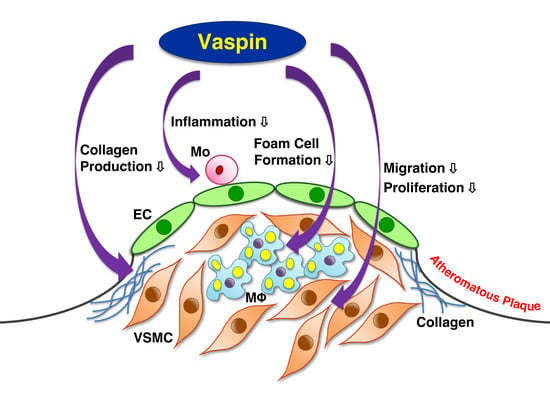
Anti-Atherogenic Effects of Vaspin on Human Aortic Smooth Muscle Cell/Macrophage Responses and Hyperlipidemic Mouse Plaque Phenotype
Abstract
1. Introduction
2. Results
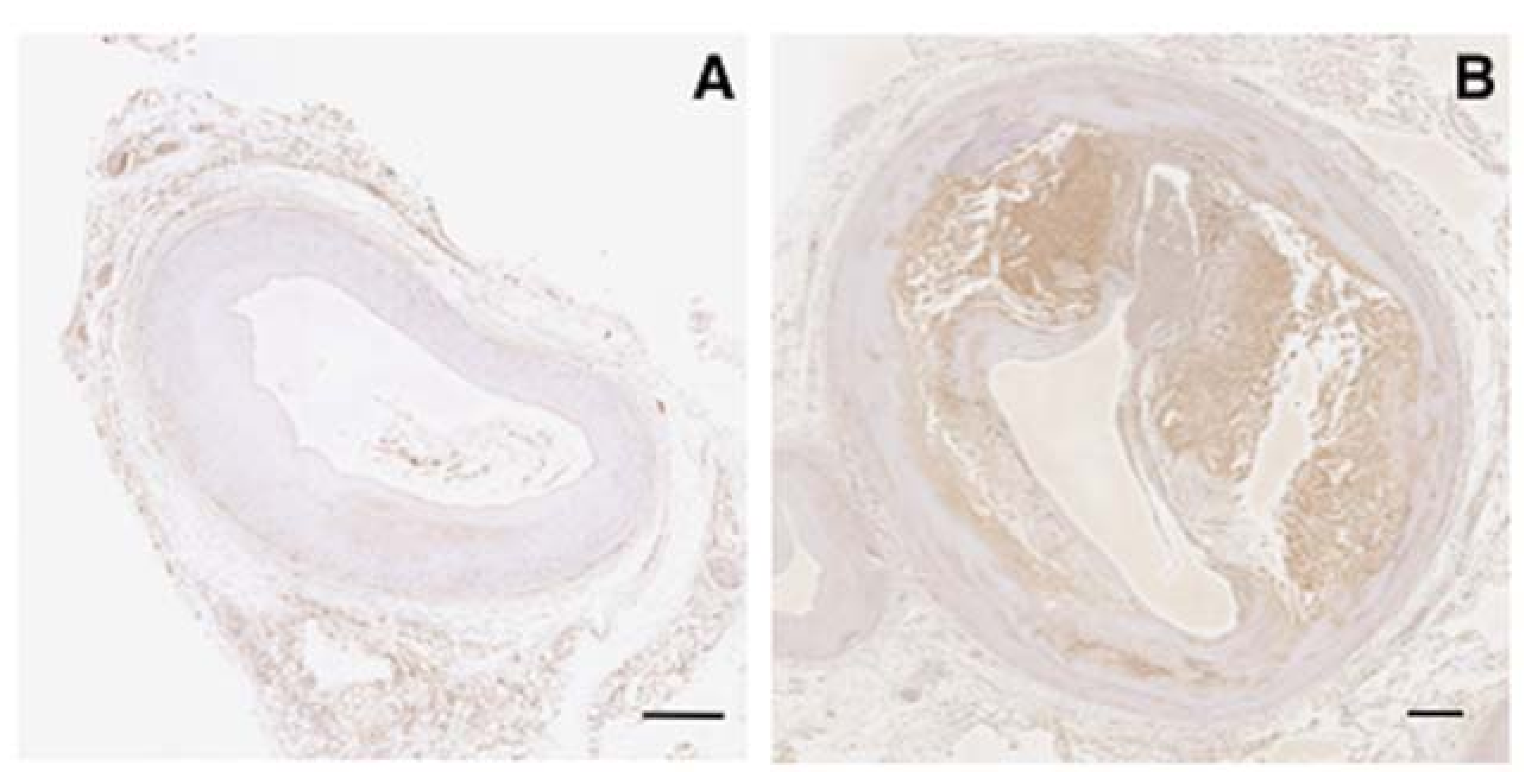
2.1. Expression of Vaspin in Human Coronary Atherosclerosis
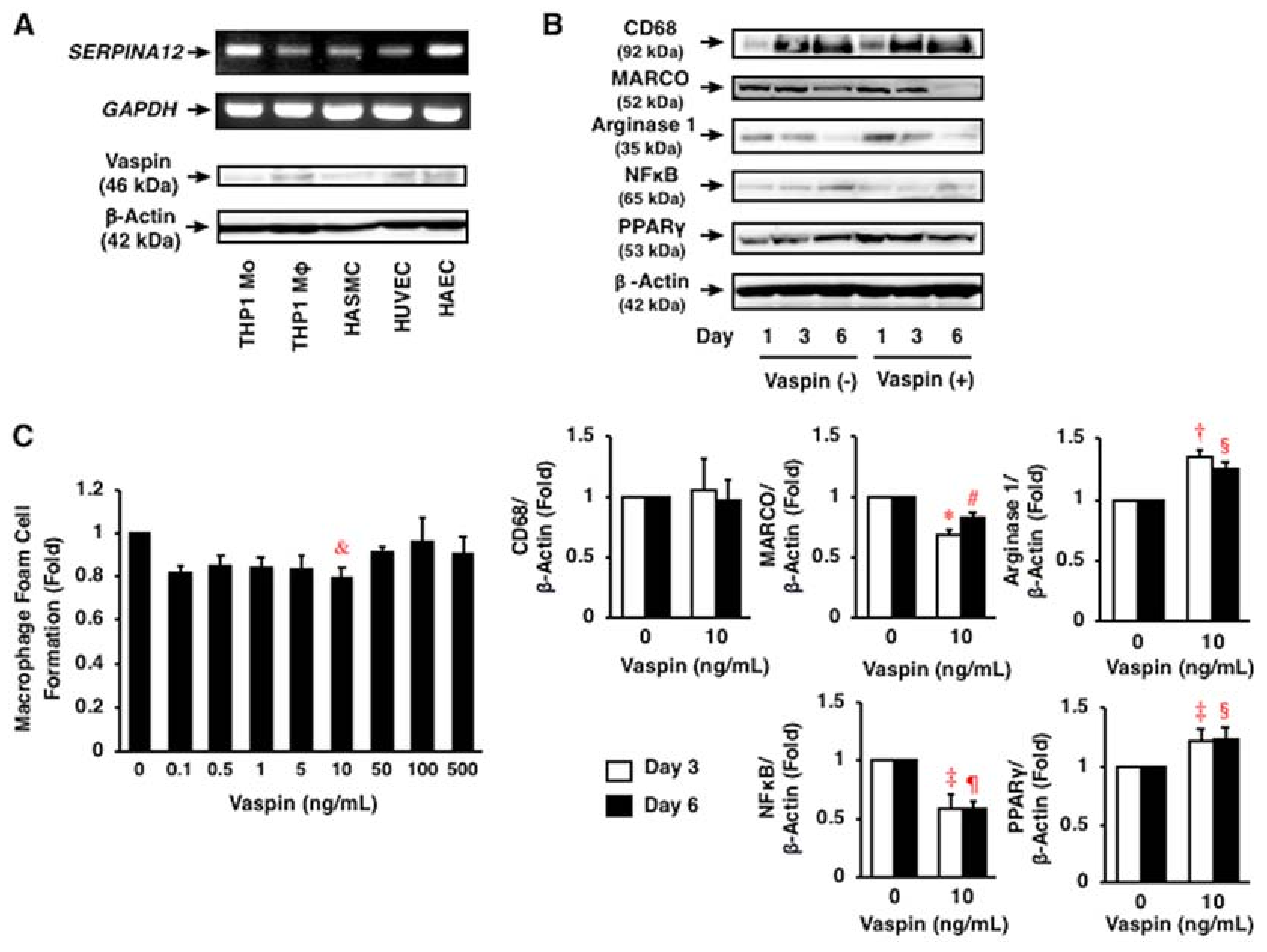
2.2. Expression of Vaspin in Human Vascular Cells
2.3. Effects of Vaspin on Inflammatory Phenotype in Human Monocytes/Macrophages
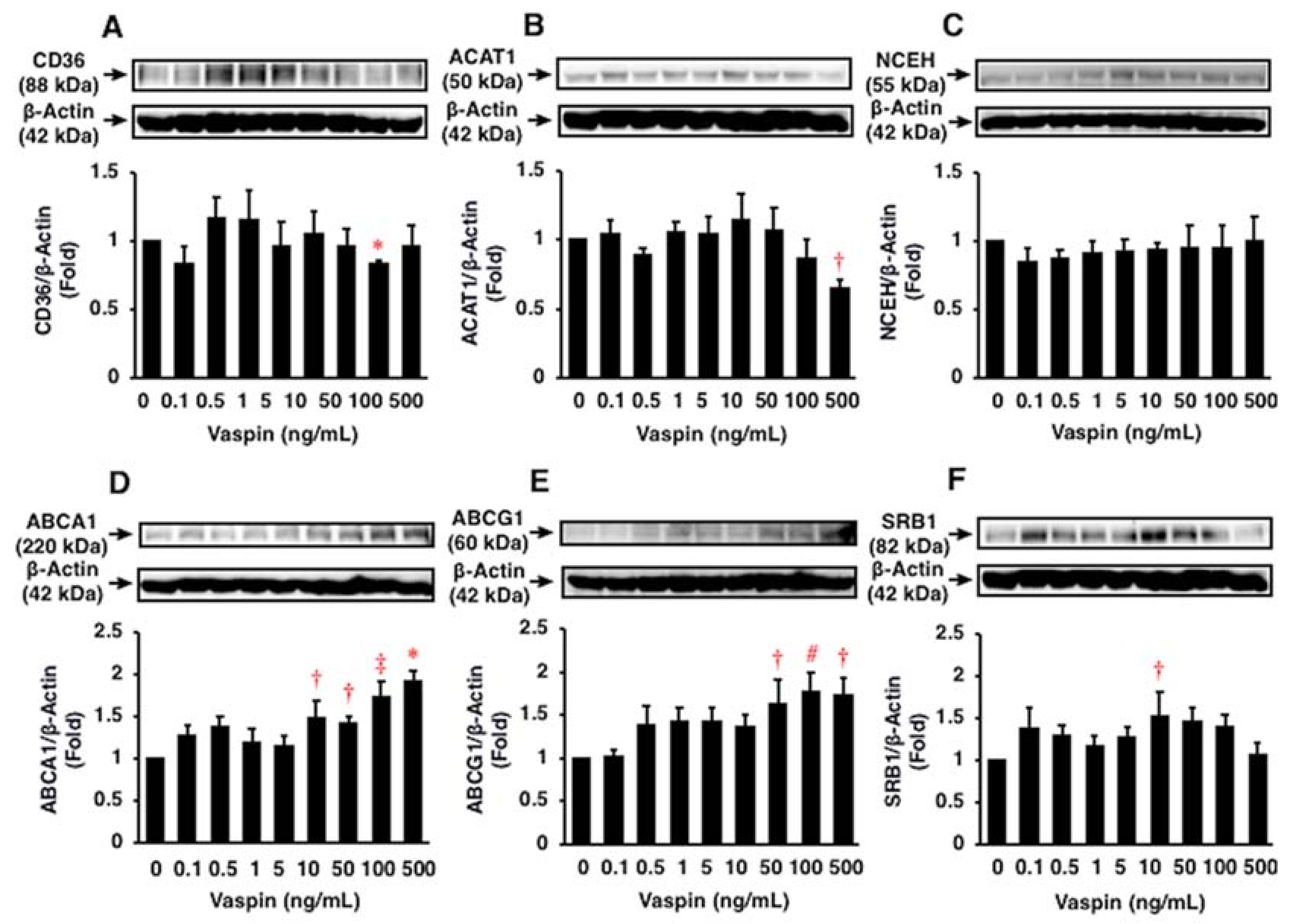
2.4. Effects of Vaspin on Human Macrophage Foam Cell Formation
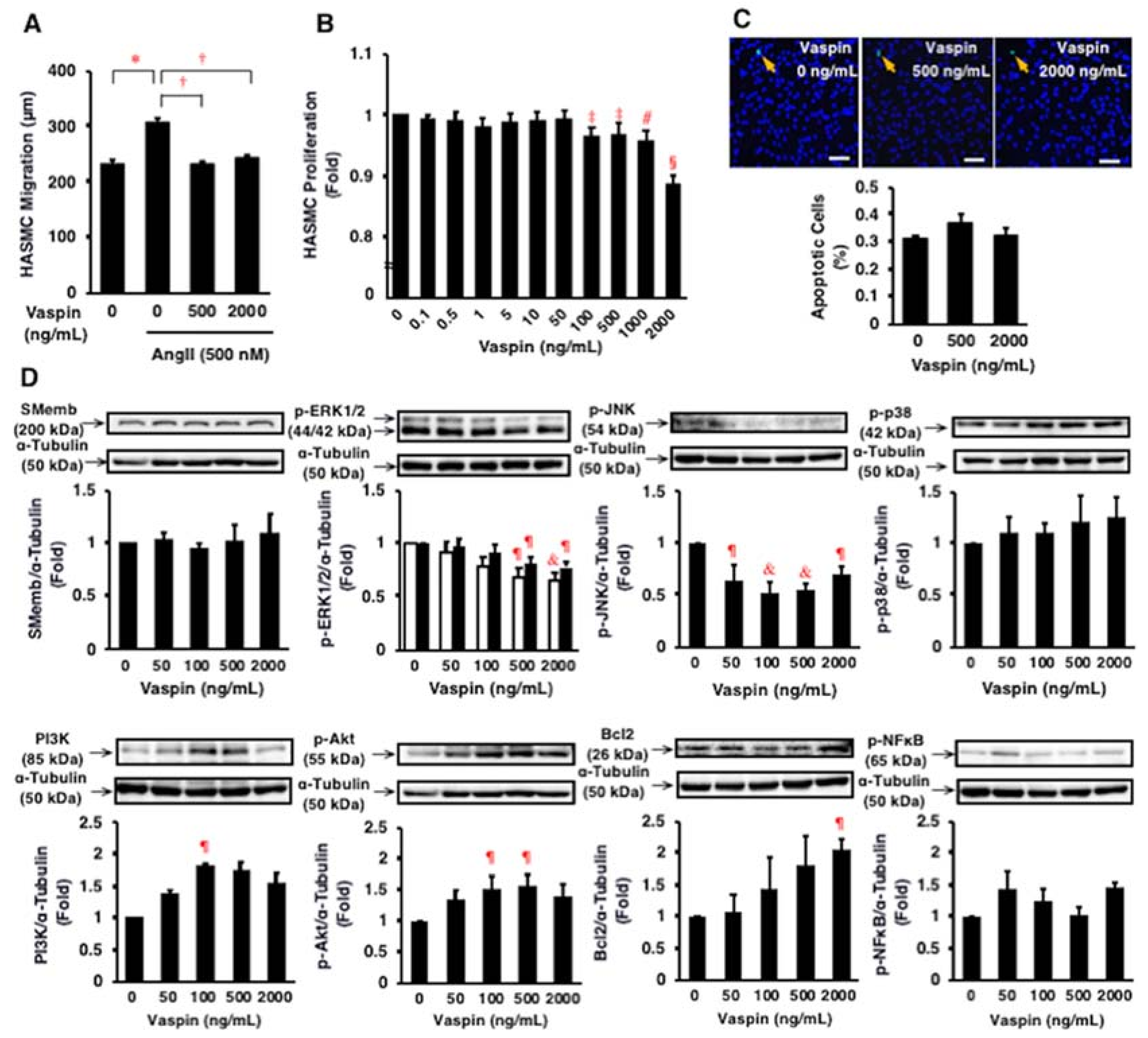
2.5. Effects of Vaspin on Migration and Proliferation of HASMCs
2.6. Effects of Vaspin on Intracellular Signaling in HASMCs
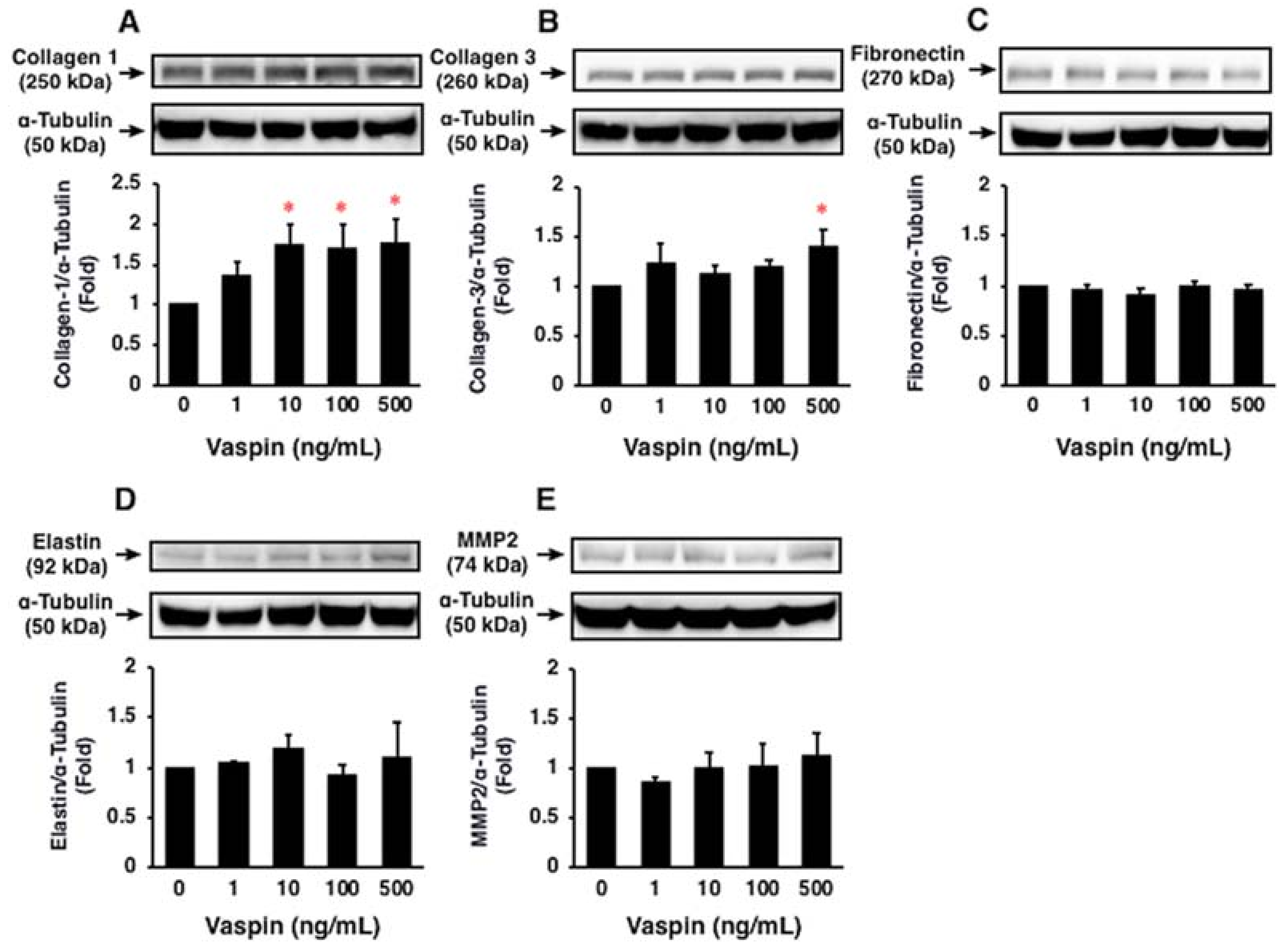
2.7. Effects of Vaspin on ECM Expression in HASMCs
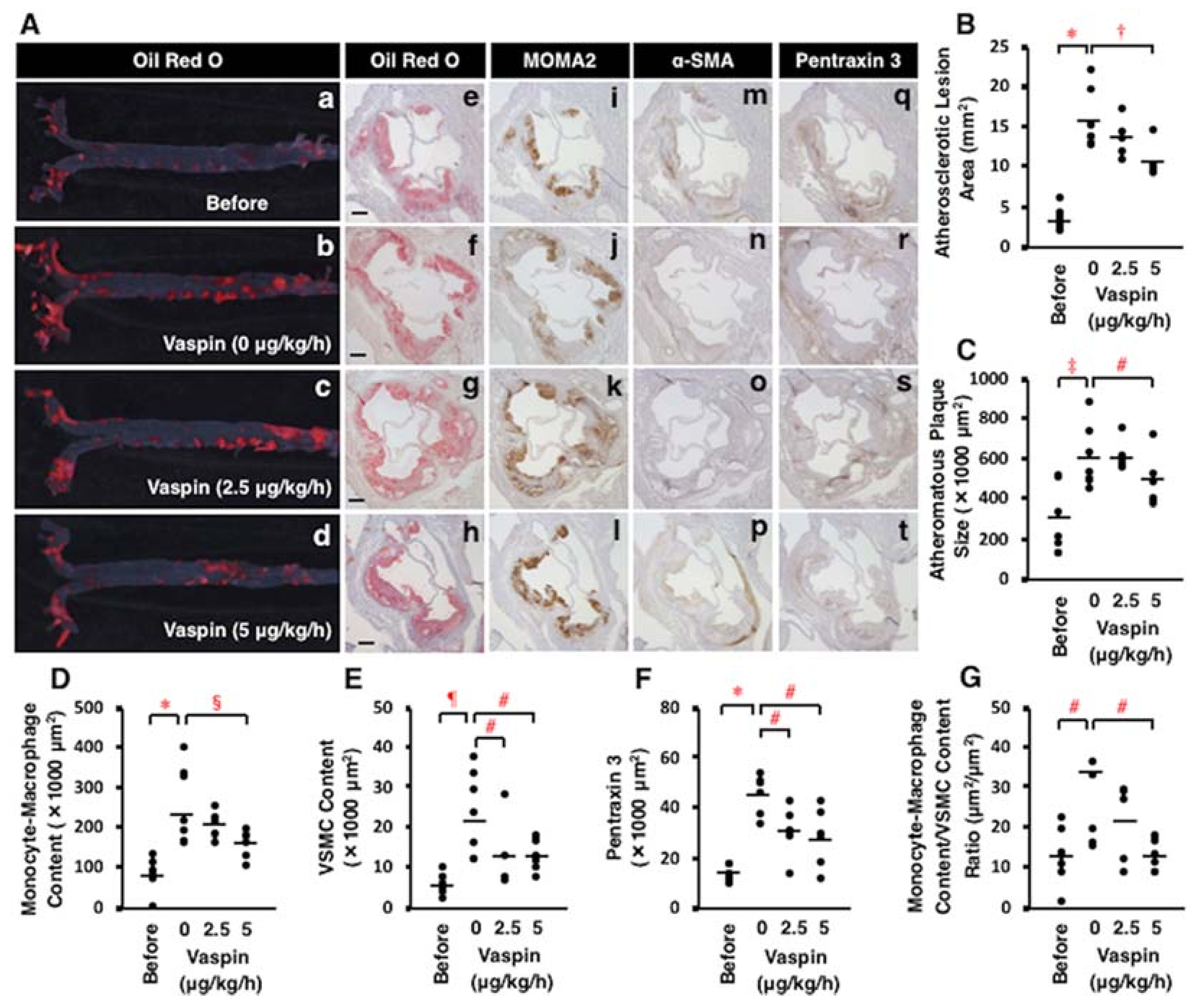
2.8. Effects of Vaspin on Atherosclerotic Lesion Development in Apoe−/− Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Human Coronary Artery Immunostaining
4.3. Migration Assay
4.4. Proliferation Assay
4.5. Apoptosis Assay
4.6. Foam Cell Formation Assay
4.7. Western Blotting
4.8. Administration of Vaspin into Mice
4.9. Animal Measurements
4.10. Assessment of Atherosclerotic Lesions
4.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter A1 |
| ABCG1 | ATP-binding cassette transporter G1 |
| ACAT1 | Acyl-coenzyme A: cholesterol acyltransferase 1 |
| CAD | Coronary artery disease |
| ECM | Extracellular matrix |
| ER | Endoplasmic reticulum |
| HAEC | Human aortic endothelial cell |
| HASMC | Human aortic smooth muscle cell |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| HUVEC | Human umbilical vein endothelial cell |
| MCM | Monocyte/macrophage conditioning medium |
| MMP | Matrix metalloproteinase |
| NCEH | Neutral cholesterol ester hydrolase |
| NFκB | Nuclear factor κB |
| LDL | Low-density lipoprotein |
| PI3K | Phosphoinositide 3-kinase |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| ROS | Reactive oxygen species |
| SRB1 | Scavenger receptor class B type 1 |
| VSMC | vascular smooth muscle cell |
References
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; Bornfeldt, K.E. Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.H.; Fu, Y.C.; Zhang, D.W.; Yin, K.; Tang, C.K. Foam cells in atherosclerosis. Clin. Chim. Acta 2013, 424, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Osuga, J.; Uozaki, H.; Sekiya, M.; Nagashima, S.; Takahashi, M.; Takase, S.; Takanashi, M.; Li, Y.; Ohta, K.; et al. The critical role of neutral cholesterol ester hydrolase 1 in cholesterol removal from human macrophages. Circ. Res. 2010, 107, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Rudijanto, A. The role of vascular smooth muscle cells on the pathogenesis of atherosclerosis. Acta Med. Indones. 2007, 39, 86–93. [Google Scholar] [PubMed]
- Businaro, R.; Tagliani, A.; Buttari, B.; Profumo, E.; Ippoliti, F.; di Cristofano, C.; Capoano, R.; Salvati, B.; Riganò, R. Cellular and molecular players in the atherosclerotic plaque progression. Ann. N. Y. Acad. Sci. 2012, 1262, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Berndt, J.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Vaspin gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 2006, 339, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Heutling, D.; Chen, J.; Farhatullah, S.; Adya, R.; Keay, S.D.; Kennedy, C.R.; Lehnert, H.; Randeva, H.S. Metformin decreases the adipokine vaspin in overweight women with polycystic ovary syndrome concomitant with improvement in insulin sensitivity and a decrease in insulin resistance. Diabetes 2008, 57, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Peng, W.H.; Cui, S.T.; Lei, H.; Wei, Y.D.; Li, W.M.; Xu, Y.W. Vaspin plasma concentrations and mRNA expressions in patients with stable and unstable angina pectoris. Clin. Chem. Lab. Med. 2011, 49, 1547–1554. [Google Scholar] [PubMed]
- Spiroglou, S.G.; Kostopoulos, C.G.; Varakis, J.N.; Papadaki, H.H. Adipokines in periaortic and epicardial adipose tissue: Differential expression and relation to atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Kovacs, P.; Kern, M.; Heiker, J.T.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Beck-Sickinger, A.G.; Blüher, M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011, 54, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Patel, K.; Moore, A.E.; Dulnoan, D.; Edwards, S.; Hampson, G. The relationship between circulating adiponectin, leptin and vaspin with bone mineral density (BMD), arterial calcification and stiffness: A cross-sectional study in post-menopausal women. J. Endocrinol. Investig. 2017, 40, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Phalitakul, S.; Okada, M.; Hara, Y.; Yamawaki, H. Vaspin prevents methyglyoxal-induced apoptosis in human vascular endothelial cells by inhibiting reactive oxygen species generation. Acta Physiol. 2013, 209, 212–219. [Google Scholar]
- Liu, S.; Dong, Y.; Wang, T.; Zhao, S.; Yang, K.; Chen, X.; Zheng, C. Vaspin inhibited proinflammatory cytokine induced activation of nuclear factor-κB and its downstream molecules in human endothelial EA.hy926 cells. Diabetes Res. Clin. Pract. 2014, 103, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; Lee, Y.L.; Yoon, H.K.; Kang, S.W.; Lee, W.J.; Park, J.Y. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc. Diabetol. 2014, 13, 41. [Google Scholar] [CrossRef] [PubMed]
- Phalitakul, S.; Okada, M.; Hara, Y.; Yamawaki, H. A novel adipocytokine, vaspin inhibits platelet-derived growth factor-BB-induced migration of vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2012, 423, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Peng, W.; Zhuang, J.; Lu, Y.; Jian, W.; Wei, Y.; Li, W.; Xu, Y. Vaspin attenuates high glucose-induced vascular smooth muscle cells proliferation and chemokinesis by inhibiting the MAPK, PI3K/Akt, and NF-κB signaling pathways. Atherosclerosis 2013, 228, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.H.; Zeng, M.Y.; Yu, X.H.; Zeng, G.F.; He, L.H.; Zheng, X.L.; Zhang, D.W.; Ouyang, X.P.; Tang, C.K. Visceral adipose tissue-derived serine protease inhibitor accelerates cholesterol efflux by up-regulating ABCA1 expression via the NF-κB/miR-33a pathway in THP-1 macropahge-derived foam cells. Biochem. Biophys. Res. Commun. 2018, 500, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 2012, 61, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhuang, J.; Li, H.; Zhu, G.; Zhou, S.; Li, W.; Peng, W.; Xu, Y. Vaspin attenuates the progression of atherosclerosis by inhibiting ER stress-induced macrophage apoptosis in apoE−/− mice. Mol. Med. Rep. 2016, 13, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Visceral adipose tissue-derived serine proteinase inhibitor inhibits apoptosis of endothelial cells as a ligand for the cell-surface GRP78/voltage-dependent anion channel complex. Circ. Res. 2013, 112, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Youn, B.S.; Klöting, N.; Kratzsch, J.; Lee, N.; Park, J.W.; Song, E.S.; Ruschke, K.; Oberbach, A.; Fasshauer, M.; Stumvoll, M.; Blüher, M. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008, 57, 372–377. [Google Scholar] [CrossRef] [PubMed]
- El-Mesallamy, H.O.; Kassem, D.H.; El-Demerdash, E.; Amin, A.I. Vaspin and visfatin/Nampt are interesting interrelated adipokines playing a role in the pathogenesis of type 2 diabetes mellitus. Metabolism 2011, 60, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Esteghamati, A.; Noshad, S.; Mousavizadeh, M.; Zandieh, A.; Nakhjavani, M. Association of vaspin with metabolic syndrome: The pivotal role of insulin resistance. Diabetes Metab. J. 2014, 38, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Kwak, S.H.; Lee, Y.; Moon, M.K.; Lim, S.; Park, Y.J.; Jang, H.C.; Kim, M.S. Plasma vaspin concentrations are elevated in metabolic syndrome in men and are correlated with coronary atherosclerosis in women. Clin. Endocrinol. 2011, 75, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Sathyaseelan, A.J.; Adole, P.S.; Wyawahare, M.; Saya, R.P. Assessment of serum vaspin levels among type 2 diabetes mellitus patients with or without acute coronary syndrome. J. Clin. Diagn. Res. 2016, 10, BC07–BC10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Peng, W.; Li, H.; Lu, Y.; Zhuang, J.; Wang, K.; Su, Y.; Xu, Y. Plasma vaspin concentrations are decreased in acute coronary syndrome, but unchanged in patients without coronary lesions. Clin. Biochem. 2013, 46, 1520–1525. [Google Scholar] [CrossRef] [PubMed]
- Kobat, M.A.; Celik, A.; Balin, M.; Altas, Y.; Baydas, A.; Bulut, M.; Aydin, S.; Dagli, N.; Yavuzkir, M.F.; Ilhan, S. The investigation of serum vaspin level in atherosclerotic coronary artery disease. J. Clin. Med. Res. 2012, 4, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Gkontopoulos, A.; Kapelouzou, A.; Fotiadis, G.; Theofilogiannakos, E.K.; Kottas, G.; Lampropoulos, S. Serum levels of vaspin and visfatin in patients with coronary artery disease–Kozani study. Clin. Chim. Acta 2011, 412, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, X.; Wu, Y.; Duan, R.; Zhang, J.; Du, F.; Zhang, Q.; Li, Y.; Li, N. Effects of vaspin on pancreatic β cell secretion via PI3K/Akt and NF-κB signaling pathways. PLoS ONE 2017, 12, e0189722. [Google Scholar] [CrossRef] [PubMed]
- Heiker, J.H.; Klöting, N.; Kovacs, P.; Kuettner, E.B.; Sträter, N.; Schultz, S.; Kern, M.; Stumvoll, M.; Blüher, M.; Beck-Sickinger, A.G. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell. Mol. Life Sci. 2013, 70, 2569–2583. [Google Scholar] [CrossRef] [PubMed]
- Zieger, K.; Weiner, J.; Krause, K.; Schwarz, M.; Kohn, M.; Stumvoll, M.; Blüher, M.; Heiker, J.T. Vaspin suppresses cytokine-induced inflammation in 3T3-L1 adipocytes via inhibition of NFκB pat hway. Mol. Cell. Endocrinol. 2018, 460, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, W.J.; Hwang, J.Y.; Seol, S.M.; Kim, Y.M.; Lee, Y.L.; Park, J.Y. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem. Biophys. Res. Commun. 2011, 413, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, H.; Wang, L. Vaspin alleviates dysfunction of endothelial progenitor cells induced by high glucose via PI3K/Akt/eNOS pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 482–489. [Google Scholar] [PubMed]
- Watanabe, K.; Watanabe, R.; Konii, H.; Shirai, R.; Sato, K.; Matsuyama, T.; Ishibashi-Ueda, H.; Koba, S.; Kobayashi, Y.; Hirano, T.; et al. Counteractive effects of omentin-1 against atherogenesis. Cardiovasc. Res. 2016, 110, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Watanabe, H.; Takahashi, Y.; Kojima, M.; Konii, H.; Watanabe, K.; Shirai, R.; Sato, K.; Matsuyama, T.; Ishibashi-Ueda, H.; et al. Atheroprotective effects of tumor necrosis factor-stimulated gene-6. JACC. Basic Transl. Sci. 2016, 6, 494–509. [Google Scholar] [CrossRef]
- Shirai, R.; Sato, K.; Yamashita, T.; Yamaguchi, M.; Okano, T.; Watanabe-Kominato, K.; Watanabe, R.; Matsuyama, T.; Ishibashi-Ueda, H.; Koba, S.; et al. Neopterin counters vascular inflammation and atherosclerosis. J. Am. Heart Assoc. 2018, 7, e007359. [Google Scholar] [CrossRef] [PubMed]
- Loppnow, H.; Libby, P. Adult human vascular endothelial cells express the IL6 gene differentially in response to LPS or IL1. Cell. Immunol. 1989, 122, 493–503. [Google Scholar] [CrossRef]
- Holst, J.J.; Ehrhart-Bornstein, M.; Messell, T.; Poulsen, S.S.; Harling, H. Release of galanin from isolated perfused porcine adrenal glands: Role of splanchnic nerves. Am. J. Physiol. 1991, 261, E31–E40. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Mahdirejei, H.A. Effects of 8 weeks resistance training on plasma vaspin and lipid profile levels in adult men with type 2 diabetes. Caspian J. Intern. Med. 2014, 5, 103–108. [Google Scholar] [PubMed]
- Esteghamati, A.; Mousavizadeh, M.; Noahad, S.; Zandieh, A.; Zarei, H.; Nakhjavani, M. Gender-dependent effects of metformin on vaspin and adiponectin in type 2 diabetes patients: A randomized clinical trial. Horm. Metab. Res. 2013, 45, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Buhadilly, A.K. Rosuvastatin improves vaspin serum levels in obese patients with acute coronary syndrome. Diseases 2018, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Takayama, T.; Hiro, T.; Yoda, S.; Fukamachi, D.; Haruta, H.; Kogo, T.; Mineki, T.; Murata, H.; Oshima, T.; Hirayama, A. Effect of Aggressive lipid-lowering treatment with Rosuvastatin on vascular endoTHelium function: Evaluation of vascular endothelium function (EARTH study). Heart Vessel. 2017, 33, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Naito, C.; Hashimoto, M.; Watanabe, K.; Shirai, R.; Takahashi, Y.; Kojima, M.; Watanabe, R.; Sato, K.; Iso, Y.; Matsuyama, T.; et al. Facilitatory effects of fetuin-A on atherosclerosis. Atherosclerosis 2016, 246, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Ozawa, N.; Mori, Y.; Takahashi, Y.; Watanabe-Kominato, K.; Shirai, R.; Watanabe, R.; Sato, K.; Matsuyama, T.; Ishibashi-Ueda, H.; et al. Catestatin prevents macrophage-driven atherosclerosis but not arterial injury-induced neointimal hyperplasia. Thromb. Haemost. 2018, 118, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Watanabe, R.; Sato, Y.; Ozawa, N.; Kojima, M.; Watanabe-Kominato, K.; Shirai, R.; Sato, K.; Hirano, T.; Watanabe, T. Novel phytopeptide osmotin mimics preventive effects of adiponectin on vascular inflammation and atherosclerosis. Metabolism 2018, 83, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Shirai, R.; Hontani, M.; Shinooka, R.; Hasegawa, A.; Kichise, T.; Yamashita, T.; Yoshizawa, H.; Watanabe, R.; Matsuyama, T.; et al. Potent vasoconstrictor kisspeptin-10 induces atherosclerotic plaque progression and instability: Reversal by its receptor GPR54 antagonist. J. Am. Heart Assoc. 2017, 6, e005790. [Google Scholar] [CrossRef] [PubMed]
- Konii, H.; Sato, K.; Kikuchi, S.; Okiyama, H.; Watanabe, R.; Hasegawa, A.; Yamamoto, K.; Itoh, F.; Hirano, T.; Watanabe, T. Stimulatory effects of cardiotrophin 1 on atherosclerosis. Hypertension 2013, 62, 942–950. [Google Scholar] [CrossRef] [PubMed]

| Parameter | 17 Weeks Old | 21 Weeks Old | ||
|---|---|---|---|---|
| Before | Vaspin 0 μg/kg/h | Vaspin 2.5 μg/kg/h | Vaspin 5.0 μg/kg/h | |
| N | 7 | 7 | 5 | 6 |
| Body weight (g) | 26.2 ± 0.6 | 28.4 ± 0.5 * | 28.7 ± 0.3 * | 28.7 ± 0.9 * |
| Food intake (g/day) | 4.0 ± 0.4 | 4.1 ± 0.1 | 4.3 ± 0.1 | 4.1 ± 0.1 |
| Systolic blood pressure (mm Hg) | 93.3 ± 1.6 | 92.1 ± 1.2 | 89.5 ± 0.2 | 91.6 ± 2.5 |
| Diastolic blood pressure (mm Hg) | 70.3 ± 1.2 | 71.2 ± 1.6 | 68.4 ± 0.9 | 70.4 ± 2.5 |
| Total cholesterol (mg/dL) | 2282.3 ± 167.8 | 2209.7 ± 126.5 | 2036.8 ± 119.0 | 2176.5 ± 44.6 |
| Non-HDL cholesterol (mg/dL) | 2269.9 ± 168.5 | 2198.0 ± 125.2 | 2030.6 ± 118.2 | 2168.3 ± 44.8 |
| HDL cholesterol (mg/dL) | 12.5 ± 2.5 | 11.7 ± 4.5 | 6.3 ± 2.0 | 8.2 ± 1.0 |
| Triglyceride (mg/dL) | 287.0 ± 48.8 | 283.4 ± 38.4 | 270.8 ± 48.5 | 174.3 ± 40.7 |
| Free fatty acid (mEq/L) | 4.1 ± 0.7 | 2.7 ± 0.8 | 2.2 ± 0.5 | 1.0 ± 0.2 † |
| Glucose (mg/dL) | 295.4 ± 49.0 | 259.3 ± 31.3 | 281.3 ± 18.9 | 172.9 ± 32.3 |
| Insulin (pmol/L) | 24.5 ± 7.4 | 26.2 ± 6.6 | 65.4 ± 16.4 ‡ | 26.6 ± 10.4 |
| HOMA-IR | 2.3 ± 0.6 | 3.5 ± 1.1 | 6.6 ± 1.8 † | 1.8 ± 0.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, K.; Shirai, R.; Yamaguchi, M.; Yamashita, T.; Shibata, K.; Okano, T.; Mori, Y.; Matsuyama, T.-a.; Ishibashi-Ueda, H.; Hirano, T.; et al. Anti-Atherogenic Effects of Vaspin on Human Aortic Smooth Muscle Cell/Macrophage Responses and Hyperlipidemic Mouse Plaque Phenotype. Int. J. Mol. Sci. 2018, 19, 1732. https://doi.org/10.3390/ijms19061732
Sato K, Shirai R, Yamaguchi M, Yamashita T, Shibata K, Okano T, Mori Y, Matsuyama T-a, Ishibashi-Ueda H, Hirano T, et al. Anti-Atherogenic Effects of Vaspin on Human Aortic Smooth Muscle Cell/Macrophage Responses and Hyperlipidemic Mouse Plaque Phenotype. International Journal of Molecular Sciences. 2018; 19(6):1732. https://doi.org/10.3390/ijms19061732
Chicago/Turabian StyleSato, Kengo, Remina Shirai, Maho Yamaguchi, Tomoyuki Yamashita, Koichiro Shibata, Taisuke Okano, Yusaku Mori, Taka-aki Matsuyama, Hatsue Ishibashi-Ueda, Tsutomu Hirano, and et al. 2018. "Anti-Atherogenic Effects of Vaspin on Human Aortic Smooth Muscle Cell/Macrophage Responses and Hyperlipidemic Mouse Plaque Phenotype" International Journal of Molecular Sciences 19, no. 6: 1732. https://doi.org/10.3390/ijms19061732
APA StyleSato, K., Shirai, R., Yamaguchi, M., Yamashita, T., Shibata, K., Okano, T., Mori, Y., Matsuyama, T.-a., Ishibashi-Ueda, H., Hirano, T., & Watanabe, T. (2018). Anti-Atherogenic Effects of Vaspin on Human Aortic Smooth Muscle Cell/Macrophage Responses and Hyperlipidemic Mouse Plaque Phenotype. International Journal of Molecular Sciences, 19(6), 1732. https://doi.org/10.3390/ijms19061732