Metformin in Pregnancy: Mechanisms and Clinical Applications
Abstract
1. Introduction
2. Mechanisms of Action of Metformin
3. Clinical Applications
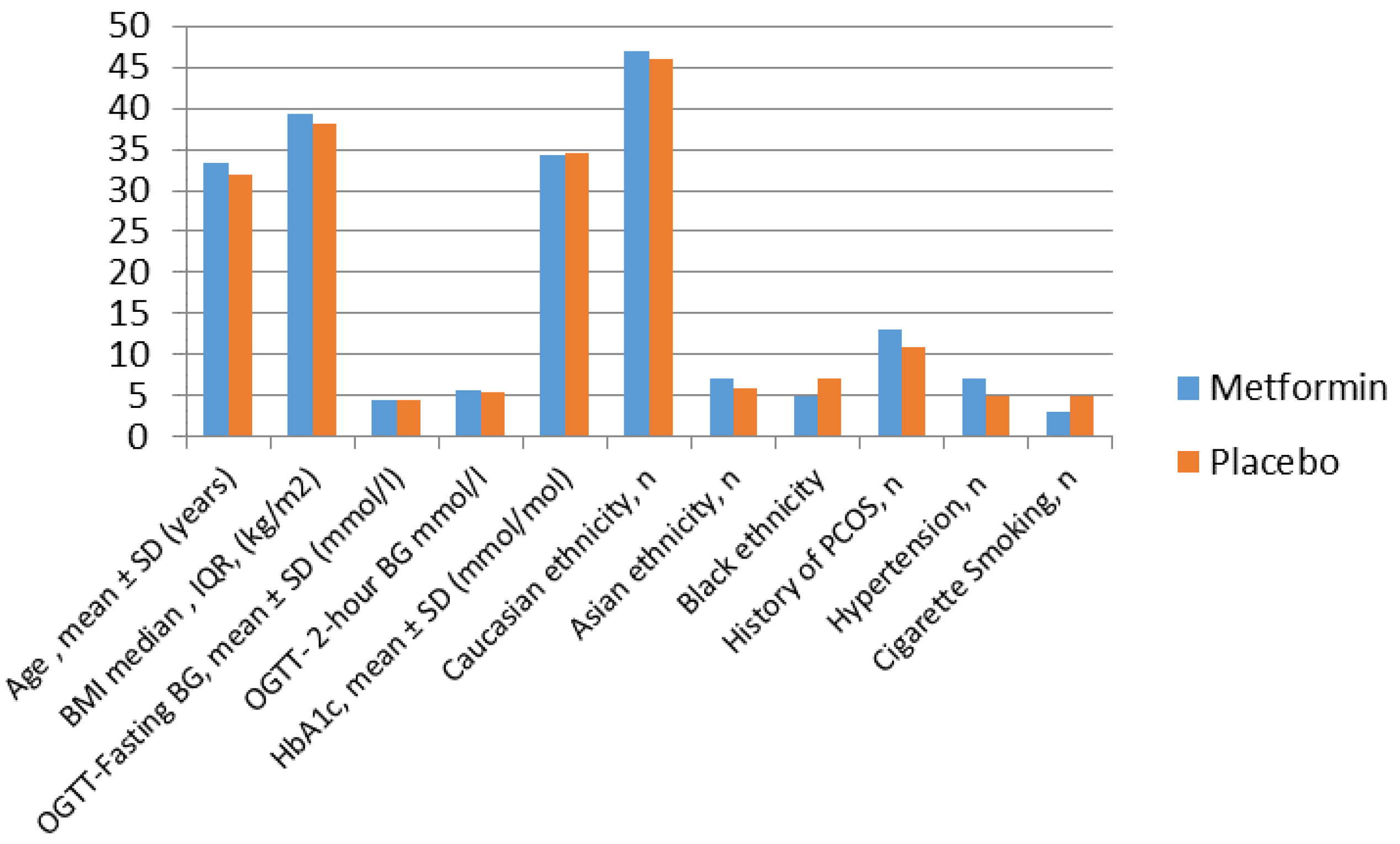
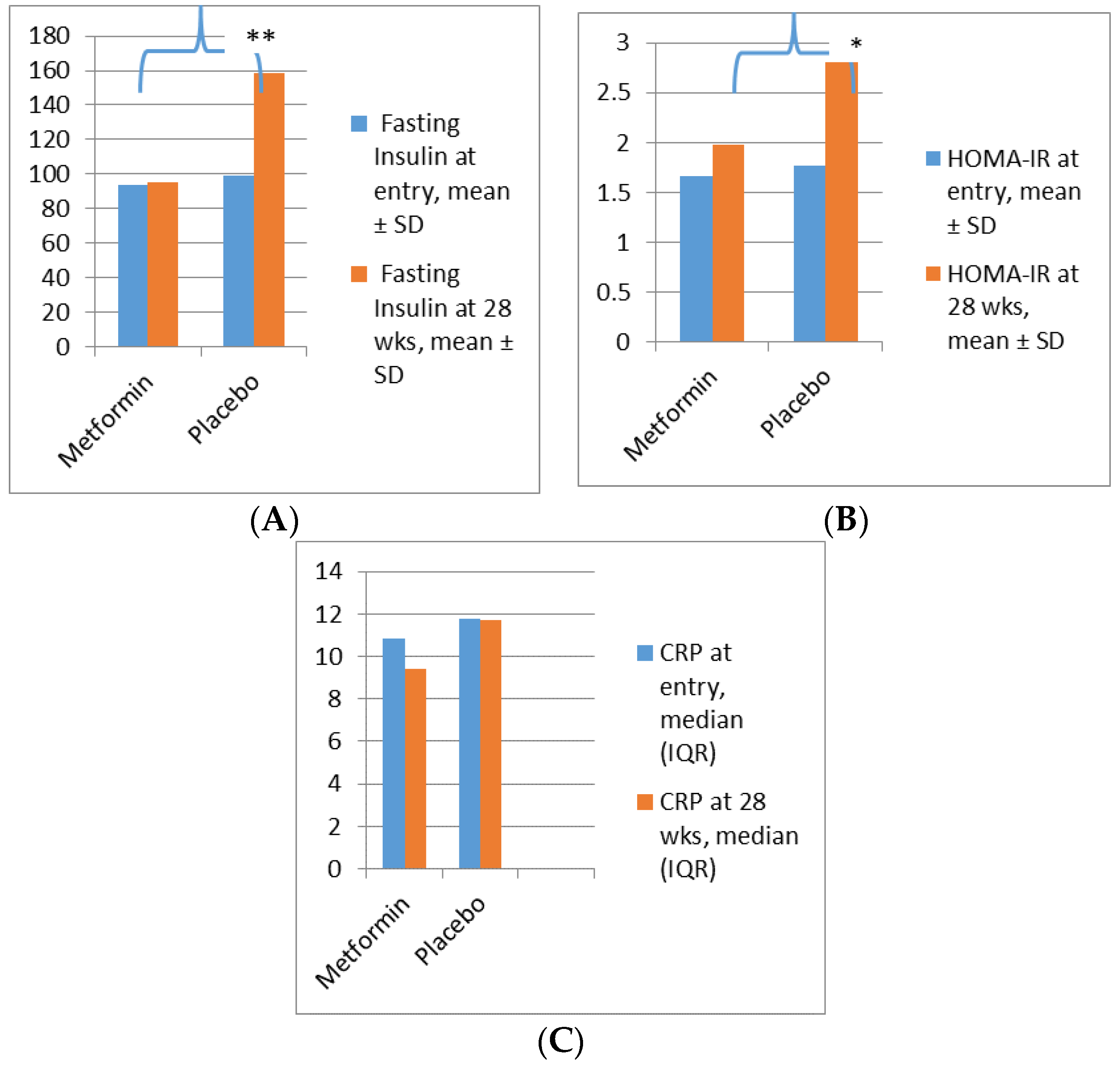
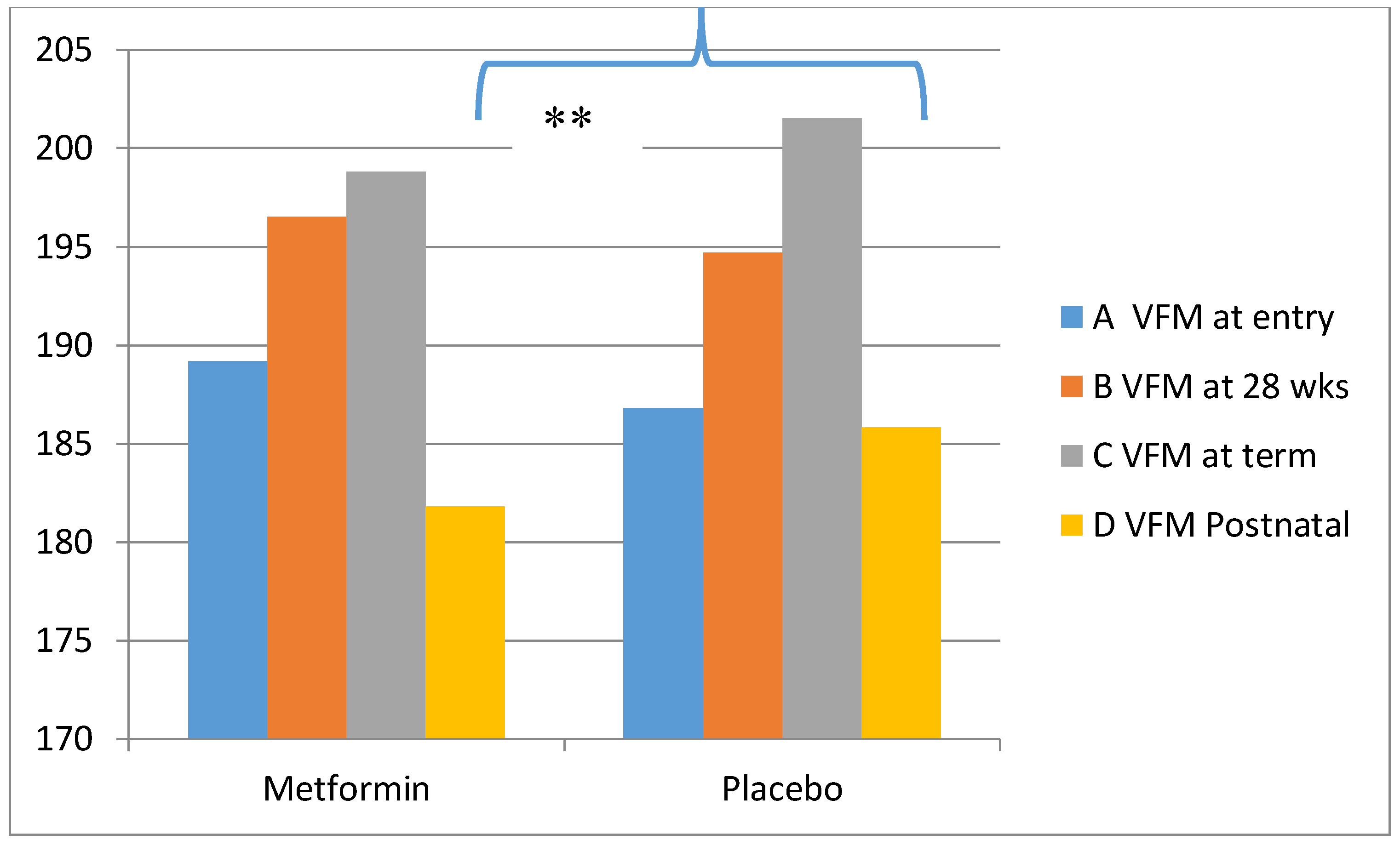
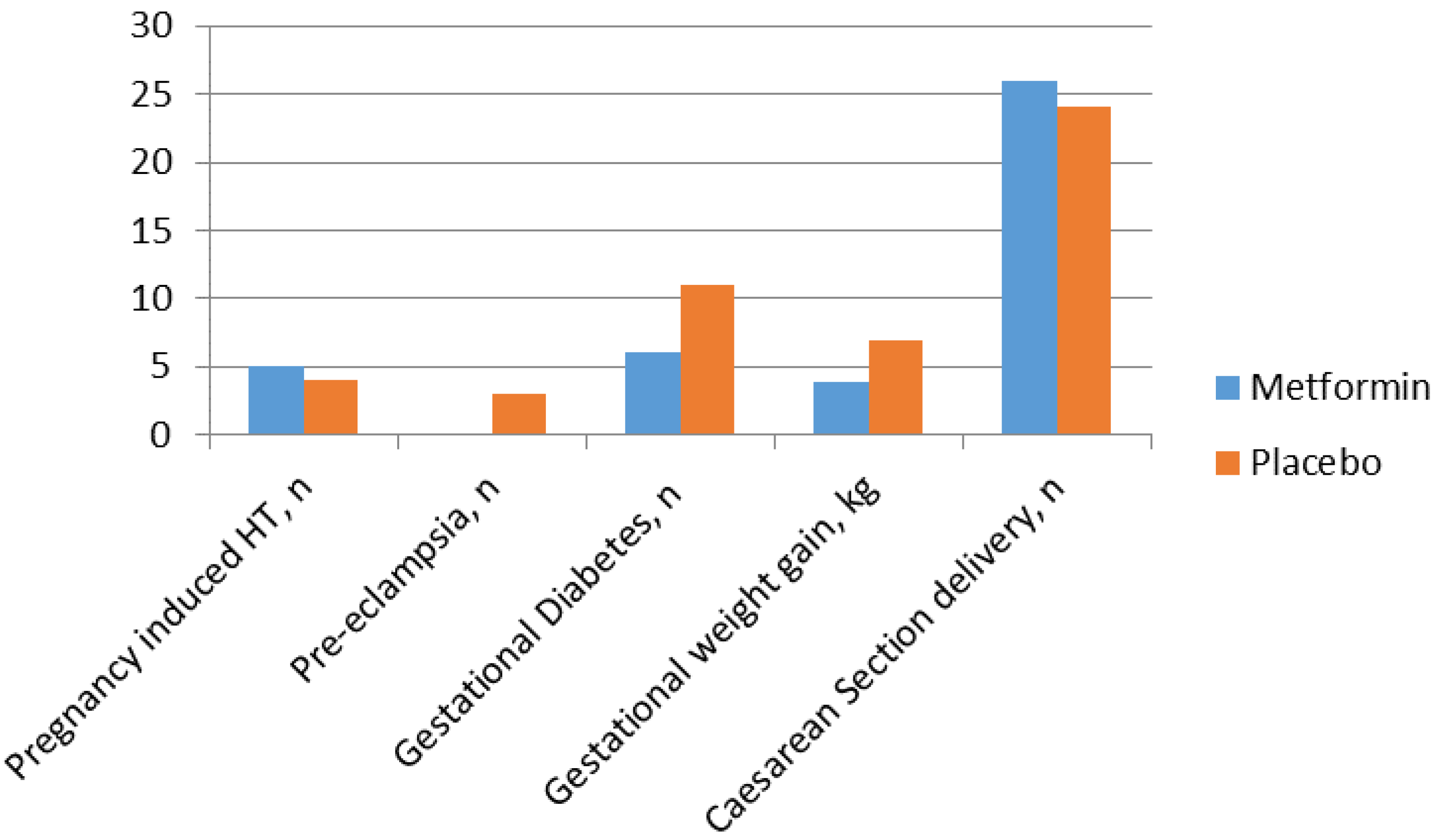
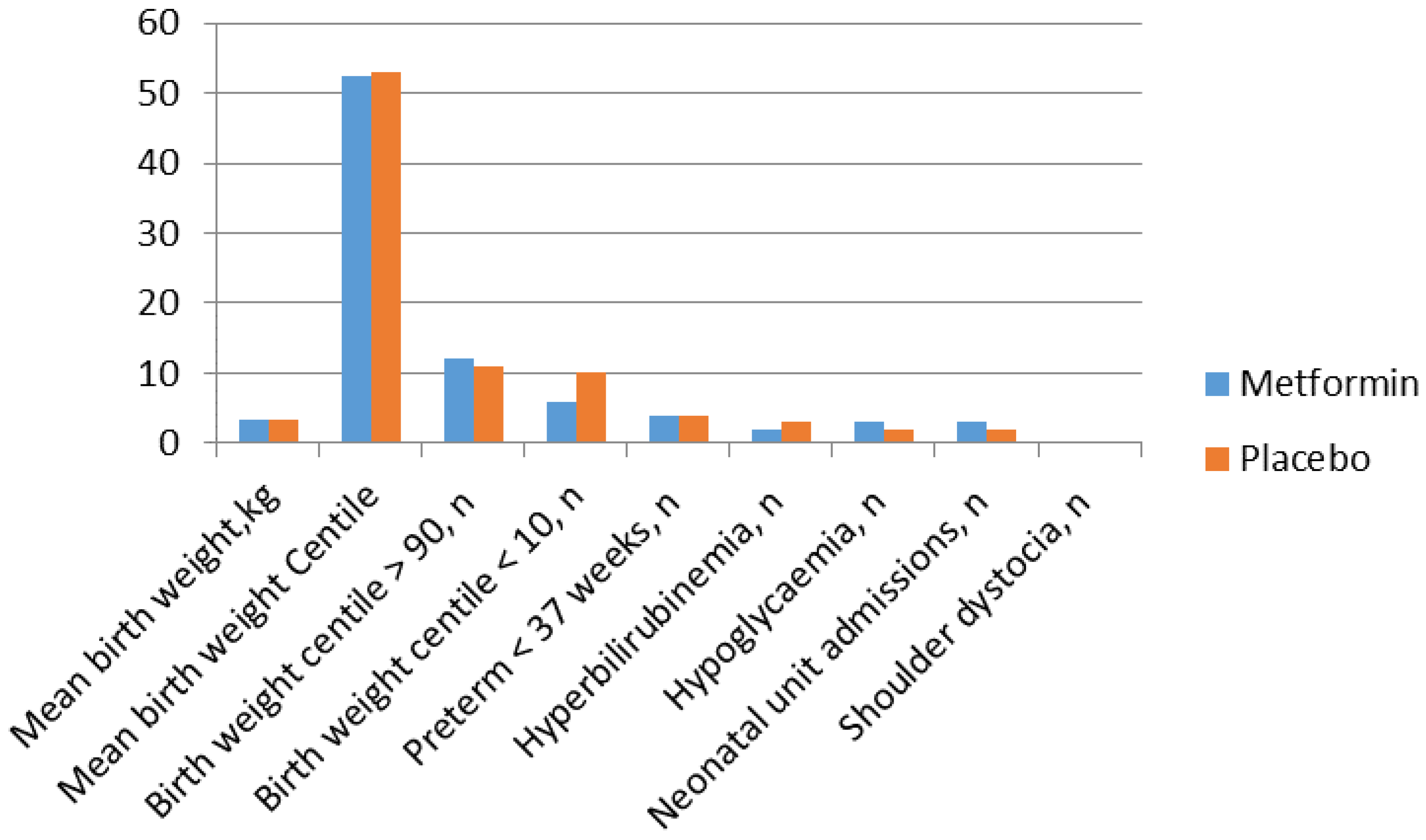
Metformin in Obese Non-Diabetic Pregnant Women
4. Adverse Events
4.1. Metformin in Gestational Diabetes
4.2. Metformin for Women with Type 2 Diabetes
4.3. Metformin for Women with PCOS
4.4. Potential Impact for Children after in Utero Exposure to Metformin
5. Summary
Author Contributions
Acknowledgments
Conflicts of Interest
References
- National Institute for Health and Care Excellence (NICE). Diabetes in pregnancy: Management from preconception to the postnatal period. In NICE Guideline (NG3); NICE: London, UK, 2015. [Google Scholar]
- Guide, Q.R. Management of Diabetes Updated November 2017. Available online: http://www.sign.ac.uk/assets/qrg116.pdf (accessed on 4 July 2018).
- Prebtani, A.P.H.; Bajaj, H.S.; Mbbs, S.A.I.; Edin, F.; Mbbs, G.A. A Publication of the Professional Section of Diabetes Canada Une Publication de la Section Professionnelle CONTENTS: April 2018 Volume 42 Supplement 1 Introduction Reducing the Risk of Developing Diabetes CONTENTS (Continued): April 2018 Volume 42 Supplement 1 Hyperglycemic Emergencies in Adults Weight Management in Diabetes Macrovascular and Microvascular Complications. Available online: http://guidelines.diabetes.ca/docs/CPG-2018-full-EN.pdf (accessed on 4 July 2018).
- American Diabetes Association. management of diabetes in pregnancy: Standards of medical care in diabetes d 2018. Diabetes Care 2018, 41, 137–143. [Google Scholar]
- Wiernsperger, N.F.; Bailey, C.J. The antihyperglycaemic effect of metformin: Therapeutic and cellular mechanisms. Drugs 1999, 58, 31–82. [Google Scholar] [CrossRef] [PubMed]
- Scarpello, J.H.; Howlett, H.C. Metformin therapy and clinical uses. Diabetes Vasc. Dis. Res. 2008, 5, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Goldenberg, N.; Wang, P.; Loftspring, M.; Sherman, A. Metformin during pregnancy reduces insulin, insulin resistance, insulin secretion, weight, testosterone and development of gestational diabetes: Prospective longitudinal assessment of women with polycystic ovary syndrome from preconception throughout preg. Hum. Reprod. 2004, 19, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Giannarelli, R.; Aragona, M.; Coppelli, A.; Del Prato, S. Reducing insulin resistance with metformin: The evidence today. Diabetes Metab. 2003, 29, 6S28–6S35. [Google Scholar] [CrossRef]
- Buse, J.B.; DeFronzo, R.A.; Rosenstock, J.; Kim, T.; Burns, C.; Skare, S.; Baron, A.; Fineman, M. The Primary Glucose-Lowering Effect of Metformin Resides in the Gut, Not the Circulation: Results From Short-term Pharmacokinetic and 12-Week Dose-Ranging Studies. Diabetes Care 2016, 39, 198–205. [Google Scholar] [CrossRef] [PubMed]
- King, P.; Peacock, I.; Donnelly, R. The UK Prospective Diabetes Study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. 1999, 45, 643–648. [Google Scholar] [CrossRef]
- Beisswenger, P.; Ruggiero-Lopez, D. Metformin inhibition of glycation processes. Diabetes Metab. 2003, 29, 6S95–6S103. [Google Scholar] [CrossRef]
- Ahmadimoghaddam, D.; Zemankova, L.; Nachtigal, P.; Dolezelova, E.; Neumanova, Z.; Cerveny, L.; Ceckova, M.; Kacerovský, M.; Micuda, S.; Staud, F. Organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter in the placenta and fetal tissues: Expression profile and fetus protective role at different stages of gestation. Biol. Reprod. 2013, 88, 55. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Kwon, S.; Pak, Y.K.; Seol, H.W.; Choi, Y.M.; Park, D.J.; Park, K.S.; Lee, H.K. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2006, 348, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Viana, M.; Thirumangalathu, S.; Loeken, M.R. AMP-activated protein kinase mediates effects of oxidative stress on embryo gene expression in a mouse model of diabetic embryopathy. Diabetologia 2012, 55, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Wei, D.; Loeken, M.R. Lack of metformin effect on mouse embryo AMPK activity: Implications for metformin treatment during pregnancy. Diabetes Metab. Res. Rev. 2014, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.; The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving Mothers’ Lives: Reviewing Maternal Deaths to Make Motherhood Safer–2003–2005; Volume 7th Report; CEMACH: London, UK, 2007. [Google Scholar]
- Galtier-Dereure, F.; Boegner, C.; Bringer, J. Obesity and pregnancy: Complications and cost. Am. J. Clin. Nutr. 2000, 71 (Suppl. 5), 1242. [Google Scholar] [CrossRef] [PubMed]
- Centre for Maternal and Child Enquiries (CMACE). Maternal Obesity in the UK: Findings from a National Project; CMACE: London, UK, 2010. [Google Scholar]
- Heslehurst, N.; Ells, L.J.; Simpson, H.; Batterham, A.; Wilkinson, J.; Summerbell, C.D. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG 2007, 114, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Jolly, M.; Harris, J.P.; Wadsworth, J.; Joffe, M.; Beard, R.W.; Regan, L.; Robinson, S. Maternal obesity and pregnancy outcome: A study of 287,213 pregnancies in London. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Chiswick, C.; Reynolds, R.M.; Denison, F.; Drake, A.J.; Forbes, S.; Newby, D.E.; Walker, B.R.; Quenby, S.; Wray, S.; Weeks, A.; et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2015, 3, 778–786. [Google Scholar] [CrossRef]
- Stanford, F.C.; Alfaris, N.; Misra, M. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. N. Engl. J. Med. 2016, 374, 434–443. [Google Scholar]
- Chen, S.; Zhou, J.; Xi, M.; Jia, Y.; Wong, Y.; Zhao, J.; Ding, L.; Zhang, J.; Wen, A. Pharmacogenetic variation and metformin response. Curr. Drug Metab. 2013, 14, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Rush, E.C.; Obolonkin, V.; Battin, M.; Wouldes, T.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition at 2 years of age. Diabetes Care 2011, 34, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Balani, J. Obesity in Pregnancy: Risk of Gestational Diabetes. Ph.D. Thesis, Cranfield University, Bedfordshire, UK, 2018. [Google Scholar]
- Ogawa, H.; Fujitani, K.; Tsujinaka, T.; Imanishi, K.; Shirakata, H.; Kantani, A.; Hirao, M.; Kurokawa, Y.; Utsumi, S. InBody 720 as a new method of evaluating visceral obesity. Hepatogastroenterology 2011, 58, 42–44. [Google Scholar] [PubMed]
- Malavolti, M.; Mussi, C.; Poli, M.; Fantuzzi, A.L.; Salvioli, G.; Battistini, N.; Bedogni, G. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21–82 years. Ann. Hum. Biol. 2003, 30, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; McKinley, M.C.; McNally, R.; Graham, U.; Watson, C.J.; Lyons, T.J.; McClements, L. Risk of pre-eclampsia in women taking metformin: A systematic review and meta-analysis. Diabet. Med. 2018, 35, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Gabbe, S.G.; Persson, B.; Buchanan, T.A.; Catalano, P.A.; Damm, P.; Dyer, A.R.; Leiva Ad Hod, M.; Kitzmiler, J.L. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Investigators, M.T. Metformin versus insulin for the treatment of gestational diabetes. N. Engl. J. Med. 2008, 358, 2003–2015. [Google Scholar]
- Balani, J.; Hyer, S.L.; Rodin, D.A.; Shehata, H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: A case-control study. Diabet. Med. 2009, 26, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Balani, J.; Hyer, S.; Johnson, A.; Shehata, H. Pregnancy outcomes after metformin treatment for gestational diabetes: A case-control study. Obstet. Med. 2012, 5, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Liu, Q.; Feng, L. Metformin vs insulin in the management of gestational diabetes: A meta-analysis. PLoS ONE 2013, 8, e64585. [Google Scholar] [CrossRef] [PubMed]
- Butalia, S.; Gutierrez, L.; Lodha, A.; Aitken, E.; Zakariasen, A.; Donovan, L. Short- and long-term outcomes of metformin compared with insulin alone in pregnancy: A systematic review and meta-analysis. Diabet. Med. 2017, 34, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gutzin, S.J.; Kozer, E.; Magee, L.A.; Feig, D.S.; Koren, G. The safety of oral hypoglycemic agents in the first trimester of pregnancy: A meta-analysis. Can. J. Clin. Pharmacol. 2003, 10, 179–183. [Google Scholar] [PubMed]
- Gilbert, C.; Valois, M.; Koren, G. Pregnancy outcome after first-trimester exposure to metformin: A meta-analysis. Fertil. Steril. 2006, 86, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, E.; Damm, P.; Molsted-Pedersen, L. Oral hypoglycaemic agents in 118 diabetic pregnancies. Diabet. Med. 2000, 17, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C.E.; Rowan, J.A. Pregnancy in women with Type 2 diabetes: Who takes metformin and what is the outcome? Diabet. Med. 2006, 23, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Ekpebegh, C.O.; Coetzee, E.J.; van der Merwe, L.; Levitt, N.S. A 10-year retrospective analysis of pregnancy outcome in pregestational Type 2 diabetes: Comparison of insulin and oral glucose-lowering agents. Diabet. Med. 2007, 24, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; Murphy, K.; Asztalos, E.; Tomlinson, G.; Sanchez, J.; Zinman, B.; Ohlsson, A.; Ryan, E.A.; Fantus, I.G.; Armson, A.B.; et al. Metformin in women with type 2 diabetes in pregnancy (MiTy): A multi-center randomized controlled trial. BMC Pregnancy Childbirth 2016, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Jakubowicz, D.J.; de Vargas, A.F.; Brik, C.; Quintero, N.; Medina, F. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositolglycan mediators as the signal transduction system. J. Clin. Endocrinol. Metab. 1998, 83, 2001–2005. [Google Scholar] [PubMed]
- Legro, R.S.; Castracane, V.D.; Kauffman, R.P. Detecting insulin resistance in polycystic ovary syndrome: Purposes and pitfalls. Obstet. Gynecol. Surv. 2004, 59, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Anderson, R.A. Impact of obesity on female reproductive health: British Fertility Society, Policy and Practice Guidelines. Hum. Fertil. 2007, 10, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Khan, K. Effects of metformin use in pregnant patients with polycystic ovary syndrome. J. Hum. Reprod. Sci. 2012, 5, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Vanky, E.; Stridsklev, S.; Heimstad, R.; Romundstad, P.; Skogøy, K.; Kleggetveit, O.; Hjelle, S.; von Brandis, P.; Eikeland, T.; Flo, K.; et al. Metformin versus placebo from first trimester to delivery in polycystic ovary syndrome: A randomized, controlled multicenter study. J. Clin. Endocrinol. Metab. 2010, 95, E448–E455. [Google Scholar] [CrossRef] [PubMed]
- De Leo, V.; Musacchio, M.C.; Piomboni, P.; Di Sabatino, A.; Morgante, G. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Khattab, S.; Mohsen, I.A.; Aboul Foutouh, I.; Ashmawi, H.S.; Mohsen, M.N.; van Wely, M.; van der Veen, F.; Youssef, M.A. Can metformin reduce the incidence of gestational diabetes mellitus in pregnant women with polycystic ovary syndrome? Prospective cohort study. Gynecol. Endocrinol. 2011, 27, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.H.; Khalid, R.; Naru, T.; Rizvi, J. Does continuous use of metformin throughout pregnancy improve pregnancy outcomes in women with polycystic ovarian syndrome? J. Obstet. Gynaecol. Res. 2008, 34, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kim, B.V.; van Wely, M.; Johnson, N.P.; Costello, M.F.; Zhang, H.; Ng, E.H.Y.; Legro, R.S.; Bhattacharya, S.; Norman, R.J.; et al. Treatment strategies for women with WHO group II anovulation: Systematic review and network meta-analysis. BMJ 2017, 356, j138. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Hanson, R.L.; Lindsay, R.S.; Pettitt, D.J.; Imperatore, G.; Gabir, M.M.; Roumain, J.; Bennett, P.H.; Knowler, W.C. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: A study of discordant sibships. Diabetes 2000, 49, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 2007, 30 (Suppl. 2), S169–S174. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.S.; Rosenfeld, C.R.; Rice, J.; Rice, M.; Hardin, D.S. Insulin resistance in Hispanic large-for-gestational-age neonates at birth. J. Clin. Endocrinol. Metab. 2007, 92, 3836–3843. [Google Scholar] [CrossRef] [PubMed]
- Ro, T.B.; Ludvigsen, H.V.; Carlsen, S.M.; Vanky, E. Growth, body composition and metabolic profile of 8-year-old children exposed to metformin in utero. Scand. J. Clin. Lab. Investig. 2012, 72, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Rush, E.C.; Plank, L.D.; Lu, J.; Obolonkin, V.; Coat, S.; Hague, W.M. Metformin in gestational diabetes: The offspring follow-up (MiG TOFU): Body composition and metabolic outcomes at 7–9 years of age. BMJ Open Diabetes Res. Care 2018, 6, e000456. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; Moses, R.G. Metformin therapy during pregnancy: Good for the goose and good for the gosling too? Diabetes Care 2011, 34, 2329–2330. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyer, S.; Balani, J.; Shehata, H. Metformin in Pregnancy: Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2018, 19, 1954. https://doi.org/10.3390/ijms19071954
Hyer S, Balani J, Shehata H. Metformin in Pregnancy: Mechanisms and Clinical Applications. International Journal of Molecular Sciences. 2018; 19(7):1954. https://doi.org/10.3390/ijms19071954
Chicago/Turabian StyleHyer, Steve, Jyoti Balani, and Hassan Shehata. 2018. "Metformin in Pregnancy: Mechanisms and Clinical Applications" International Journal of Molecular Sciences 19, no. 7: 1954. https://doi.org/10.3390/ijms19071954
APA StyleHyer, S., Balani, J., & Shehata, H. (2018). Metformin in Pregnancy: Mechanisms and Clinical Applications. International Journal of Molecular Sciences, 19(7), 1954. https://doi.org/10.3390/ijms19071954



