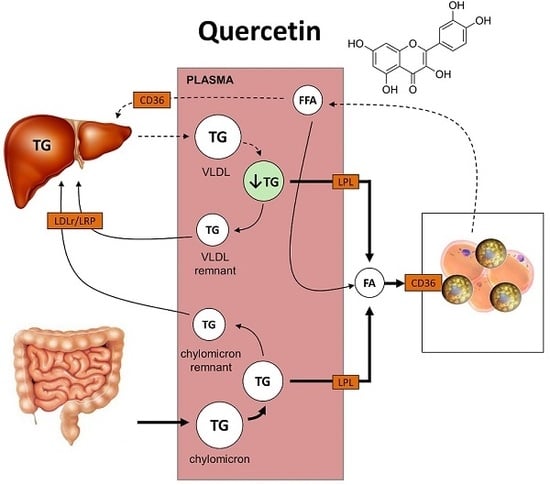
Quercetin Lowers Plasma Triglycerides Accompanied by White Adipose Tissue Browning in Diet-Induced Obese Mice
Abstract
1. Introduction
2. Results
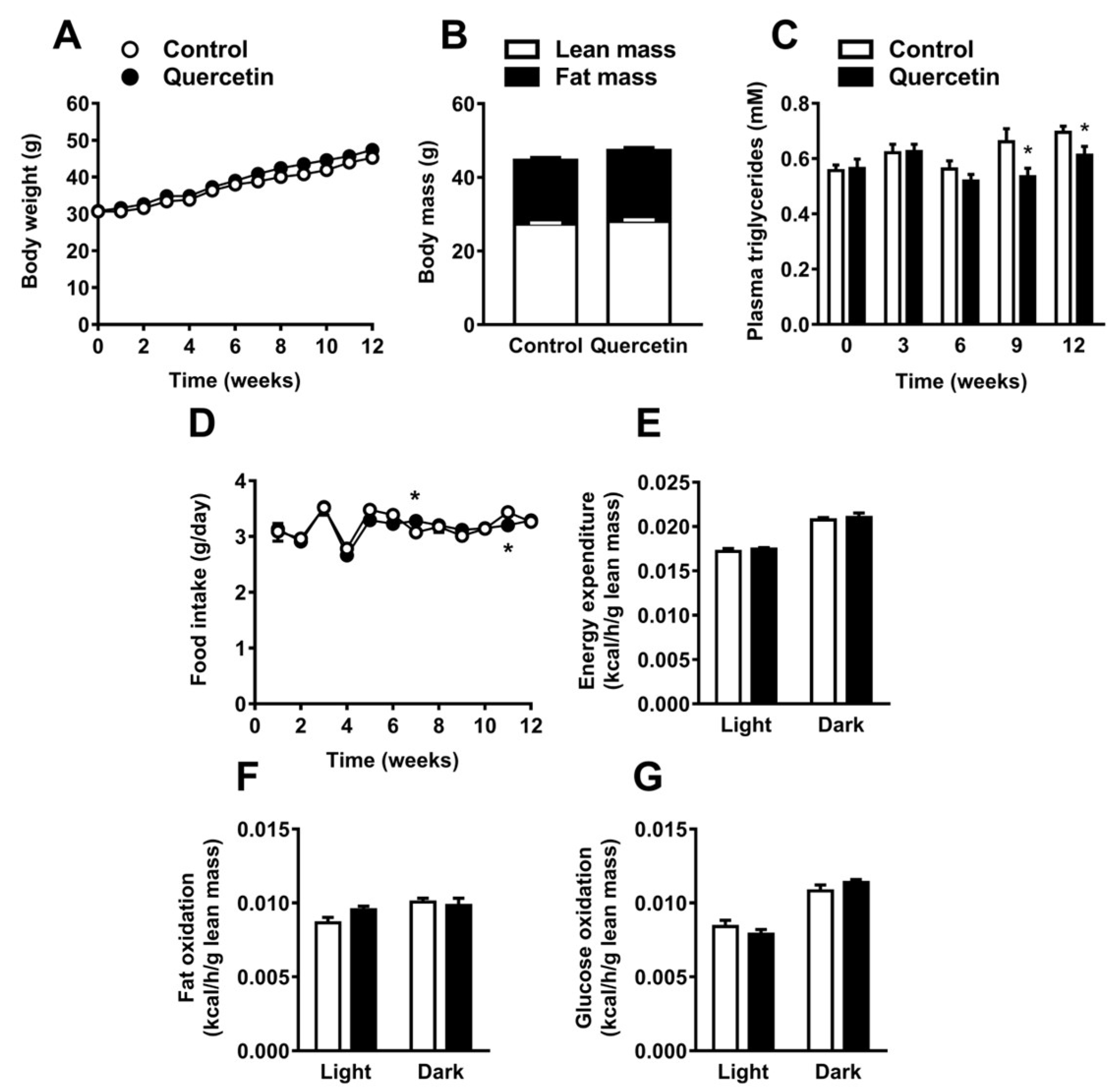
2.1. Quercetin Reduces Plasma Triglyceride Levels without Affecting Body Composition, Food Intake, and Energy Expenditure
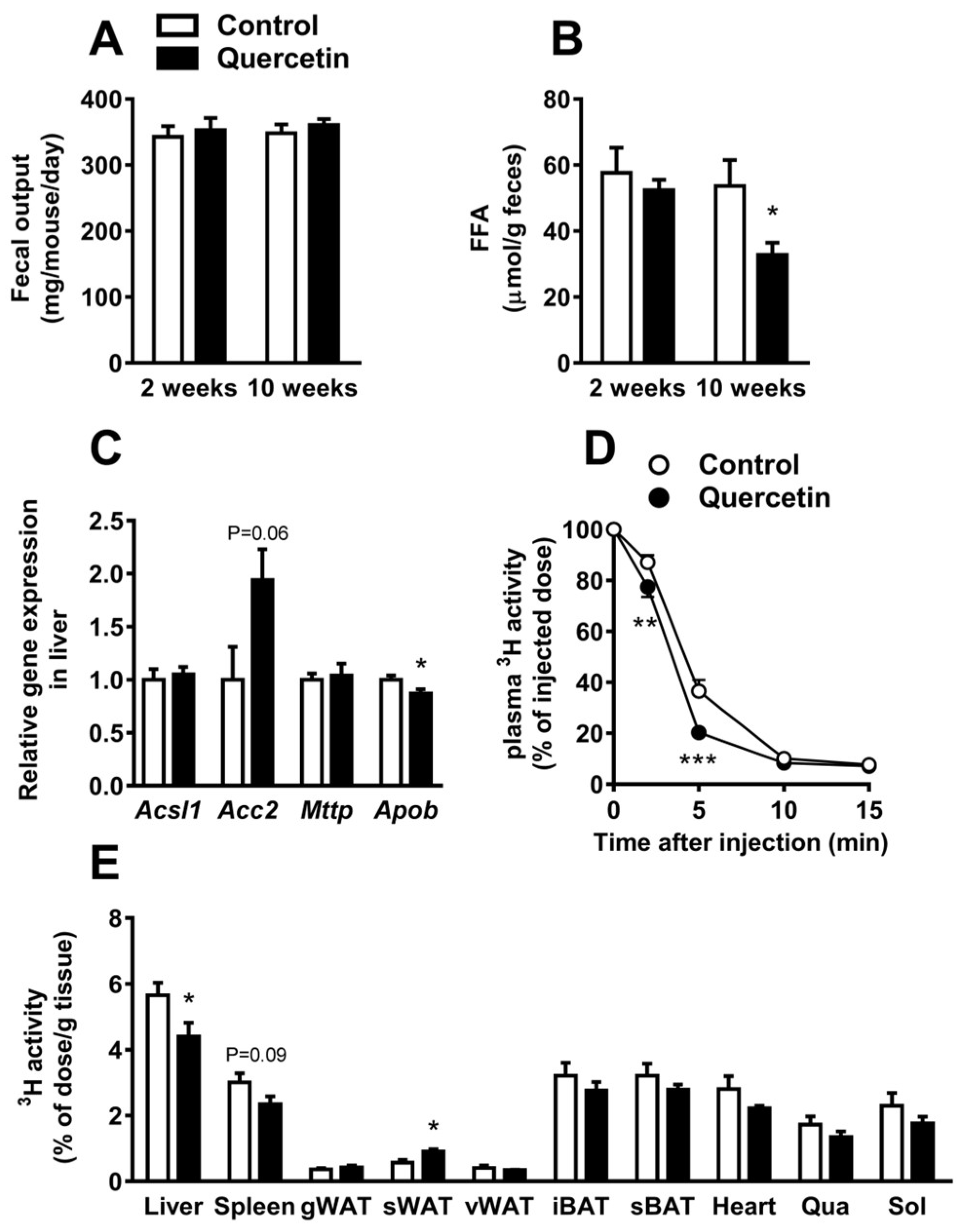
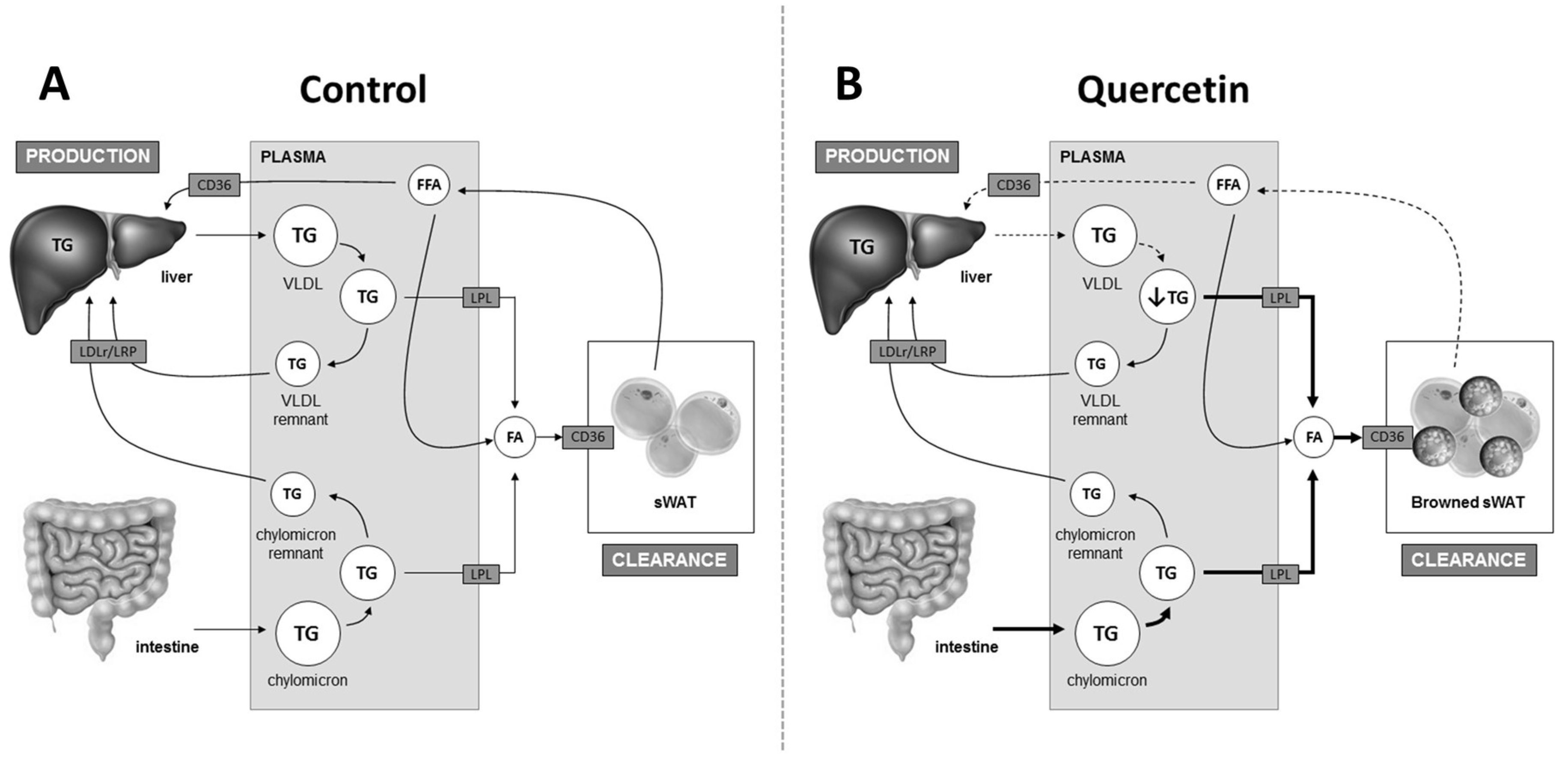
2.2. Quercetin Reduces Hepatic ApoB Expression and Increases Uptake of TG-Derived FA by sWAT
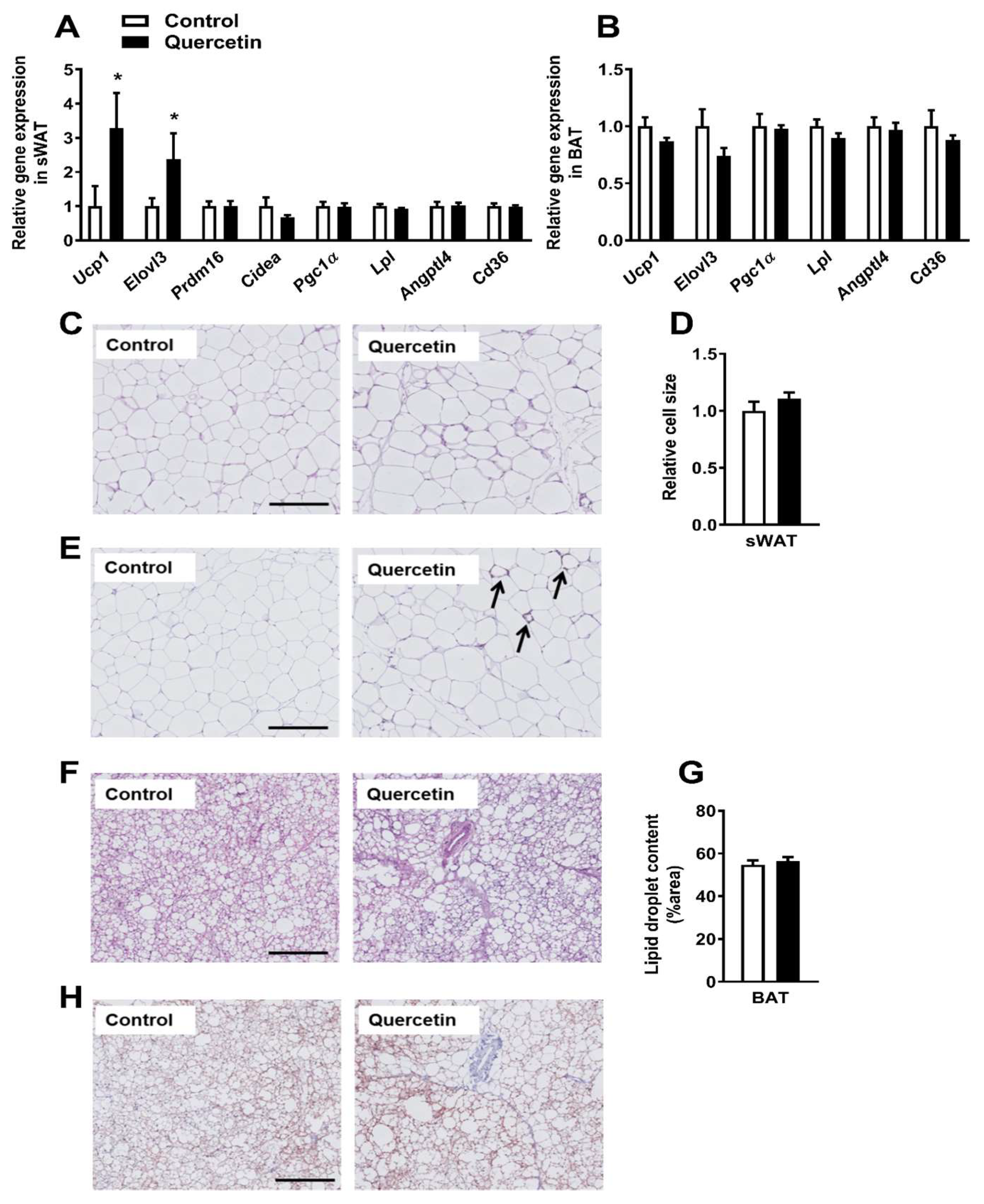
2.3. Quercetin Increases Ucp1 Gene Expression Specifically in sWAT
3. Discussion
4. Materials and Methods
4.1. Animals and Diet
4.2. Body Weight, Body Composition and Food Intake
4.3. Plasma Parameters
4.4. Lipoprotein Profiles
4.5. Indirect Calorimetry and Physical Activity
4.6. Feces Collection and Fecal FFA Concentration
4.7. RNA Isolation and qRT-PCR Analysis
4.8. In Vivo Clearance of Radiolabeled Lipoprotein-Like Particles
4.9. Histology
4.10. Quantification of Lipid Content in Liver
4.11. Protein Isolation and Western Blot
4.12. Determination of mtDNA/nDNA Ratio
4.13. Statistical Analysis
Supplementary Materials
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC2; Acc2 | acetyl-CoA carboxylase 2 |
| ACSL1; Acsl1 | long-chain-fatty-acid-CoA ligase 1 |
| AMPK | AMP-activated protein kinase |
| APOB; Apob | apolipoprotein B |
| ANGPTL4; Angptl4 | angiopoietin-like 4 |
| (i)BAT | (interscapular) brown adipose tissue |
| CD36; Cd36 | fatty acid translocase |
| EE | energy expenditure |
| ELOVL3; Elovl3 | fatty acid elongase 3 |
| (F)FA | (free) fatty acids |
| HFD | high fat diet |
| LDLr | low density lipoprotein receptor |
| LPL; Lpl | lipoprotein lipase |
| LRP | low density lipoprotein receptor-related protein |
| MTP; Mttp | microsomal triglyceride transfer protein |
| PGC1α; Pgc1α | peroxisome proliferator-activated receptor γ coactivator 1α |
| PXR; Pxr | pregnane X receptor |
| RER | resting energy expenditure |
| SIRT1 | sirtuin 1 |
| TC | total cholesterol |
| TG | triglyceride |
| TO | triolein |
| UCP-1; Ucp1 | uncoupling protein-1 |
| VLDL | very-low density lipoprotein |
| (g,s,v)WAT | (gonadal, subcutaneous, visceral) white adipose tissue |
References
- Knight, J.A. Diseases and disorders associated with excess body weight. Ann. Clin. Lab. Sci. 2011, 41, 107–121. [Google Scholar] [PubMed]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ. Res. 2016, 118, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.J.; Dwyer, J.T.; Jacques, P.F.; McCullough, M.L. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr. Rev. 2012, 70, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ward, N.C.; Hodgson, J.M.; Puddey, I.B.; Wang, Y.; Zhang, D.; Maghzal, G.J.; Stocker, R.; Croft, K.D. Dietary quercetin attenuates oxidant-induced endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: A critical role for heme oxygenase-1. Free Radic. Biol. Med. 2013, 65, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Masumoto, S.; Akimoto, Y.; Oike, H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol. Nutr. Food Res. 2011, 55, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Kobori, M.; Takahashi, Y.; Sakurai, M.; Akimoto, Y.; Tsushida, T.; Oike, H.; Ippoushi, K. Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.; Nistal, E.; Martinez-Florez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; Gonzalez-Gallego, J.; Garcia-Mediavilla, M.V.; Sanchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2016, 102, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Kwon, Y.; Choe, S.Y.; Hong, S.M.; Yoo, H.; Goto, T.; Kawada, T.; Choi, H.S.; Joe, Y.; Chung, H.T.; et al. Quercetin reduces obesity-induced hepatosteatosis by enhancing mitochondrial oxidative metabolism via heme oxygenase-1. Nutr. Metab. 2015, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Cho, I.; Ahn, J.; Jeon, T.I.; Ha, T.Y. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother. Res. 2013, 27, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Hoek-van den Hil, E.F.; Keijer, J.; Bunschoten, A.; Vervoort, J.J.; Stankova, B.; Bekkenkamp, M.; Herreman, L.; Venema, D.; Hollman, P.C.; Tvrzicka, E.; et al. Quercetin induces hepatic lipid omega-oxidation and lowers serum lipid levels in mice. PLoS ONE 2013, 8, e51588. [Google Scholar] [CrossRef]
- Sahebkar, A. Effects of quercetin supplementation on lipid profile: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2017, 57, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Parks, J.S.; Kang, H.W. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J. Nutr. Biochem. 2017, 42, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Rosell, M.; Kaforou, M.; Frontini, A.; Okolo, A.; Chan, Y.W.; Nikolopoulou, E.; Millership, S.; Fenech, M.E.; MacIntyre, D.; Turner, J.O.; et al. Brown and white adipose tissues: Intrinsic differences in gene expression and response to cold exposure in mice. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E945–E964. [Google Scholar] [CrossRef] [PubMed]
- Berbee, J.F.; Boon, M.R.; Khedoe, P.P.; Bartelt, A.; Schlein, C.; Worthmann, A.; Kooijman, S.; Hoeke, G.; Mol, I.M.; John, C.; et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015, 6, 6356. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, S.; Wang, Y.; Parlevliet, E.T.; Boon, M.R.; Edelschaap, D.; Snaterse, G.; Pijl, H.; Romijn, J.A.; Rensen, P.C. Central GLP-1 receptor signalling accelerates plasma clearance of triacylglycerol and glucose by activating brown adipose tissue in mice. Diabetologia 2015, 58, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Festuccia, W.T.; Soucy, G.; Blanchard, P.G.; Renaud, A.; Berger, J.P.; Olivecrona, G.; Deshaies, Y. Tissue-specific postprandial clearance is the major determinant of PPARγ-induced triglyceride lowering in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R57–R66. [Google Scholar] [CrossRef] [PubMed]
- Khedoe, P.P.; Hoeke, G.; Kooijman, S.; Dijk, W.; Buijs, J.T.; Kersten, S.; Havekes, L.M.; Hiemstra, P.S.; Berbee, J.F.; Boon, M.R.; et al. Brown adipose tissue takes up plasma triglycerides mostly after lipolysis. J. Lipid Res. 2015, 56, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bakker, L.E.; Boon, M.R.; van der Linden, R.A.; Arias-Bouda, L.P.; van Klinken, J.B.; Smit, F.; Verberne, H.J.; Jukema, J.W.; Tamsma, J.T.; Havekes, L.M.; et al. Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: A prospective, case-controlled observational study. Lancet Diabetes Endocrinol. 2014, 2, 210–217. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Porras, A.; Alvarez, A.M.; Valladares, A.; Benito, M. TNF-α induces apoptosis in rat fetal brown adipocytes in primary culture. FEBS Lett. 1997, 416, 324–328. [Google Scholar] [CrossRef]
- Couto, M.R.; Goncalves, P.; Catarino, T.; Araujo, J.R.; Correia-Branco, A.; Martel, F. The effect of oxidative stress upon the intestinal uptake of folic acid: In vitro studies with Caco-2 cells. Cell Biol. Toxicol. 2012, 28, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, G.V.; Paglialonga, G.; Siculella, L. Quercetin inhibits fatty acid and triacylglycerol synthesis in rat-liver cells. Eur. J. Clin. Investig. 2009, 39, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Miranda, J.; Portillo, M.P. Liver delipidating effect of a combination of resveratrol and quercetin in rats fed an obesogenic diet. J. Physiol. Biochem. 2015, 71, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Milton, I.; Portillo, M.P. The combination of resveratrol and quercetin enhances the individual effects of these molecules on triacylglycerol metabolism in white adipose tissue. Eur. J. Nutr. 2016, 55, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Labbe, S.M.; Caron, A.; Bakan, I.; Laplante, M.; Carpentier, A.C.; Lecomte, R.; Richard, D. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J. 2015, 29, 2046–2058. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Browning of white adipose tissue: Lessons from experimental models. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.M.; Grunberg, J.R.; Church, C.; Elias, I.; Palsdottir, V.; Jansson, J.O.; Bosch, F.; Hammarstedt, A.; Hedjazifar, S.; Smith, U. BMP4 Gene Therapy in Mature Mice Reduces BAT Activation but Protects from Obesity by Browning Subcutaneous Adipose Tissue. Cell Rep. 2017, 20, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Ma, Y.; Chanturiya, T.; Cao, Q.; Wang, Y.; Kadegowda, A.K.G.; Jackson, R.; Rumore, D.; Xue, B.; Shi, H.; et al. Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab. 2017, 26, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, S.; Tran, Q.T.; Harvey, I.; Smallwood, H.S.; Thiyagarajan, T.; Banerjee, S.; Johnson, D.L.; Dalton, J.T.; Sullivan, R.D.; Miller, D.D.; et al. Pharmacologic activation of estrogen receptor β increases mitochondrial function, energy expenditure, and brown adipose tissue. FASEB J. 2017, 31, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.F.; Su, W.; Dai, Y.B.; Wu, W.F.; Huang, B.; Barros, R.P.; Nguyen, H.; Maneix, L.; Guan, Y.F.; Warner, M.; et al. An ERβ agonist induces browning of subcutaneous abdominal fat pad in obese female mice. Sci. Rep. 2016, 6, 38579. [Google Scholar] [CrossRef] [PubMed]
- Weir, J.B. New methods for calculating metabolic rate with special reference to protein metabolism. J. Physiol. 1949, 109, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Van Klinken, J.B.; van den Berg, S.A.; Havekes, L.M.; Willems Van Dijk, K. Estimation of activity related energy expenditure and resting metabolic rate in freely moving mice from indirect calorimetry data. PLoS ONE 2012, 7, e36162. [Google Scholar] [CrossRef] [PubMed]
- van Dam, A.D.; Nahon, K.J.; Kooijman, S.; van den Berg, S.M.; Kanhai, A.A.; Kikuchi, T.; Heemskerk, M.M.; van Harmelen, V.; Lombes, M.; van den Hoek, A.M.; et al. Salsalate activates brown adipose tissue in mice. Diabetes 2015, 64, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Rensen, P.C.; van Dijk, M.C.; Havenaar, E.C.; Bijsterbosch, M.K.; Kruijt, J.K.; van Berkel, T.J. Selective liver targeting of antivirals by recombinant chylomicrons—A new therapeutic approach to hepatitis B. Nat. Med. 1995, 1, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Boon, M.R.; Kooijman, S.; van Dam, A.D.; Pelgrom, L.R.; Berbee, J.F.; Visseren, C.A.; van Aggele, R.C.; van den Hoek, A.M.; Sips, H.C.; Lombes, M.; et al. Peripheral cannabinoid 1 receptor blockade activates brown adipose tissue and diminishes dyslipidemia and obesity. FASEB J. 2014, 28, 5361–5375. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, J.M.; Ramakers, C.; Hoogaars, W.M.; Karlen, Y.; Bakker, O.; van den Hoff, M.J.; Moorman, A.F. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009, 37, e45. [Google Scholar] [CrossRef] [PubMed]
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| β2-microglobulin | TGACCGGCTTGTATGCTATC | CAGTGTGAGCCAGGATATAG |
| Angptl4 | GGAAAGAGGCTTCCCAAGAT | TCCCAGGACTGGTTGAAGTC |
| Apob | GCCCATTGTGGACAAGTTGATC | CCAGGACTTGGAGGTCTTGGA |
| Acc2 | AGATGGCCGATCAGTACGTC | GGGGACCTAGGAAAGCAATC |
| Acsl1 | TGCCAGAGCTGATTGACATTC | GGCATACCAGAAGGTGGTGAG |
| Cd36 | GCAAAGAACAGCAGCAAAATC | CAGTGAAGGCTCAAAGATGG |
| Cidea | CTCGGCTGTCTCAATGTCAA | CCGCATAGACCAGGAACTGT |
| Cyp3a11 | CTTTCCTTCACCCTGCATTCC | CTCATCCTGCAGTTTTTTCTGGAT |
| Elovl3 | GGATGACGCCGTAGTCAGTA | GACAGAATGGACGCCAAAGT |
| Gapdh | GGGGCTGGCATTGCTCTCAA | TTGCTCAGTGTCCTTGCTGGGG |
| Hprt | TTGCTCGAGATGTCATGAAGGA | AGCAGGTCAGCAAAGAACTTATAG |
| Lpl | CCCTAAGGACCCCTGAAGAC | GGCCCGATACAACCAGTCTA |
| Mttp | CTCTTGGCAGTGCTTTTTCTCT | GAGCTTGTATAGCCGCTCATT |
| Pgc1a | TGCTAGCGGTTCTCACAGAG | AGTGCTAAGACCGCTGCATT |
| Prdm16 | ACTTTGGATGGGAGCAGATG | CTCCAGGCTCGATGTCCTTA |
| Pxr | GAGCGGAGAAGACGGCAGCATC | CCCAGGTTCCCGTTTCCGTGTC |
| Ucp1 | TCAGGATTGGCCTCTACGAC | TGCATTCTGACCTTCACGAC |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| 16S | CCGCAAGGGAAAGATGAAAGAC | TCGTTTGGTTTCGGGGTTTC |
| Cox2 | GTTGATAACCGAGTCGTTCTGC | CCTGGGATGGCATCAGTTTT |
| Hk2 | TCTGGCTCTGAGATCCATCTTCA | CCGGCCTCTTAACCACATTCC |
| Ucp2 | CTACAGATGTGGTAAAGGTCCGC | GCAATGGTCTTGTAGGCTTCG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuipers, E.N.; Dam, A.D.v.; Held, N.M.; Mol, I.M.; Houtkooper, R.H.; Rensen, P.C.N.; Boon, M.R. Quercetin Lowers Plasma Triglycerides Accompanied by White Adipose Tissue Browning in Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 19, 1786. https://doi.org/10.3390/ijms19061786
Kuipers EN, Dam ADv, Held NM, Mol IM, Houtkooper RH, Rensen PCN, Boon MR. Quercetin Lowers Plasma Triglycerides Accompanied by White Adipose Tissue Browning in Diet-Induced Obese Mice. International Journal of Molecular Sciences. 2018; 19(6):1786. https://doi.org/10.3390/ijms19061786
Chicago/Turabian StyleKuipers, Eline N., Andrea D. van Dam, Ntsiki M. Held, Isabel M. Mol, Riekelt H. Houtkooper, Patrick C.N. Rensen, and Mariëtte R. Boon. 2018. "Quercetin Lowers Plasma Triglycerides Accompanied by White Adipose Tissue Browning in Diet-Induced Obese Mice" International Journal of Molecular Sciences 19, no. 6: 1786. https://doi.org/10.3390/ijms19061786
APA StyleKuipers, E. N., Dam, A. D. v., Held, N. M., Mol, I. M., Houtkooper, R. H., Rensen, P. C. N., & Boon, M. R. (2018). Quercetin Lowers Plasma Triglycerides Accompanied by White Adipose Tissue Browning in Diet-Induced Obese Mice. International Journal of Molecular Sciences, 19(6), 1786. https://doi.org/10.3390/ijms19061786