Tapetal-Delayed Programmed Cell Death (PCD) and Oxidative Stress-Induced Male Sterility of Aegilops uniaristata Cytoplasm in Wheat
Abstract
:1. Introduction
2. Results
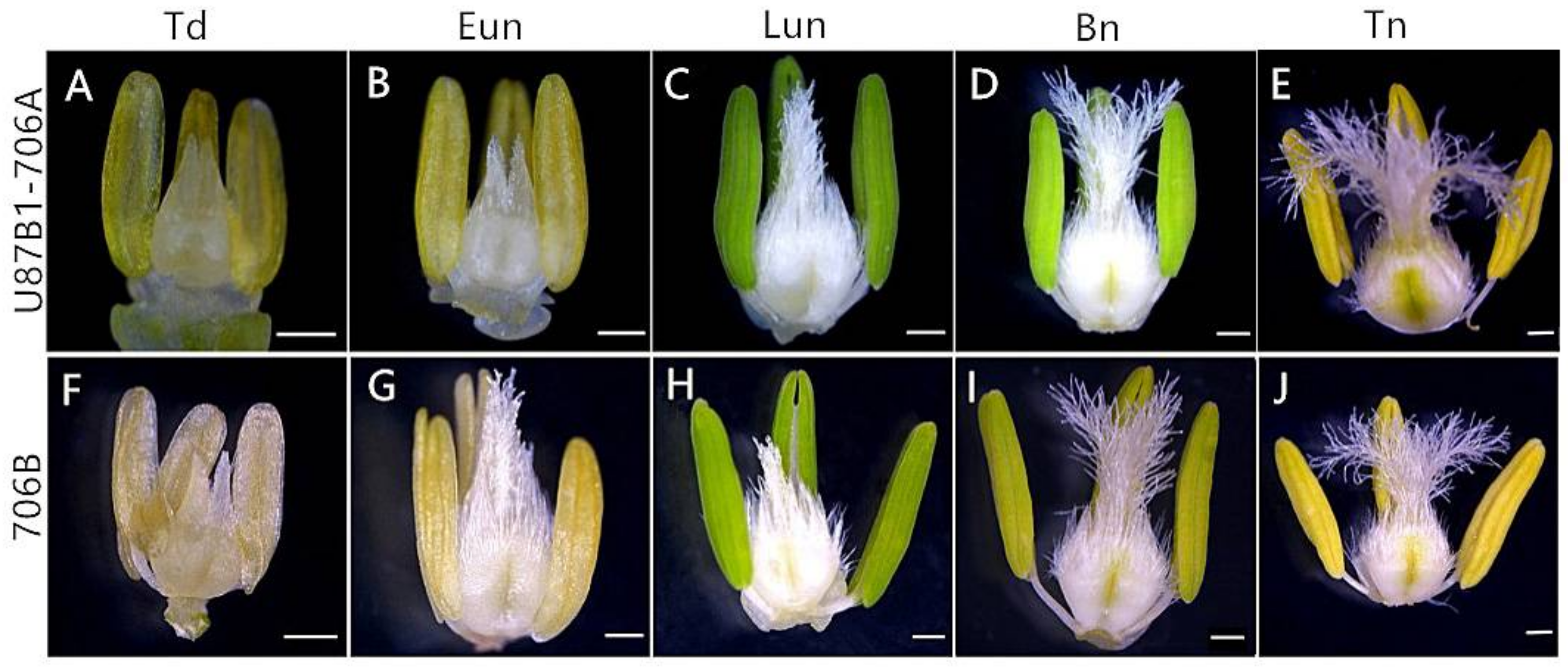
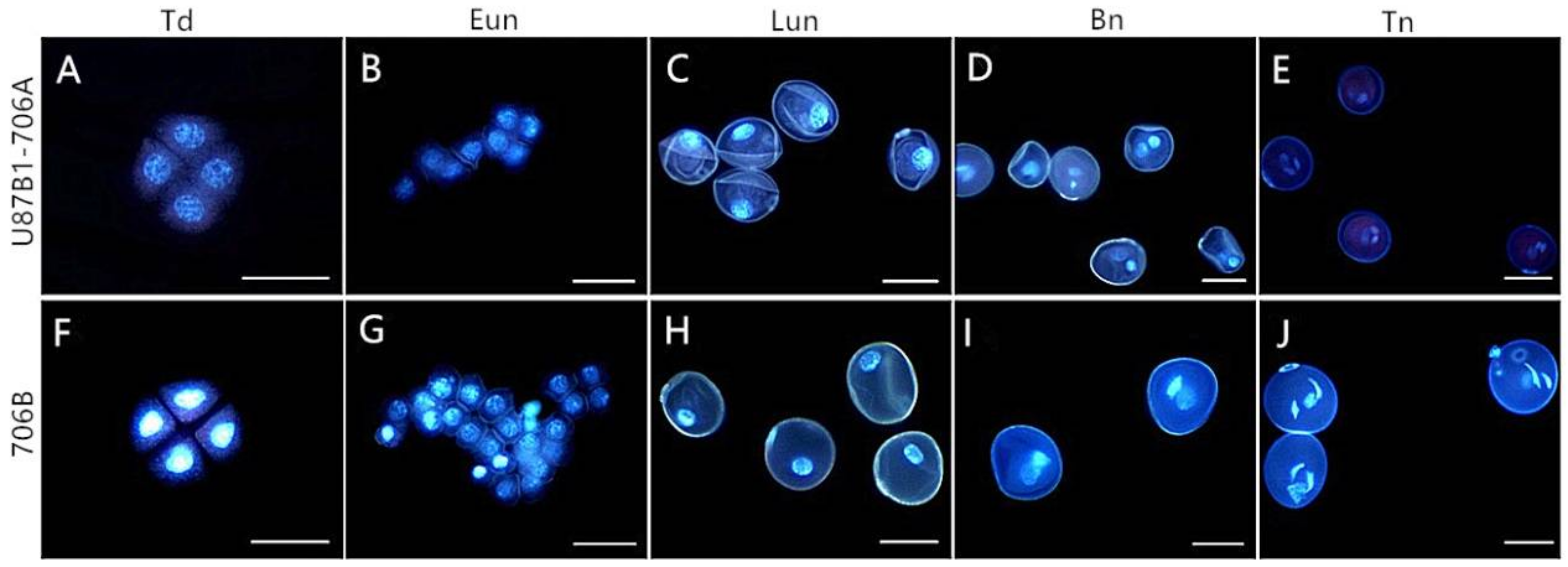
2.1. Abortive Morphological Features in U87B1-706A
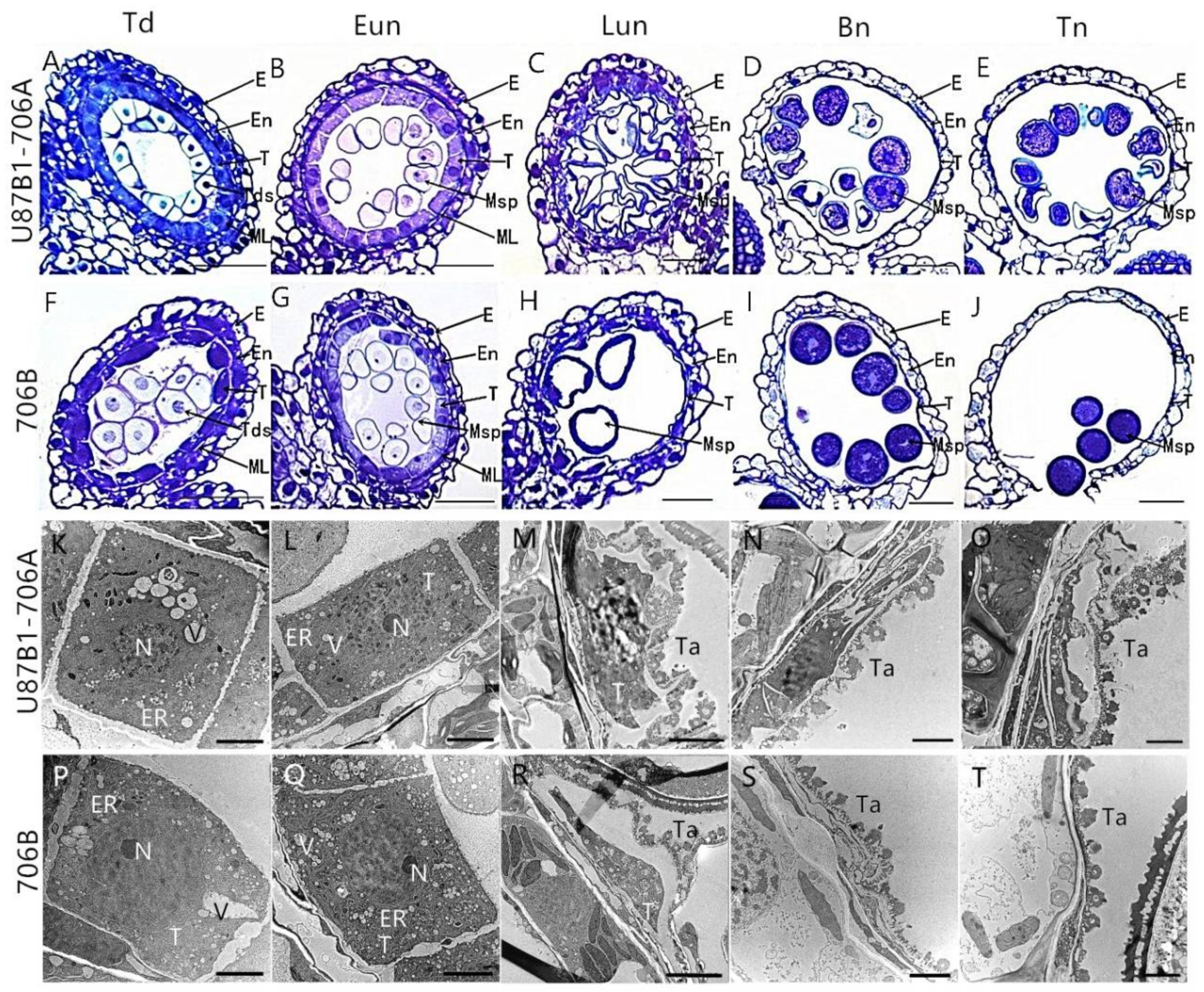
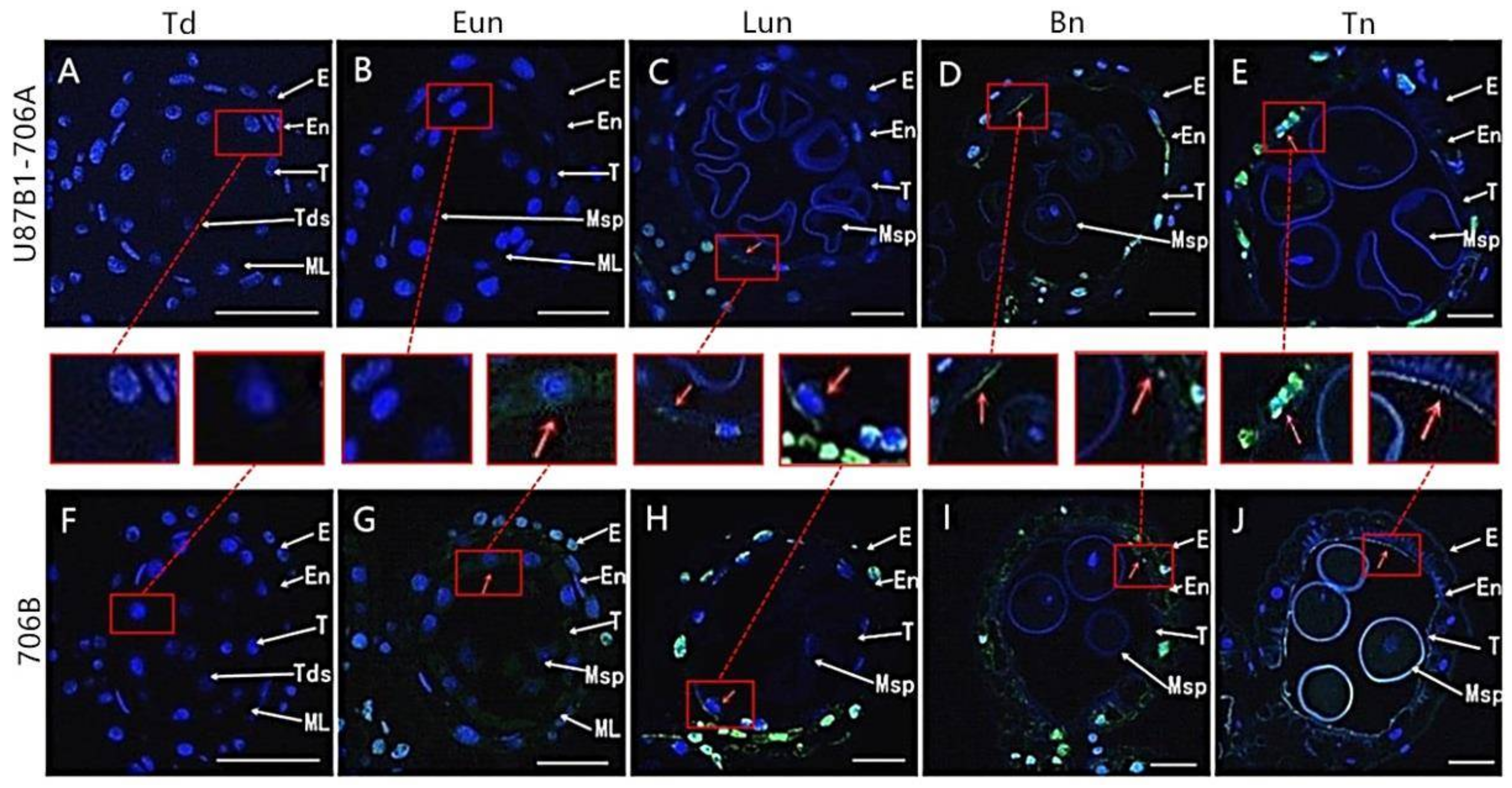
2.2. Tapetal Development in Anthers
2.3. Tapetal PCD Detection in Anthers
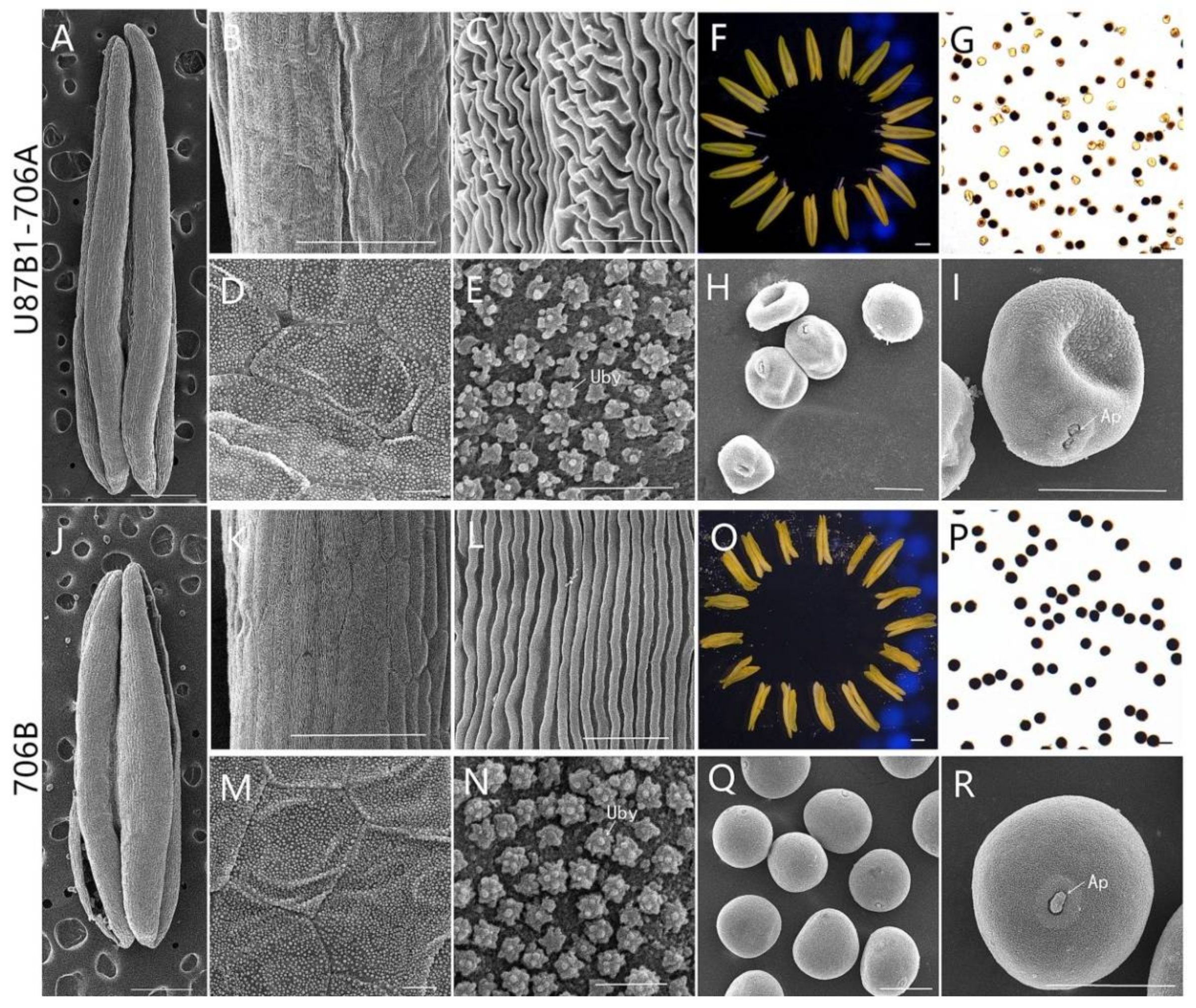
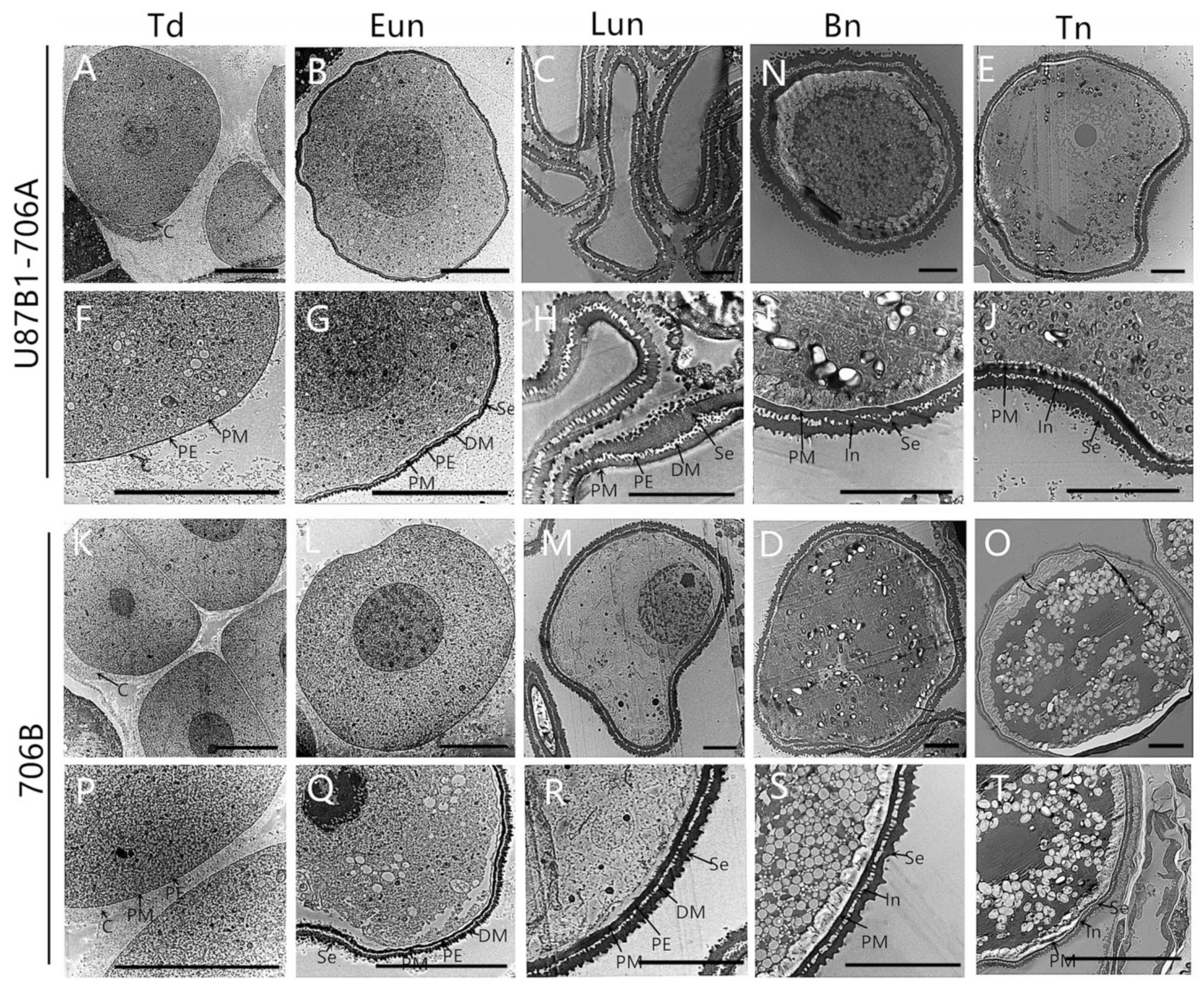
2.4. Cytological Characteristics of Microspores
2.5. O2− Generation Rate and Contents of H2O2 and MDA
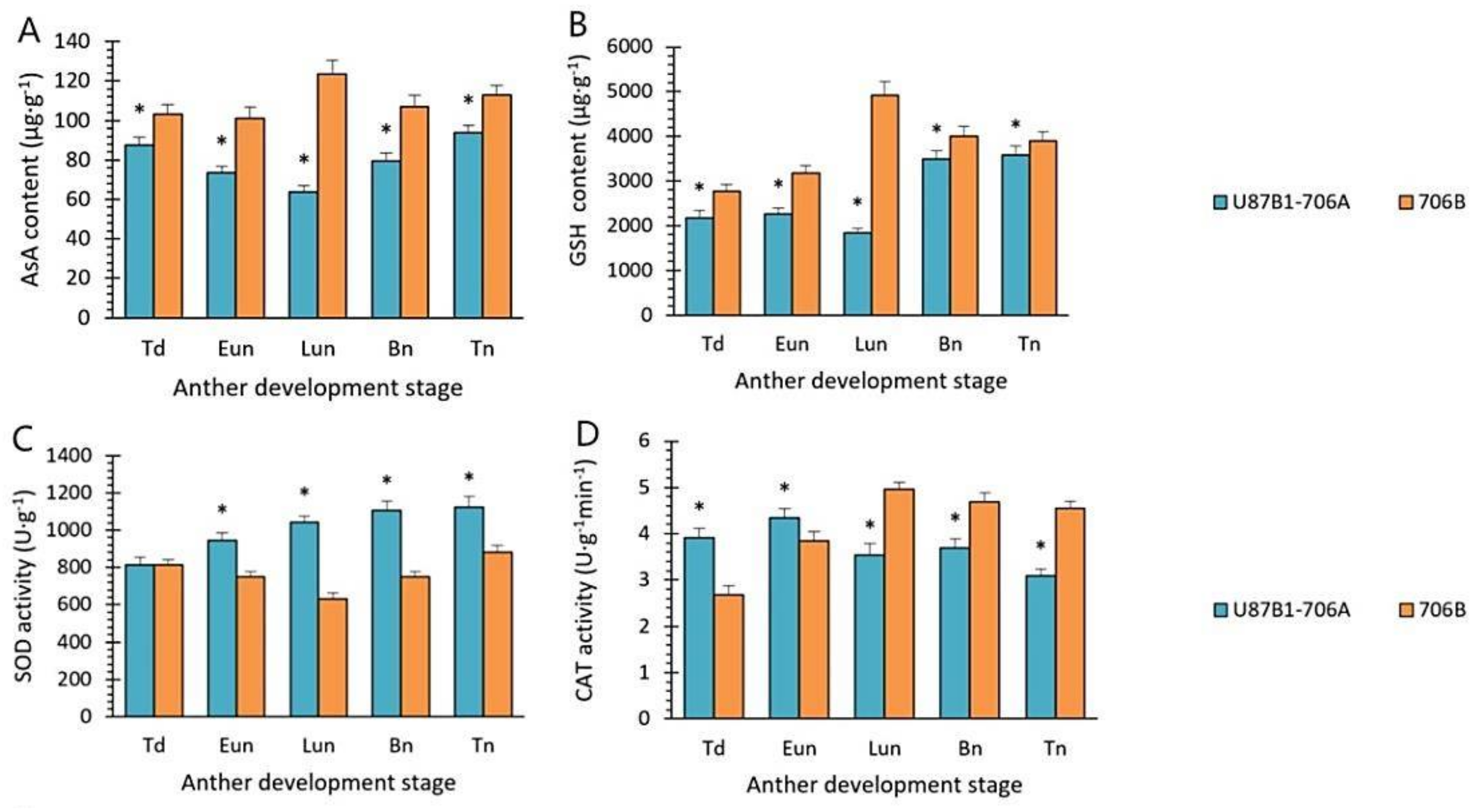
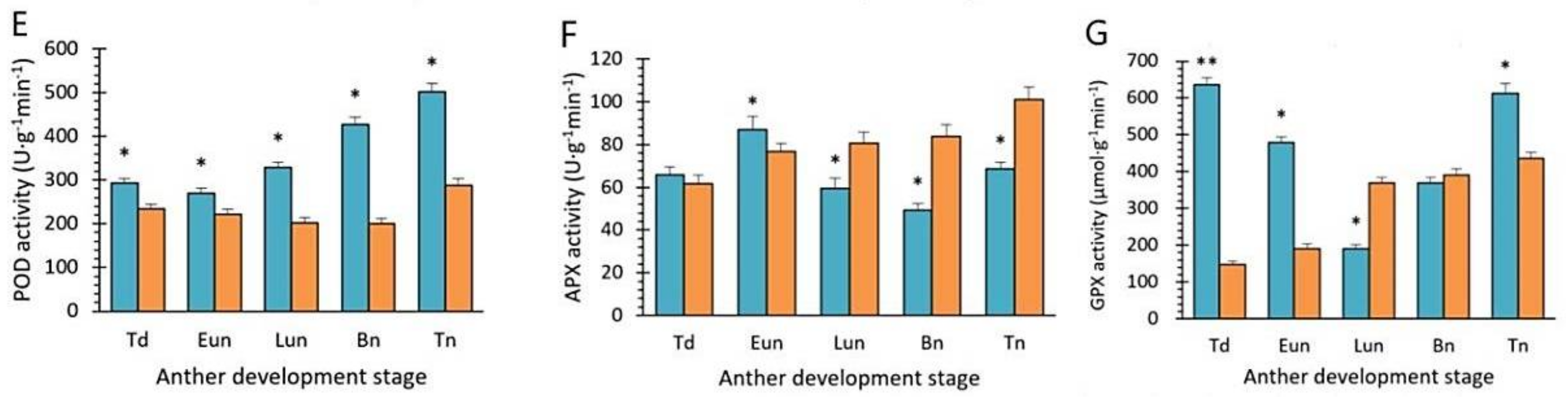
2.6. Activities of Antioxidant Enzymes and Nonenzymatic Antioxidants
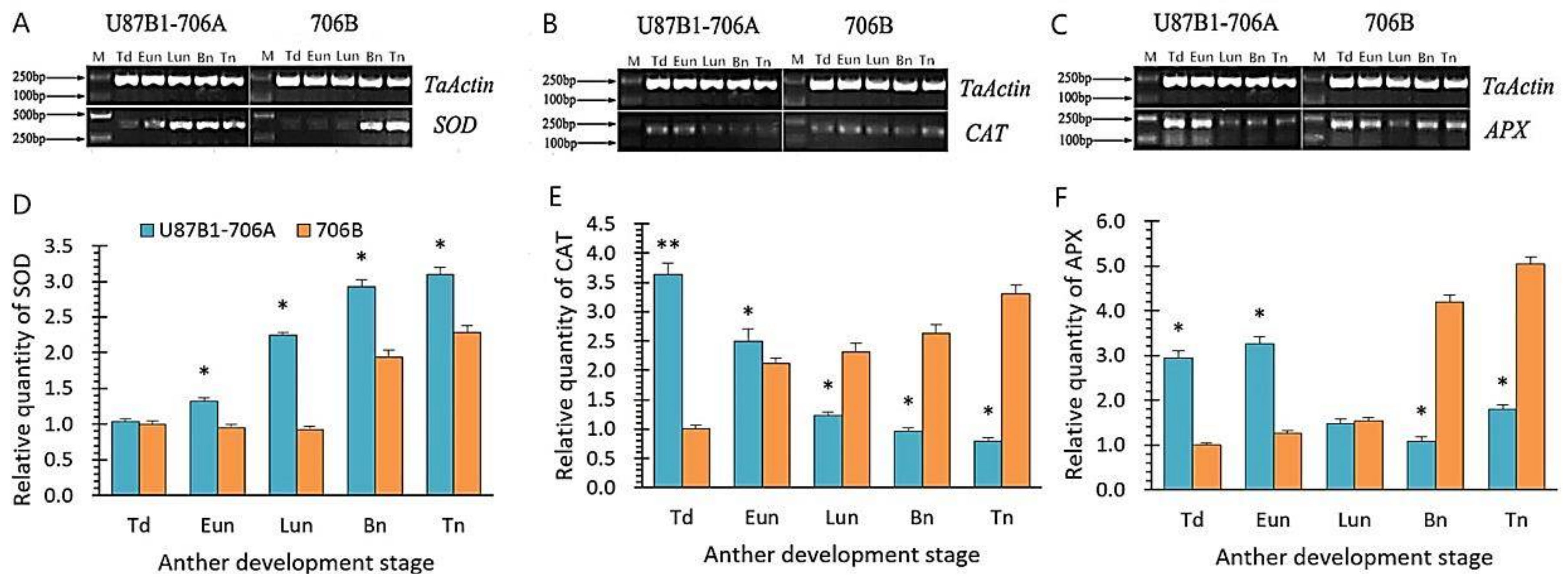
2.7. Expression Levels of Antioxidant Enzyme Genes
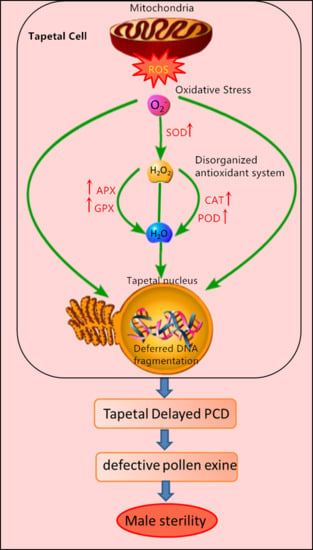
3. Discussion
3.1. The Critical Period of Abortion in Mu Type-CMS
3.2. Tapetal PCD and Pollen Abortion in Mu Type-CMS
3.3. ROS and Antioxidant Defense System in Mu Type-CMS
4. Methods
4.1. Plant Materials
4.2. Phenotypic Characterization
4.3. Histological Analysis
4.4. TUNEL and DNA Laddering Analysis
4.5. Quantification of ROS and Antioxidants
4.6. Reverse Transcription-PCR (RT-PCR) and Quantitative Real-Time-PCR (qRT-PCR) Analysis of Antioxidant Enzyme Genes
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CMS | cytoplasmic male sterility |
| PCD | programmed cell death |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| CAT | catalase |
| POD | peroxidase |
| APX | ascorbate peroxidase |
| GPX | glutathione peroxidase |
| ASA | ascorbic acid |
| GSH | glutathione |
| TUNEL | terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling |
| SEM | scanning electron microscopy |
| DAPI | 4′,6-diamidino-2-phenylindole |
| TEM | transmission electron microscopy |
| MDA | malondialdehyde |
| Uby | Ubisch bodies |
| Ap | germination aperture |
| E | epidermis |
| En | endothecium |
| ML | middle layer |
| T | tapetum |
| Tds | tetrads |
| Msp | microspores |
| ER | endoplasmic reticulum |
| N | nucleus |
| Ta | tapetosome |
| V | vacuole |
| PE | primexine |
| Se | sexine |
| DM | deposit materials |
| PM | plasma membrane |
| In | intine |
References
- Singh, S.P.; Srivastava, R.; Kumar, J. Male Sterility Systems in Wheat and Opportunities for Hybrid Wheat Development. Acta Physiol. Plant. 2015, 37, 1713. [Google Scholar] [CrossRef]
- Gong, W.; Li, G.; Zhou, J.; Li, G.; Liu, C.; Huang, C.; Zhao, Z.; Yang, Z. Cytogenetic and Molecular Markers for Detecting Aegilops uniaristata Chromosomes in a Wheat Background. Genome 2014, 57, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Tsunewaki, K. Genome-plasmon Interactions in Wheat. Jpn. J. Genet. 1993, 68, 1–34. [Google Scholar] [CrossRef]
- Gong, W.P.; Chu, X.S.; Han, R.; Wang, C.G.; Sun, Z.J.; Cheng, D.G.; Yu, X.C.; Liu, C.; Liu, J.J. Excellent Gene Exploration from Aegilops uniaristata Genome. Shandong Agric. Sci. 2015, 47, 11–15. [Google Scholar]
- Song, X.Y.; Liu, S.D.; Zhu, L.C.; Hu, Y.G.; Xi, Y.J.; Ma, L.J.; Li, H.B.; He, P.R. The Comparative Study of the Cytoplasm Effect between Ae. Uniaristata and Ae. Kotschyi CMS Line. Acta Agric. Boreali-Occident. Sin. 1999, 8, 48–52. [Google Scholar]
- Yao, M.; Ye, J.L.; Yang, Z.Q.; Duan, Y.; Meng, L.Y.; Yan, P.J.; Liu, Z.H.; Zhang, L.L.; Song, X.Y. Abortion Feature and Fertility Restoration of Five Kinds of Cytoplasmic Male Serile Wheat Lines. J. Triticeae Crops 2015, 35, 1676–1684. [Google Scholar]
- Xie, H.T.; Wan, Z.Y.; Li, S.; Zhang, Y. Spatiotemporal Production of Reactive Oxygen Species by NADPH Oxidase Is Critical for Tapetal Programmed Cell Death and Pollen Development in Arabidopsis. Plant Cell 2014, 26, 2007–2023. [Google Scholar] [CrossRef] [PubMed]
- Falasca, G.; D’Angeli, S.; Biasi, R.; Fattorini, L.; Matteucci, M.; Canini, A.; Altamura, M.M. Tapetum and Middle Layer Control Male Fertility in Actinidia deliciosa. Ann. Bot. 2013, 112, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Flores-Renteria, L.; Orozco-Arroyo, G.; Cruz-Garcia, F.; Garcia-Campusano, F.; Alfaro, I.; Vazquez-Santana, S. Programmed Cell Death Promotes Male Sterility in the Functional Dioecious Opuntia Stenopetala (Cactaceae). Ann. Bot. 2013, 112, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Papini, A.; Mosti, S.; van Doorn, W.G. Classical Macroautophagy in Lobivia Rauschii (Cactaceae) and Possible Plastidial Autophagy in Tillandsia albida (Bromeliaceae) Tapetum Cells. Protoplasma 2014, 251, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Iacuone, S.; Li, S.F.; Parish, R.W. MYB80 Homologues in Arabidopsis, Cotton and Brassica: Regulation and Functional Conservation in Tapetal and Pollen Development. BMC Plant Biol. 2014, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dong, C.; Yu, J.; Shi, L.; Tong, C.; Li, Z.; Huang, J.; Liu, S. Cysteine Protease 51 (CP51), an Anther-specific Cysteine Protease Gene, is Essential for Pollen Exine Formation in Arabidopsis. Plant Cell Tissue Organ Cult. 2014, 119, 383–397. [Google Scholar] [CrossRef]
- Ba, Q.S.; Zhang, G.S.; Wang, J.S.; Che, H.X.; Liu, H.Z.; Niu, N.; Ma, S.C.; Wang, J.W. Relationship between Metabolism of Reactive Oxygen Species and Chemically Induced Male Sterility in Wheat (Triticum aestivum L.). Can. J. Plant Sci. 2013, 93, 675–681. [Google Scholar] [CrossRef]
- Yu, S.X.; Feng, Q.N.; Xie, H.T.; Li, S.; Zhang, Y. Reactive Oxygen Species Mediate Tapetal Programmed Cell Death in Tobacco and Tomato. BMC Plant Biol. 2017, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.A.; Iacuone, S.; Li, S.F.; Parish, R.W. The MYB80 Transcription Factor is Required for Pollen Development and the Regulation of Tapetal Programmed Cell Death in Arabidopsis thaliana. Plant Cell 2011, 23, 2209–2224. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Zhang, G.S.; Song, Q.L.; Zhang, Y.X.; Li, Y.; Chen, Z.; Niu, N.; Ma, S.C.; Wang, J.W. Programmed Cell Death, Antioxidant Response and Oxidative Stress in Wheat Flag Leaves Induced by Chemical Hybridization Agent SQ-1. J. Integr. Agric. 2016, 15, 76–86. [Google Scholar] [CrossRef]
- Jimenez-Quesada, M.J.; Traverso, J.A.; Alche Jde, D. NADPH Oxidase-Dependent Superoxide Production in Plant Reproductive Tissues. Front. Plant Sci. 2016, 7, 359. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, S.; Wen, L.; Kong, J.; Wang, K.; Zhu, Y. Damage of Oxidative Stress on Mitochondria during Microspores Development in Honglian CMS line of rice. Plant Cell Rep. 2007, 26, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Mhadhbi, H.; Fotopoulos, V.; Mylona, P.V.; Jebara, M.; Aouani, M.E.; Polidoros, A.N. Alternative Oxidase 1 (Aox1) Gene Expression in Roots of Medicago Truncatula is a Genotype-specific Component of Salt Stress Tolerance. J. Plant Physiol. 2013, 170, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Sahoo, L.; Katsuhara, M.; Matsumoto, H. Overexpression of Alternative Oxidase Gene Confers Aluminum Tolerance by Altering the Respiratory Capacity and the Response to Oxidative Stress in Tobacco Cells. Mol. Biotechnol. 2013, 54, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Bhoomika, K.; Pyngrope, S.; Dubey, R.S. Differential Responses of Antioxidant Enzymes to Aluminum Toxicity in Two Rice (Oryza sativa L.) Cultivars with Marked Presence and Elevated Activity of Fe SOD and Enhanced Activities of Mn SOD and Catalase in Aluminum Tolerant Cultivar. Plant Growth Regul. 2013, 71, 235–252. [Google Scholar] [CrossRef]
- Li, J.; Dai, X.; Li, L.; Jiao, Z.; Huang, Q. Metabolism of Reactive Oxygen Species in Cytoplasmic Male Sterility of Rice by Marking Upmost Pulvinus Interval. Appl. Biochem. Biotechnol. 2015, 175, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y. Studies on Male Sterile Lines of Rice with Different Cytoplasms. Acta Agron. Sin. 1979, 5, 29–37. [Google Scholar]
- Sun, Z.; Tan, X.; Tao, G.; Guo, A.; Lei, W.; Zhu, G.; Zhao, Y.; Wang, C.; Cheng, C. Pollen Abortive Characteristics of Japonica CMS Lines with Different Cytoplasmic Sources. Hybrid Rice 2013, 28, 68–71. [Google Scholar]
- Yi, J.; Moon, S.; Lee, Y.S.; Zhu, L.; Liang, W.; Zhang, D.; Jung, K.H.; An, G. Defective Tapetum Cell Death 1 (DTC1) Regulates ROS Levels by Binding to Metallothionein during Tapetum Degeneration. Plant Physiol. 2016, 170, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhu, J.; Cui, Y.L.; Yang, Z.N. Ultrastructure Analysis Reveals Sporopollenin Deposition and Nexine Formation at Early Stage of Pollen Wall Development in Arabidopsis. Sci. Bull. 2015, 60, 273–276. [Google Scholar] [CrossRef]
- Rao, Y. Cytohistology of Cytoplasmic Male Sterile Lines in Hybrid Rice. In Hybrid Rice, Proceedings of the International Symposium on Hybrid Rice; Smith, W.H., Bostian, L.R., Cervantes, E.P., Eds.; International Rice Research Institute: Manila, Philippines, 1988; pp. 115–128. [Google Scholar]
- Shi, Y.; Zhao, S.; Yao, J. Premature Tapetum Degeneration: A Major Cause of Abortive Pollen Development in Photoperiod Sensitive Genic Male Sterility in Rice. J. Integr. Plant Biol. 2009, 51, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Senatore, A.; Trobacher, C.P.; Greenwood, J.S. Ricinosomes Predict Programmed Cell Death Leading to Anther Dehiscence in Tomato. Plant Physiol. 2009, 149, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Zhu, L.; Tu, L.; Deng, F.; Yuan, D.; Zhang, X. Cotton GhCKI Disrupts Normal Male Reproduction by Delaying Tapetum Programmed Cell Death via Inactivating Starch Synthase. Plant J. 2013, 75, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, G.; Song, Q.; Zhang, Y.; Li, Z.; Guo, J.; Niu, N.; Ma, S.; Wang, J. Abnormal Development of Tapetum and Microspores Induced by Chemical Hybridization Agent SQ-1 in Wheat. PLoS ONE 2015, 10, e0119557. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.J.; Huang, W.; Li, Z.; Chai, W.G.; Yin, Y.X.; Li, D.W.; Gong, Z.H. Tapetum-specific Expression of a Cytoplasmic orf507 Gene Causes Semi-male Sterility in Transgenic Peppers. Front. Plant Sci. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Varnier, A.L.; Mazeyrat-Gourbeyre, F.; Sangwan, R.S.; Clement, C. Programmed Cell Death Progressively Models the Development of Anther Sporophytic Tissues from the Tapetum and is Triggered in Pollen Drains during Maturation. J. Struct. Biol. 2005, 152, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.S.; Liang, W.Q.; Yuan, Z.; Li, N.; Shi, J.; Wang, J.; Liu, Y.M.; Yu, W.J.; Zhang, D.B. Tapetum Degeneration Retardation is Critical for Aliphatic Metabolism and Gene Regulation during Rice Pollen Development. Mol. Plant 2008, 1, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhou, Z.; Tang, S.; Zhang, Z.; Xia, S.; Qin, M.; Li, B.; Wen, J.; Yi, B.; Shen, J.; et al. Ectopic Expression of BnaC.CP20.1 Results in Premature Tapetal Programmed Cell Death in Arabidopsis. Plant Cell Physiol. 2016, 57, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, D.; Lv, X.; Wang, Y.; Xun, Z.; Liu, Z.; Li, F.; Lu, H. The Cysteine Protease CEP1, a Key Executor Involved in Tapetal Programmed Cell Death, Regulates Pollen Development in Arabidopsis. Plant Cell 2014, 26, 2939–2961. [Google Scholar] [CrossRef] [PubMed]
- Semwal, V.K.; Singh, B.; Khanna-Chopra, R. Delayed Expression of SAGs Correlates with Longevity in CMS Wheat Plants Compared to Its Fertile Plants. Physiol. Mol. Biol. Plants 2014, 20, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Goher, M.; Iqbal, N. Drought Induced Programmed Cell Death and Associated Changes in Antioxidants, Proteases, and Lipid Peroxidation in Wheat Leaves. Biol. Plant. 2012, 57, 370–374. [Google Scholar] [CrossRef]
- Deng, M.H.; Wen, J.F.; Huo, J.L.; Zhu, H.S.; Dai, X.Z.; Zhang, Z.Q.; Zhou, H.; Zou, X.X. Relationship of Metabolism of Reactive Oxygen Species with Cytoplasmic Male Sterility in Pepper (Capsicum annuum L.). Sci. Hortic. 2012, 134, 232–236. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kornyeyev, D. Effect of Chloroplastic Overproduction of Ascorbate Peroxidase on Photosynthesis and Photoprotection in Cotton Leaves Subjected to Low Temperature Photoinhibition. Plant Sci. 2003, 165, 1033–1041. [Google Scholar] [CrossRef]
- Xie, Y.; Cui, W.; Yuan, X.; Shen, W.; Yang, Q. Heme Oxygenase-1 is Associated with Wheat Salinity Acclimation by Modulating Reactive Oxygen Species Homeostasis. J. Integr. Plant Biol. 2011, 53, 653–670. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Paul, P.; Kumar, S.; Verma, S.K.; Prasad, R.; Dhaliwal, H.S. Abnormal Endosperm Development Causes Female Sterility in Rice Insertional Mutant OsAPC6. Plant Sci. 2012, 183, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Song, Q.; Fang, Z.; Chen, Z.; Zhang, Y.; Zhang, L.; Zhang, L.; Niu, N.; Ma, S.; et al. Mitochondrial Dysfunction Causes Oxidative Stress and Tapetal Apoptosis in Chemical Hybridization Reagent-Induced Male Sterility in Wheat. Front. Plant Sci. 2017, 8, 2217. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wan, C.; Hu, C.; Gao, F.; Huang, Q.; Wang, K.; Wang, T.; Zhu, Y. Mitochondrial Mutation Impairs Cytoplasmic Male Sterility Rice in Response to H2O2 Stress. Plant Sci. 2012, 195, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hu, Y.; Ma, L.; Song, X.; Xi, Y.; Liu, S.; Yang, C.; He, P. A Comparasion o f Wheat CMS Lines of Ae. Uniaristata and Ae. Kotschyi Cytoplasms. Acta Univ. Agric. Boreali-Occident. 1998, 26, 21–24. [Google Scholar]
- Meng, L.; Liu, Z.; Zhang, L.; Hu, G.; Song, X. Cytological Characterization of a Thermo-sensitive Cytoplasmic Male-sterile Wheat Line Having K-type Cytoplasm of Aegilops kotschyi. Breed. Sci. 2016, 66, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, H.; Su, Y.; Li, W.; Shi, C. G1/ELE Functions in the Development of Rice Lemmas in Addition to Determining Identities of Empty Glumes. Front. Plant Sci. 2016, 7, 1006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Zhang, G.S.; Zhao, X.L.; Yang, S.L. Screening and Analysis of Proteins Interacting with TaPDK from Physiological Male Sterility Induced by CHA in Wheat. J. Integr. Agric. 2013, 12, 941–950. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Zhang, H.; Qian, Q.; Ding, Y. Comparative Transcript Profiling of Alloplasmic Male-sterile Lines Revealed Altered Gene Expression Related to Pollen Development in Rice (Oryza sativa L.). BMC Plant Biol. 2016, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Bibi, N.; Yuan, S.; Zhu, Y.; Wang, X. Improvements of Fertility Restoration in Cytoplasmic Male Sterile Cotton by Enhanced Expression of Glutathione S-Transferase (GST) Gene. J. Plant Growth Regul. 2013, 33, 420–429. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Zhang, J.H. Effect of Abscisic Acid on Active Oxygen Species, Antioxidative Defence System and Oxidative Damage in Leaves of Maize Seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.H.; Feng, T.; Peng, X.X.; Yan, M.L.; Zhou, P.L.; Tang, X.K. Ameliorative Effects of Brassinosteroid on Excess Manganese-Induced Oxidative Stress in Zea mays L. Leaves. Agric. Sci. China 2009, 8, 1063–1074. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Shi, X.; Li, S.; Hu, G.; Zhang, L.; Song, X. Tapetal-Delayed Programmed Cell Death (PCD) and Oxidative Stress-Induced Male Sterility of Aegilops uniaristata Cytoplasm in Wheat. Int. J. Mol. Sci. 2018, 19, 1708. https://doi.org/10.3390/ijms19061708
Liu Z, Shi X, Li S, Hu G, Zhang L, Song X. Tapetal-Delayed Programmed Cell Death (PCD) and Oxidative Stress-Induced Male Sterility of Aegilops uniaristata Cytoplasm in Wheat. International Journal of Molecular Sciences. 2018; 19(6):1708. https://doi.org/10.3390/ijms19061708
Chicago/Turabian StyleLiu, Zihan, Xiaoyi Shi, Sha Li, Gan Hu, Lingli Zhang, and Xiyue Song. 2018. "Tapetal-Delayed Programmed Cell Death (PCD) and Oxidative Stress-Induced Male Sterility of Aegilops uniaristata Cytoplasm in Wheat" International Journal of Molecular Sciences 19, no. 6: 1708. https://doi.org/10.3390/ijms19061708