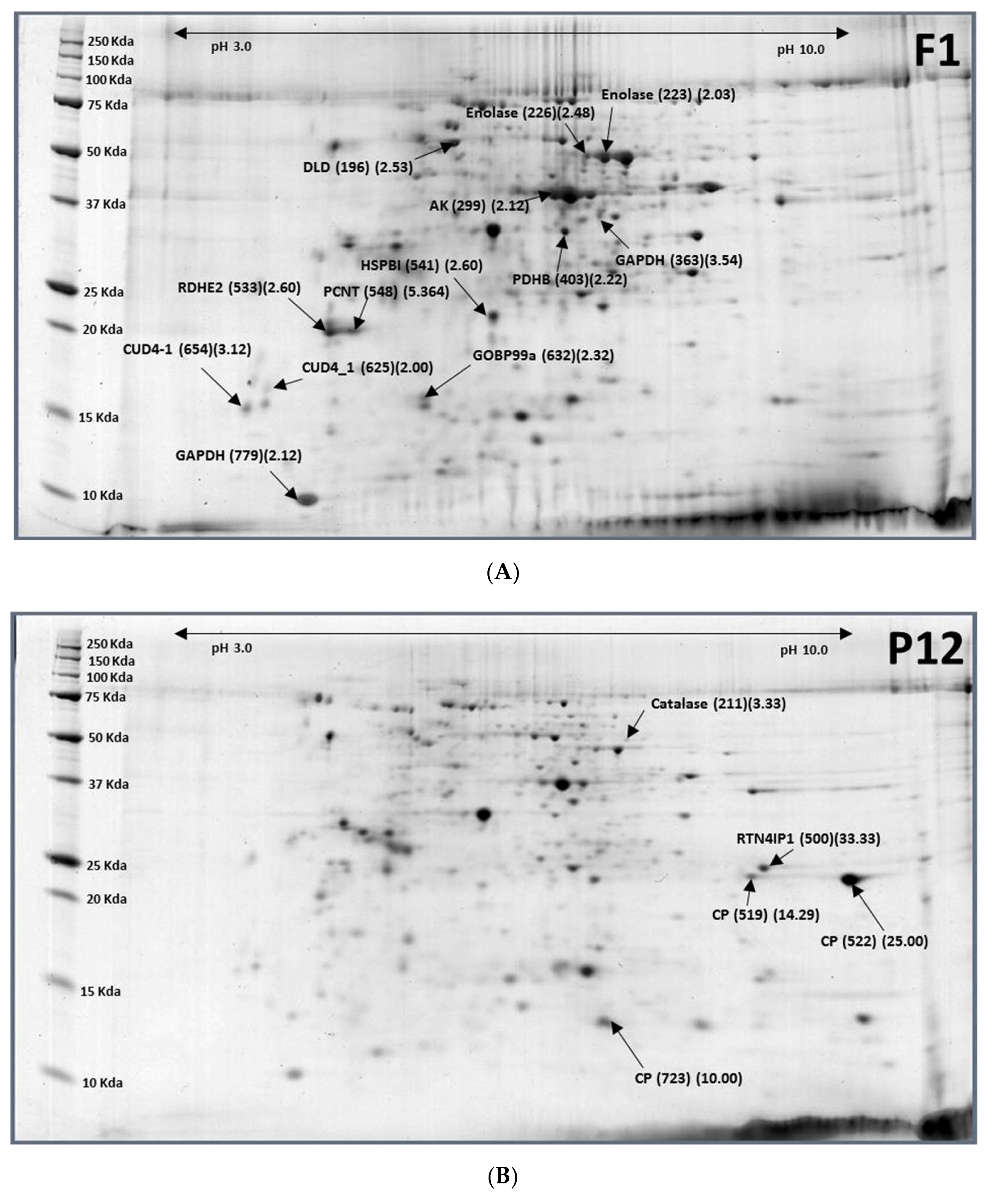
2.3. Pupae (12-Day-Old, P12) to Females (1-Day-Old, F1)
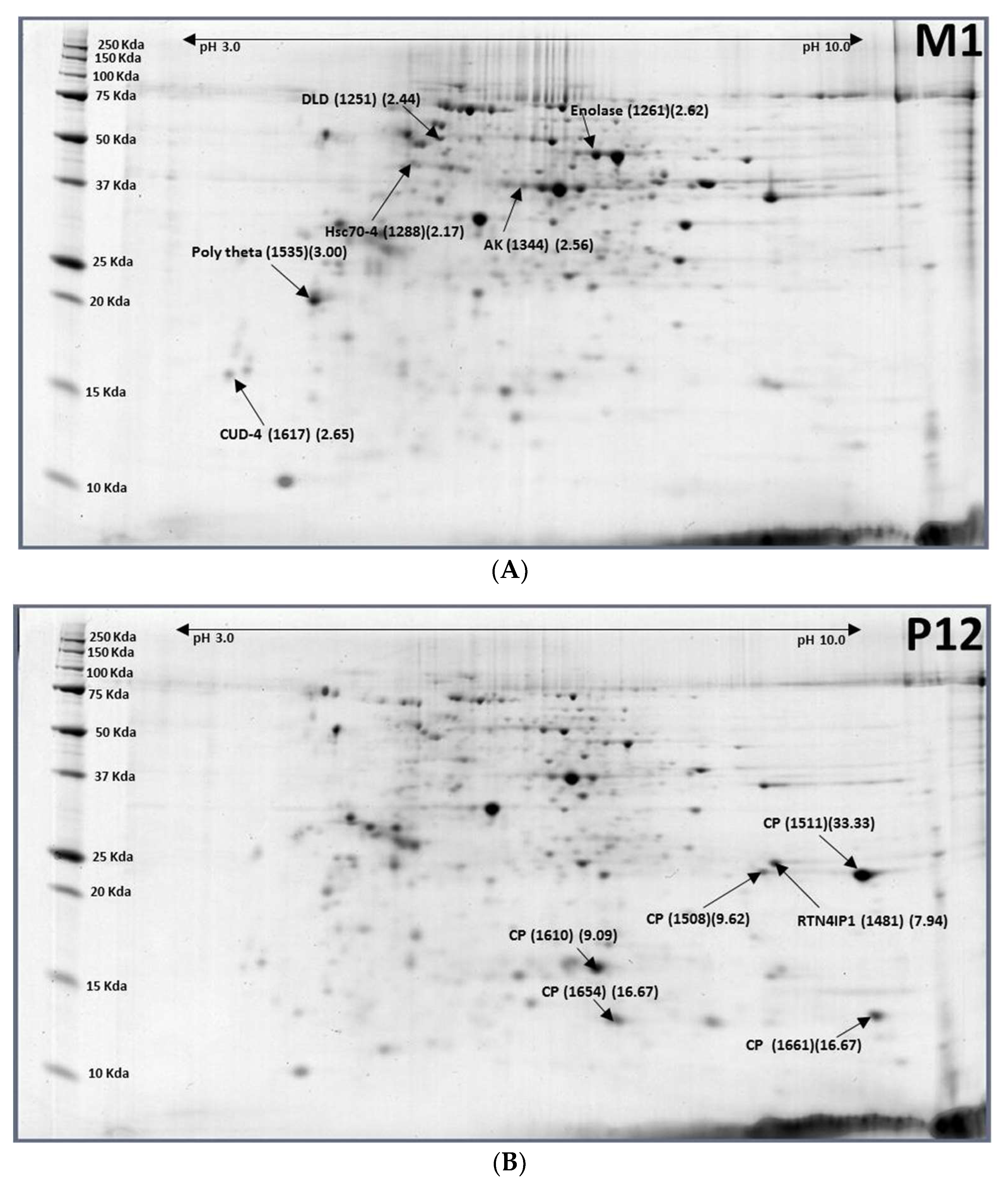
2.4. Pupae (12-Day-Old, P12) to Males (1-Day-Old, M1)
2.5. Females (1-Day-Old)–Males (1-Day-Old)
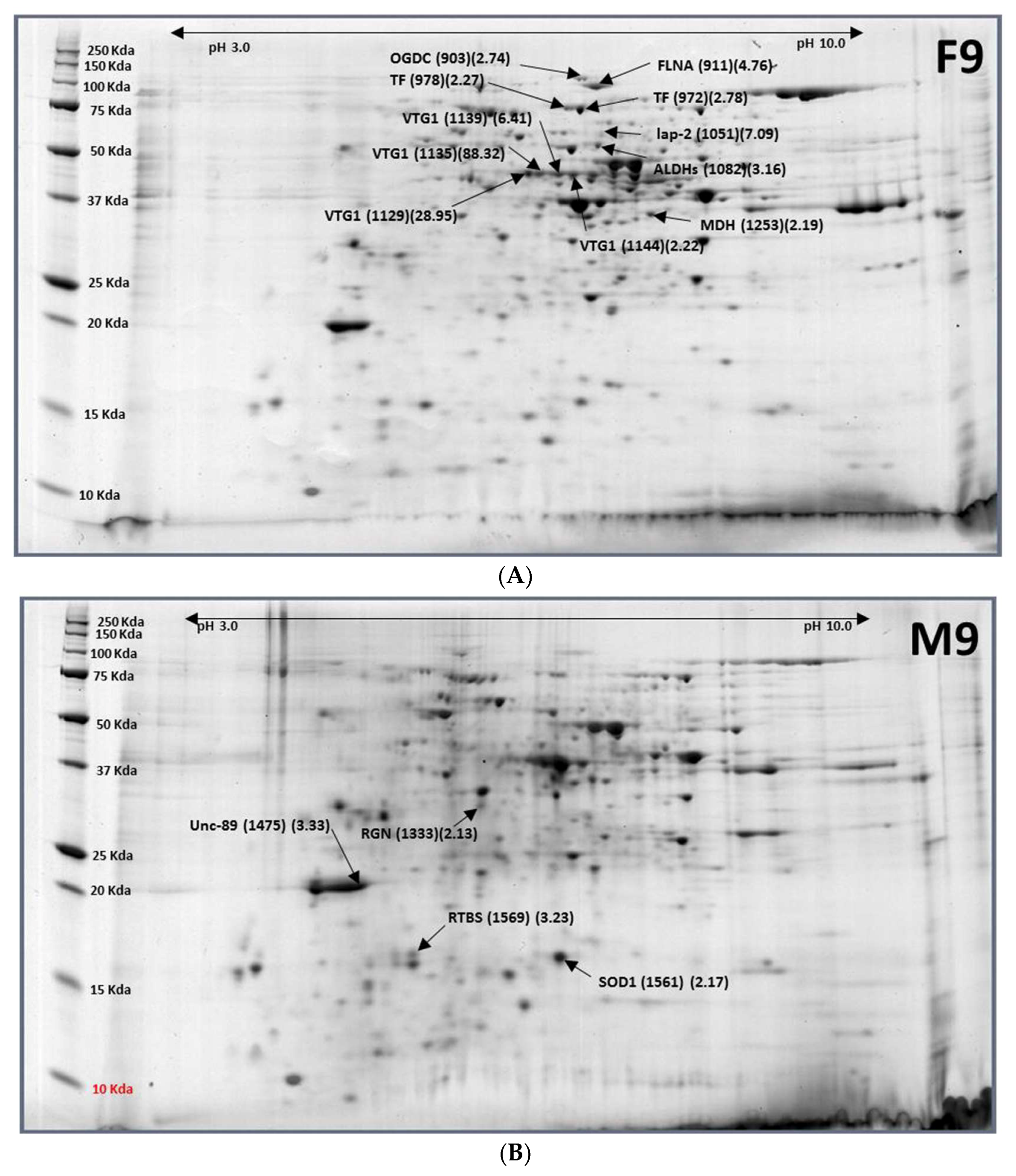
2.6. Females (9-Day-Old)–Males (9-Day-Old)
3. Materials and Methods
3.1. Insects
3.2. Protein Extraction
3.3. Protein Quantification
3.4. 2D-Electrophoresis
3.4.1. IEF (Isoelectric Focusing): Separating Different Proteins Based on pH Value
3.4.2. SDS Gel Electrophoresis Based on Molecular Weight (MW)
3.5. MS/MS Analysis: Identification of Protein Identity
3.6. Data Search and Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shashank, P.R.; Haritha, B. Insect proteomics: Present and future prospective. Curr. Biotica 2014, 7, 336–342. [Google Scholar]
- Vargas, R.I.; Nishida, T. Life history and demographic parameters of Dacus latifrons (Diptera: Tephritidae). J. Econ. Entomol. 1985, 78, 1242–1244. [Google Scholar] [CrossRef]
- Liquido, N.J.; Harris, E.J.; Dekker, L.A. Ecology of Bactrocera latifrons (Diptera:Tephritidade) populations: Host plants, natural enemies, distribution, and abundance. Ann. Entomol. Soc. Am. 1994, 87, 71–84. [Google Scholar] [CrossRef]
- McQuate, G.T.; Liquido, N.J. Annotated world bibliography of host fruits of Bactrocera latifrons (Hendel) (Diptera: Tephritidae). Insecta Mundi 2013, 289, 1–61. [Google Scholar]
- Vargas, R.I.; Walsh, W.A.; Kanehisa, D.; Jang, E.B.; Armstrong, J.W. Demography of four Hawaiian fruit flies (Diptera: Tephritidae) reared at five constant temperatures. Ann. Entomol. Soc. Am. 1997, 90, 162–168. [Google Scholar] [CrossRef]
- Seeger, M.A.; Haffley, L.; Kaufman, T.C. Characterization of amalgam: A member of the immunoglobulin superfamily from Drosophila. Cell 1988, 55, 589–600. [Google Scholar] [CrossRef]
- Zeev-Ben-Mordehai, T.; Mylonas, E.; Paz, A.; Peleg, Y.; Toker, L.; Silman, I.; Svergun, D.I.; Sussman, J.L. The quaternary structure of amalgam, a Drosophila neuronal adhesion protein, explains its dual adhesion properties. Biophys. J. 2009, 97, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, B.; Dacklin, I.; Grabbe, C. Urm1: An essential regulator of JNK signaling and oxidative stress in Drosophila melanogaster. Cell. Mol. Life Sci. 2016, 73, 1939–1954. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.A.; Mintzas, A.C. cDNA cloning, heat shock regulation and developmental expression of the hsp83 gene in the Mediterranean fruit fly Ceratitis capitata. Insect Mol. Biol. 2006, 15, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Rourke, I.J.; East, P.D. Evidence for gene conversion between tandemly duplicated cytoplasmic actin genes of Helicoverpa armigera (Lepidoptera:Noctuidae). J. Mol. Evol. 1997, 44, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.; Boin, N.G.; Valera, J.M.; Johnson, A.N. Noncanonical roles for Tropomyosin during myogenesis. Development 2015, 142, 3440–3452. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Haddad, G.G. Role of trehalose phosphate synthase and trehalose during hypoxia: From flies to mammals. J. Exp. Biol. 2004, 207, 3125–3129. [Google Scholar] [CrossRef] [PubMed]
- Alcolea, P.J.; Alonso, A.; Larraga, V. Proteome profiling of Leishmania infantum promastigotes. J. Eukaryot. Microbiol. 2011, 58, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, M.K.; Kuldeep, J.; Siddiqi, M.I.; Goyal, N. The TCP1γ subunit of Leishmania donovani forms a biologically active homo-oligomeric complex. FEBS J. 2015, 282, 4607–4619. [Google Scholar] [CrossRef] [PubMed]
- Jagla, T.; Wojtowicz, I.; Jagla, K. Lethal (2) Essential for Life Gene Regulates Lateral Muscle Shapes during Embryonic Development; GReD: Clermont, France, 2014. [Google Scholar]
- Kurzik-Dumke, U.; Lohmann, E. Sequence of the new Drosophila melanogaster small heat-shock-related gene, lethal(2) essential for life [l(2)efl], at locus 59F4,5. Gene 1995, 154, 171–175. [Google Scholar] [CrossRef]
- Feng, Y.; Walsh, C.A. The many faces of filamin: A versatile molecular scaffold for cell motility and signalling. Nat. Cell Biol. 2004, 6, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Ohta, Y.; Hartwig, J.H.; Stossel, T.P. FilGAP, a Rho- and ROCK-regulated GAP for Rac binds filamin A to control actin remodelling. Nat. Cell Biol. 2006, 8, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Popowicz, G.M.; Schleicher, M.; Noegel, A.A.; Holak, T.A. Filamins: Promiscuous organizers of the cytoskeleton. Trends Biochem. Sci. 2006, 31, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Stossel, T.P.; Condeelis, J.; Cooley, L.; Hartwig, J.H.; Noegel, A.; Schleicher, M.; Shapiro, S.S. Filamins as integrators of cell mechanics and signaling. Nat. Rev. Mol. Cell Biol. 2001, 2, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.G.; Huhn, S.; Shen, Z. Complex roles of filamin-A mediated cytoskeleton network in cancer progression. Cell Biosci. 2013, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Ayme-Southgate, A.; Lasko, P.; French, C.; Pardue, M.L. Characterization of the gene for mp20: A Drosophila muscle protein that is not found in asynchronous oscillatory flight muscle. J. Cell Biol. 1989, 108, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Brendel, S.; Cunha, P.M.; Astola, N.; Song, B.; Furlong, E.E.; Leonard, K.R.; Bullard, B. Myofilin, a protein in the thick filaments of insect muscle. J. Cell. Sci. 2005, 118, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Vera, A.; Stanic, K.; Montecinos, H.; Torrejón, M.E.; Marcellini, S.; Caprile, T. SCO-spondin from embryonic cerebrospinal fluid is required for neurogenesis during early brain development. Front. Cell. Neurosci. 2013, 7, 80. [Google Scholar] [CrossRef] [PubMed]
- Thanaphum, S.; Haymer, D.S. A member of the hsp70 gene family from the Mediterranean fruit fly, Ceratitis capitata. Insect Mol. Biol. 1998, 7, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L. Proteomic identification of a potential sex biomarker for 2 fruit fly species at pupal stage. J. Asia Pac. Entomol. 2017, 20, 125–131. [Google Scholar] [CrossRef]
- Stanley, D.W.; Goodman, C.; An, S.; McIntosh, A.; Song, Q. Prostaglandins A1 and E1 influence gene expression in an established insect cell line (BCIRLHzAM1cells). Insect Biochem. Mol. Biol. 2008, 38, 275–284. [Google Scholar] [CrossRef] [PubMed]
| Accession # | Protein Names (Abbreviations) | Spot ID (L1/E3 Ratio) |
|---|---|---|
| XP_018793231.1 | Maltase 2 (Mal-B2) | E3-98 (0.25), 104 (0.11), 121 (0.28), 129 (0.35), 149 (0.05), 157 (0.44), 183 (0.26) |
| XP_018784870.1 | Transferrin (TF) | E3-105 (0.04), 112 (0.24), 113 (0.07), 116 (0.12), 128 (0.04) |
| XP_018798053.1 | Vitellogenin-1 (VTG1) | E3-252 (0.26), 257 (0.36), 282 (0.15), 291 (0.34), 293 (0.09) |
| XP_018787380.1 | Vitellogenin-1 (VTG1) | E3-267 (0.14), 268 (0.07), 276 (0.25), 277 (0.12), 281 (0.25), 307 (0.17), 316 (0.10) |
| XP_018787290.1 | Vitellogenin-2 (VTG2) | E3-308 (0.17), 312 (0.11), 313 (0.39), 317 (0.46), 322 (0.39), 326 (0.43), 327 (0.19) |
| XP_018791497.1 | Protein amalgam (Ama) | E3-366 (0.32) |
| XP_011201883.1 | Peroxiredoxin 1 (PRDX1) | E3-564 (0.27) |
| XP_018795769.1 | Peptidyl-prolyl cis-trans isomerase 6 (PPIL6) | E3-628 (0.46) |
| XP_018788060.1 | Heat shock protein 23 (Hsp23) | E3-633 (0.16), 640 (0.12), 643 (0.08), 646 (0.19), 650 (0.23) |
| XP_018797702.1 | Peroxiredoxin-5, MC (PRDX5) | E3-685 (0.49) |
| XP_018799095.1 | Heat shock protein 83 (Hsp83) | E3-709 (0.25) |
| XP_018793742.1 | SCO-spondin (SSPO) | L1-130 (4.37) |
| XP_018793741.1 | Uncharacterized protein LOC108971872 isoform X2 | L1-136 (5.89) |
| XP_004526477.1 | Actin-2, muscle specific | L1-304 (3.82) |
| XP_018799378.1 | Tropomyosin-2 (TPM2) | L1-365 (20.31), L-378 (7.12) |
| XP_018802321.1 | L-lactate dehydrogenase (LDH) | L1-372 (2.67) |
| XP_018783435.1 | Malate dehydrogenase, cytoplasmic (MDH) | L1-379 (2.62) |
| XP_018795679.1 | Uncharacterized protein LOC108973107 isoform X1 | L1-395 (5.17) |
| XP_018789491.1 | UDP-glucose 4-epimerase | L1-401 (5.13), 407 (6.18), 485 (2.01) |
| XP_018787334.1 | Glyceraldehyde-3-phosphate dehydrogenase (G3PD) | L1-416 (4.45) |
| XP_018804543.1 | Trehalose-phosphate phosphatase (TPP) | L1-419 (15.82) |
| XP_004519608.1 | T-complex protein 1 subunit gamma (TCP1) | L1-426 (23.12) |
| XP_018804039.1 | 3-hydroxyisobutyrate dehydrogenase (HIBADH) | L1-454 (2.14) |
| XP_018804543.1 | Trehalose-phosphate phosphatase B(TPP) | L1-551 (2.04) |
| XP_018794827.1 | Uncharacterized protein LOC108972624 | L1-563 (3.30), 565 (26.86) |
| XP_011210145.1 | Uncharacterized protein LOC105230849 | L1-566 (6.57) |
| XP_018793665.1 | Protein lethal (2) essential for life (L2EFL) | L1-571 (2.13) |
| XP_018791794.1 | Filamin-A, isoform X2 (FLNA) | L1-59 (34.34) |
| XP_018787727.1 | Hypothetical protein c0_g1_i1 (c0_g1_i1) | L1-674 (15.72) |
| XP_018804351.1 | Muscle-specific protein 20 (MP20) | L1-679 (3.02) |
| gi|880832606| | Krueppel ous protein 1 | L1-699 (7.44) |
| XP_018799091.1 | General odorant-binding protein 99a (GOBP99a) | L1-708 (2.01) |
| XP_018788026.1 | Myophilin | L1-710 (12.10) |
| XP_014094514.1 | Larval cuticle protein 5 (LCP5) | L1-721 (7.70) |
| XP_018797071.1 | 60S acidic ribosomal protein P2 (RpLP2) | L1-739 (3.64) |
| XP_018803514.1 | 60S acidic ribosomal protein P1 (RpLP1) | L1-750 (3.90) |
| Accession No. | Protein Names (Abbreviations) | Spot ID (P1/L10 Ratio) |
|---|---|---|
| XP_018793742.1 | Uncharacterized protein LOC108971874 | L10-1991 (0.41) |
| XP_018793741.1 | Uncharacterized protein LOC108971872 isoform X2 | L10-2001 (0.45) |
| XP_018783509.1 | Aldehyde dehydrogenase X (ALDH1B1) | L10-2120 (0.50) |
| XP_018790778.1 | Phosphoglycerate kinase (PGK1) | L10-2187 (0.34) |
| XP_011208284.1 | Heat shock protein cognate 4 (Hsc70-4) | L10-2222 (0.35) |
| XP_018794703.1 | Calreticulin (CALR) | L10-2303 (0.10) |
| XP_018795679.1 | Uncharacterized protein LOC108973107 isoform X1 | L10-2320 (0.42) |
| XP_018789492.1 | Uncharacterized protein LOC108969313 | L10-2367 (0.33) |
| XP_018802393.1 | putative oxidoreductase TM_0325 (TM0325) | L10-2454 (0.42) |
| XP_018794827.1 | Uncharacterized protein LOC108972625 | L10-2483 (0.29), L10-2487 (0.34) |
| XP_018788990.1 | Superoxide dismutase [Mn] (SOD) | L10-2496 (0.44) |
| XP_018804351.1 | Muscle-specific protein 20 (Mp20) | L10-2569 (0.30), L10-2652 (0.05) |
| XP_018788026.1 | Myophilin | L10-2590 (0.22) |
| gi|880854006| | Larval cuticle protein 5 (Lcp5) | L10-2601 (0.41) |
| XP_018804878.1 | ATP synthase subunit delta (ATP5D) | L10-2610 (0.22) |
| XP_004517938.2 | Nucleoside diphosphate kinase (NDPK) | L10-2634 (0.13) |
| XP_018803514.1 | 60S acidic ribosomal protein P1 (RpLP1) | L10-2667(0.42) |
| XP_018800124.1 | Enolase-phosphatase E1 | P1-1954 (4.21) |
| XP_018789027.1 | Protein yellow | P1-2070 (3.64) |
| XP_018802734.1 | Putative aminopeptidase W07G4.4 (lap-2) | P1-2074 (7.03) |
| XP_018799378.1 | Tropomyosin-2 (TPM2) | P1-2261 (2.75), P1-2262 (3.71) |
| XP_018802440.1 | Regucalcin (RGN) | P1-2304 (2.96), P1-2322 (4.40) |
| XP_018799300.1 | Tropomyosin-2 (TPM2) | P1-2309 (2.51) |
| XP_018794340.1 | Uncharacterized protein LOC108972251 | P1-2424 (6.31), P1-2435 (11.17) |
| XP_018786140.1 | Dehydrogenase/reductase SDR family member 11 (DHRS11) | P1-2436 (3.27) |
| XP_018793665.1 | Triosephosphate isomerase (TPI) | P1-2466 (2.79) |
| XP_018789492.1 | Uncharacterized protein LOC108969313 | P1-2540 (4.70) |
| XP_018788064.1 | Heat shock protein 23 (Hsp23) | P1-2557 (2.30), P1-2558 (9.03) |
| XP_018805087.1 | Larval cuticle protein 5 (Lcp5) | P1-2559 (7.26) |
| XP_018793318.1 | Larval cuticle protein 5 (Lcp5) | P1-2579 (3.29) |
| XP_018793320.1 | Larval cuticle protein 5 (Lcp5) | P1-2618 (3.94) |
| XP_018805010.1 | Cuticular protein 47Eg (Cp47Eg) | P1-2647 (5.84) |
| Accession No. | Protein Name | Spot ID (F1/P12) |
|---|---|---|
| XP_018798316.1 | Catalase | P12-211 (0.30) |
| XP_018794196.1 | Reticulon-4-interacting protein 1(RTN4lPI) | P12-500 (0.03) |
| XP_018794197.1 | Cuticle protein (CP) | P12-519 (0.07) |
| XP_018794197.1 | Larval cuticle protein A2B | P12-522 (0.04) |
| XP_018788732.1 | Cuticle protein 8 (CP8) | P12-723 (0.10) |
| XP_018803223.1 | Dihydrolipoyl dehydrogenase (DLD) | F1-196 (2.53) |
| XP_018794316.1 | Enolase | F1-223 (2.03), F1-226 (2.48) |
| XP_018785511.1 | Arginine kinase (AK) | F1-299 (2.12) |
| XP_018804575.1 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F1-363 (3.54) |
| XP_018798823.1 | Pyruvate dehydrogenase E1 component subunit β (PDHB) | F1-403 (2.22) |
| XP_018801988.1 | Epidermal retinol dehydrogenase 2 (RDHE2) | F1-533 (2.60) |
| XP_018800458.1 | Heat shock protein beta-1 (HSPB1) | F1-541 (2.60) |
| XP_018801988.1 | Pericentrin (PCNT) | F1-548 (5.64) |
| XP_004520585.1 | Endocuticle structural glycoprotein ABD-4 (CUD-4) | F1-625 (2.00), F1-654 (3.12) |
| XP_018799095.1 | General odorant-binding protein 99a-like (OBP99a) | F1-632 (2.32) |
| XP_018787334.1 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | F1-779 (2.12) |
| Accession No. | Protein Name | Spot ID (M1/P12) |
|---|---|---|
| XP_018794197.1 | Cuticle protein (CP) | P12-1511 (0.03) |
| XP_018783307.1 | Cuticle protein (CP) | P12-1610 (0.11) |
| XP_018783299.1 | Cuticle protein (CP) | P12-1654 (0.06) |
| XP_018788732.1 | Cuticle protein 8 (CP8) | P12-1661 (0.06) |
| XP_018803223.1 | Dihydrolipoyl dehydrogenase (DLD) | M1-1251 (2.44) |
| XP_018794316.1 | Enolase | M1-1261 (2.62) |
| XP_014087797.1 | Heat shock protein cognate 4 (Hsp70-4) | M1-1288 (2.17) |
| XP_018785511.1 | Arginine kinase (AK) | M1-1344 (2.56) |
| XP_018801988.1 | DNA polymerase θ (Poly theta) | M1-1535 (3.00) |
| XP_004520585.1 | Endocuticle structural glycoprotein ABD-4 (CUD-4) | M1-1617 (2.65) |
| Accession No. | Protein Name | Spot ID (F9/M9 Ratio) |
|---|---|---|
| XP_018804032.1 | Regucalcin (RGN) | M9-1333 (0.47) |
| XP_018801988.1 | Muscle M-line assembly protein unc-89 (UNC-89) | M9-1475 (0.30) |
| NP_001291905.1 | Superoxide dismutase [Cu-Zn] (SOD1) | M9-1561 (0.46) |
| XP_018799095.1 | RNA-directed DNA polymerase from transposon BS (RTBS) | M9-1569 (0.31) |
| XP_018802735.1 | Putative aminopeptidase W07G4.4 (LAP-2) | F9-1051 (7.09) |
| XP_018784524.1 | Aldehyde dehydrogenase (ADH) | F9-1082 (3.16) |
| XP_018787380.1 | Vitellogenin-1 (VTG1) | F9-1129 (28.95), F9-1135 (88.32), |
| F9-1139 (6.41), F9-1144 (2.22) | ||
| XP_018783435.1 | Malate dehydrogenase, cytoplasmic (MDH) | F9-1253 (2.19) |
| XP_018793355.1 | 2-oxoglutarate dehydrogenase (OGDC) | F9-903 (2.74) |
| XP_018791794.1 | Filamin-A (FLNA) | F9-911 (4.76) |
| XP_018784870.1 | Transferrin (TF) | F9-972 (2.78), F9-978 (2.27) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.L.; Geib, S.M. Comparative Proteomic Profiling between Each of Two Consecutive Developmental Stages of the Solanum Fruit Fly, Bactrocera latifrons (Hendel). Int. J. Mol. Sci. 2018, 19, 1996. https://doi.org/10.3390/ijms19071996
Chang CL, Geib SM. Comparative Proteomic Profiling between Each of Two Consecutive Developmental Stages of the Solanum Fruit Fly, Bactrocera latifrons (Hendel). International Journal of Molecular Sciences. 2018; 19(7):1996. https://doi.org/10.3390/ijms19071996
Chicago/Turabian StyleChang, Chiou Ling, and Scott M. Geib. 2018. "Comparative Proteomic Profiling between Each of Two Consecutive Developmental Stages of the Solanum Fruit Fly, Bactrocera latifrons (Hendel)" International Journal of Molecular Sciences 19, no. 7: 1996. https://doi.org/10.3390/ijms19071996
APA StyleChang, C. L., & Geib, S. M. (2018). Comparative Proteomic Profiling between Each of Two Consecutive Developmental Stages of the Solanum Fruit Fly, Bactrocera latifrons (Hendel). International Journal of Molecular Sciences, 19(7), 1996. https://doi.org/10.3390/ijms19071996