The Extracts and Major Compounds Derived from Astragali Radix Alter Mitochondrial Bioenergetics in Cultured Cardiomyocytes: Comparison of Various Polar Solvents and Compounds
Abstract
:1. Introduction
2. Results
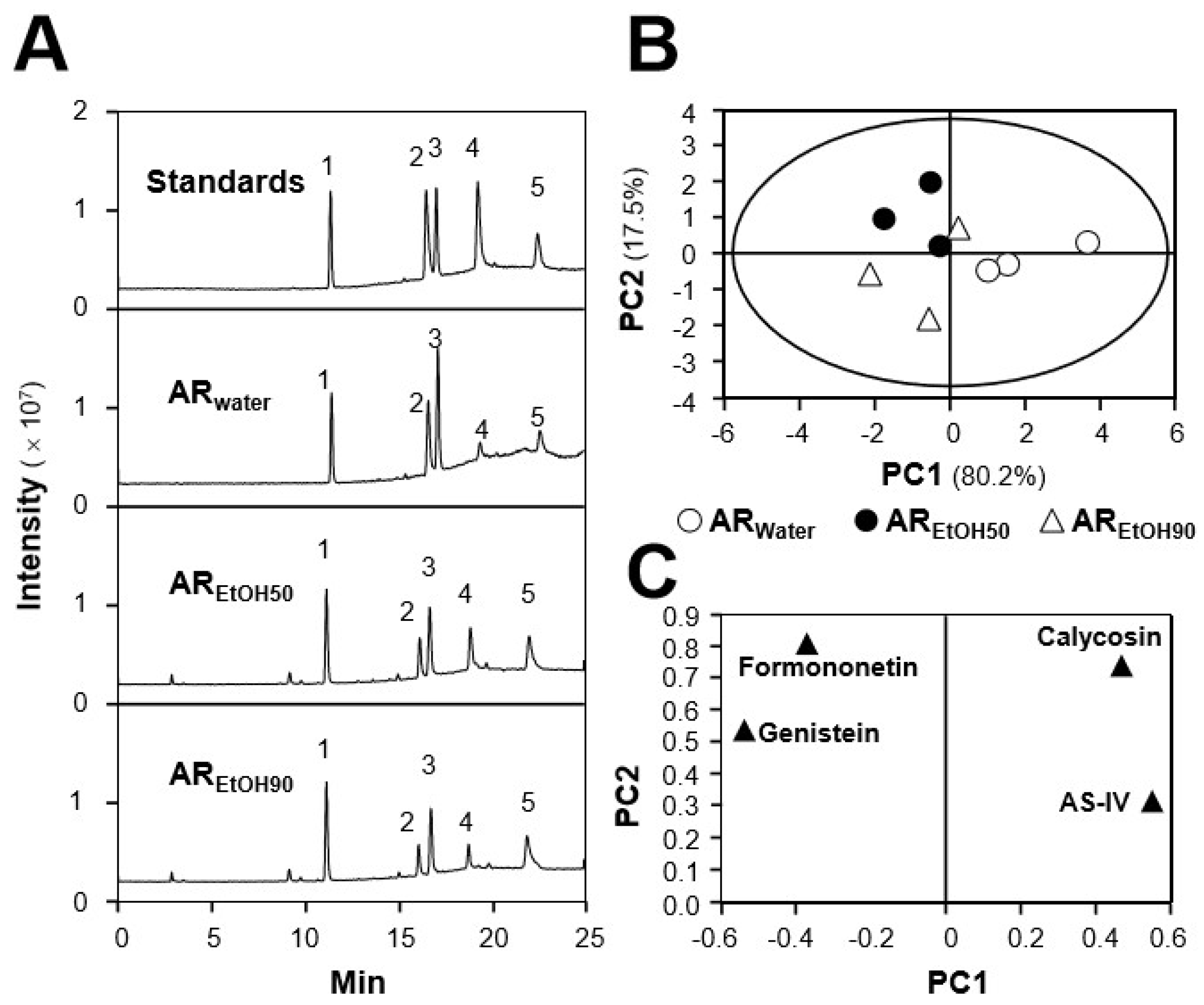
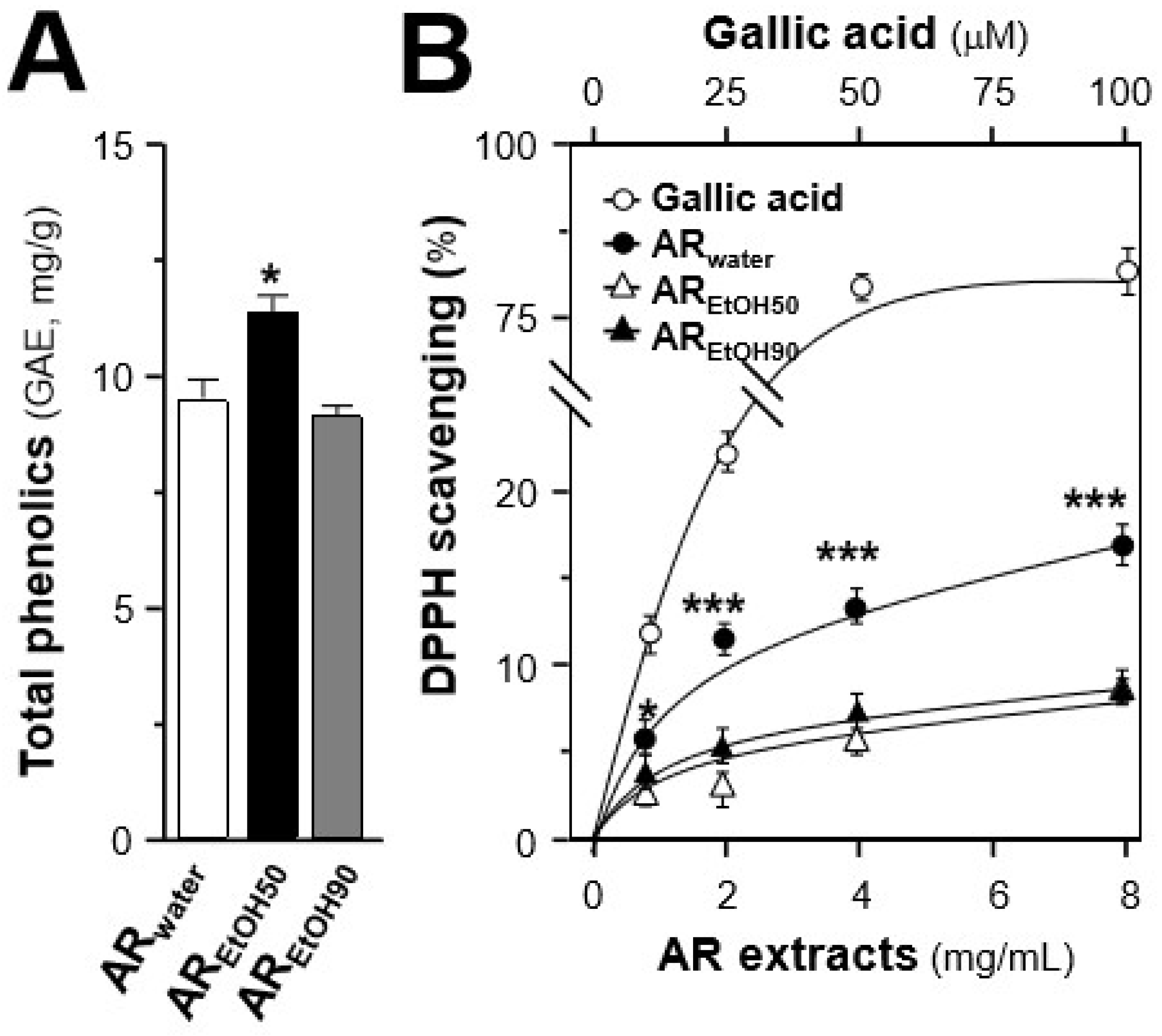
2.1. Quantification of Chemicals and Total Phenolic Compounds in Different Extracts
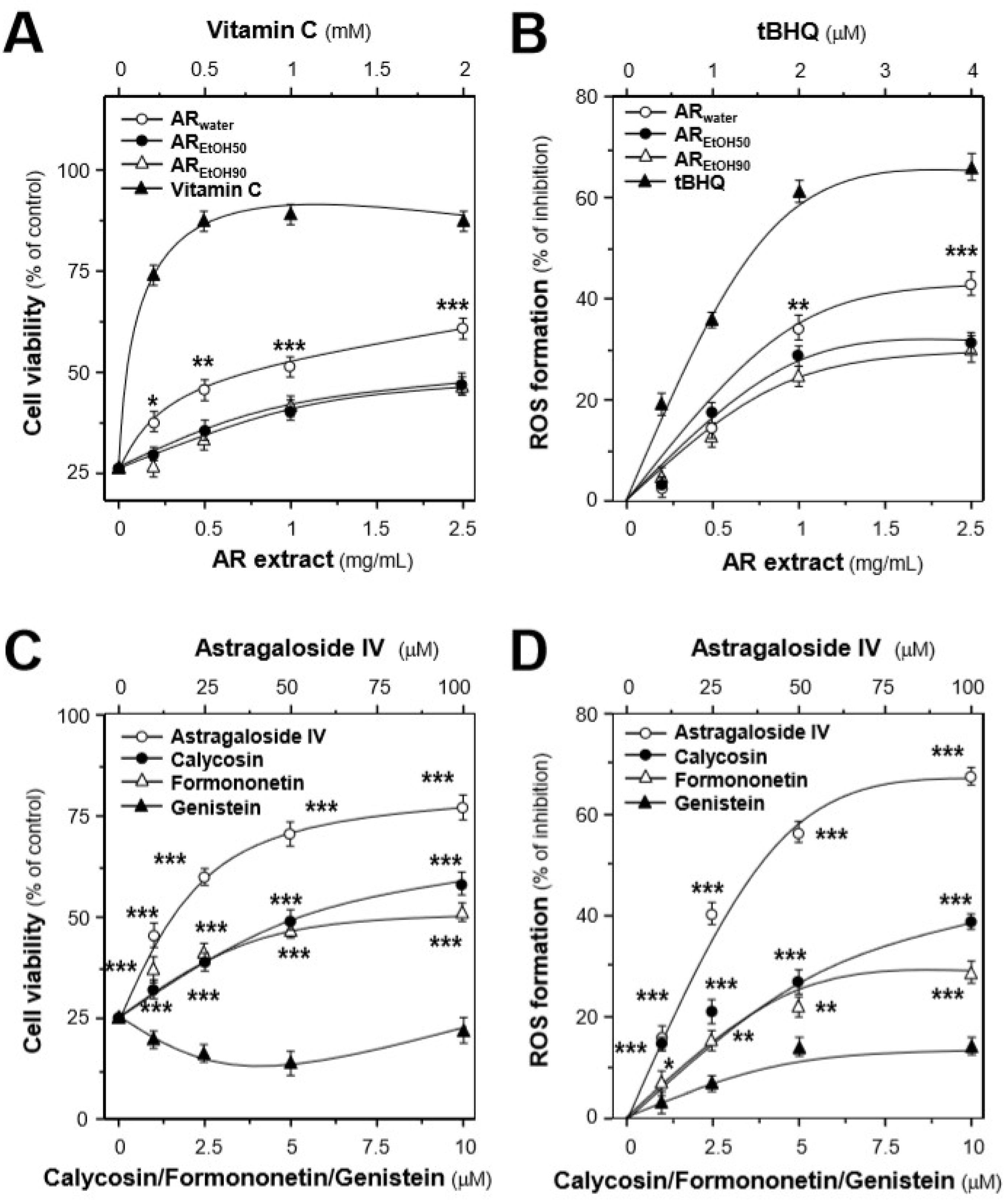
2.2. Protection Effects of the AR Extracts in H9C2 Cells Subjected to Oxidative Stressed
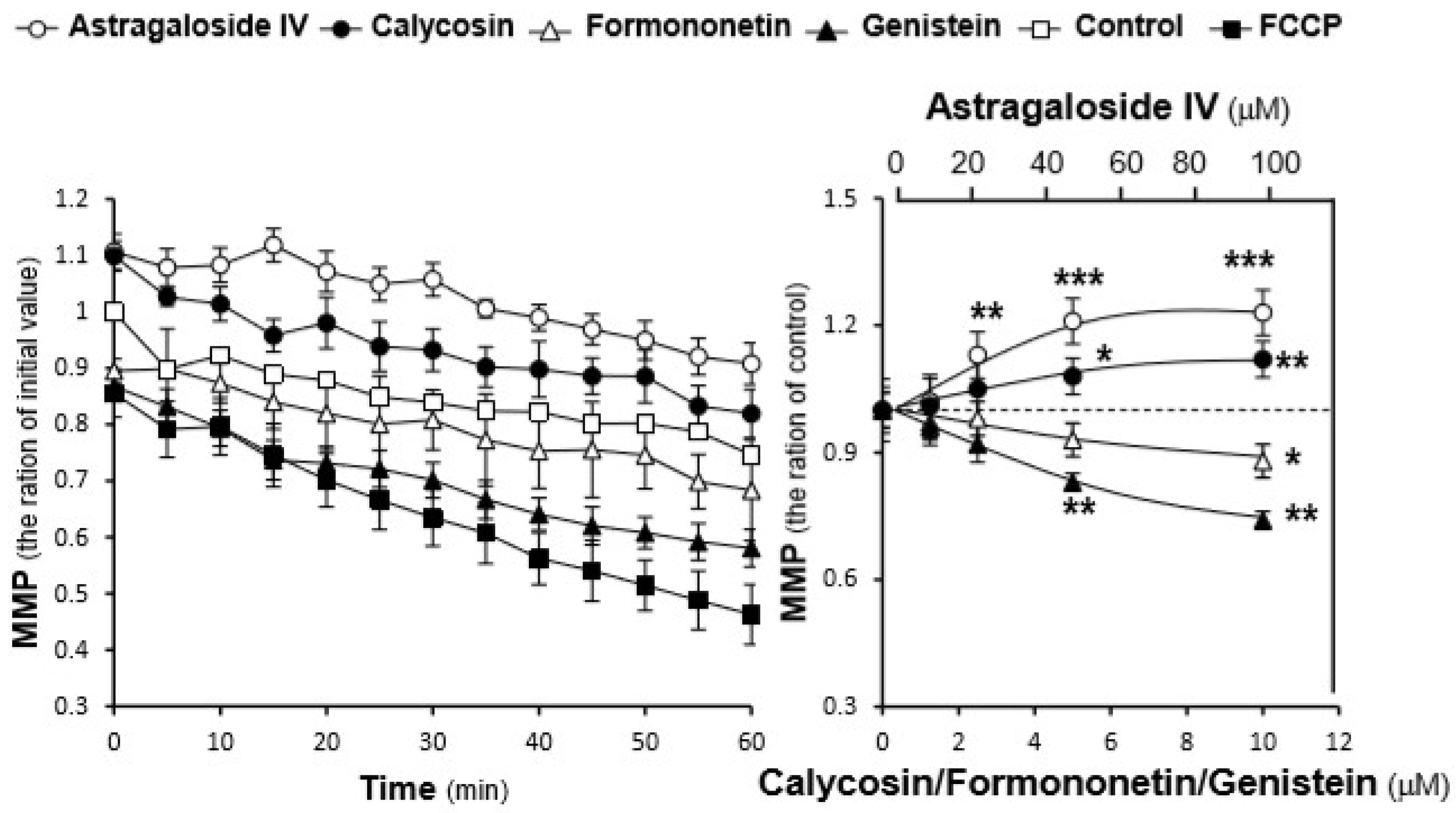
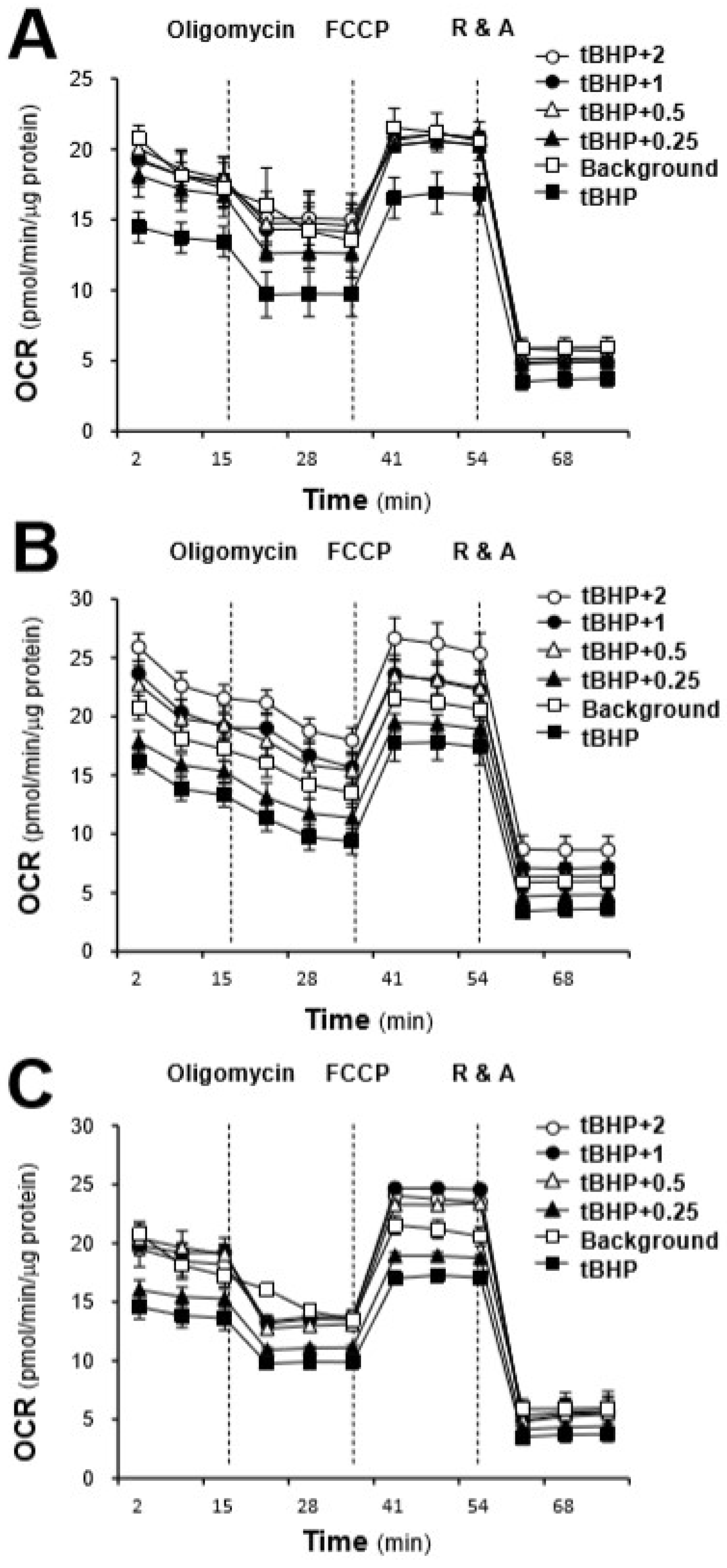
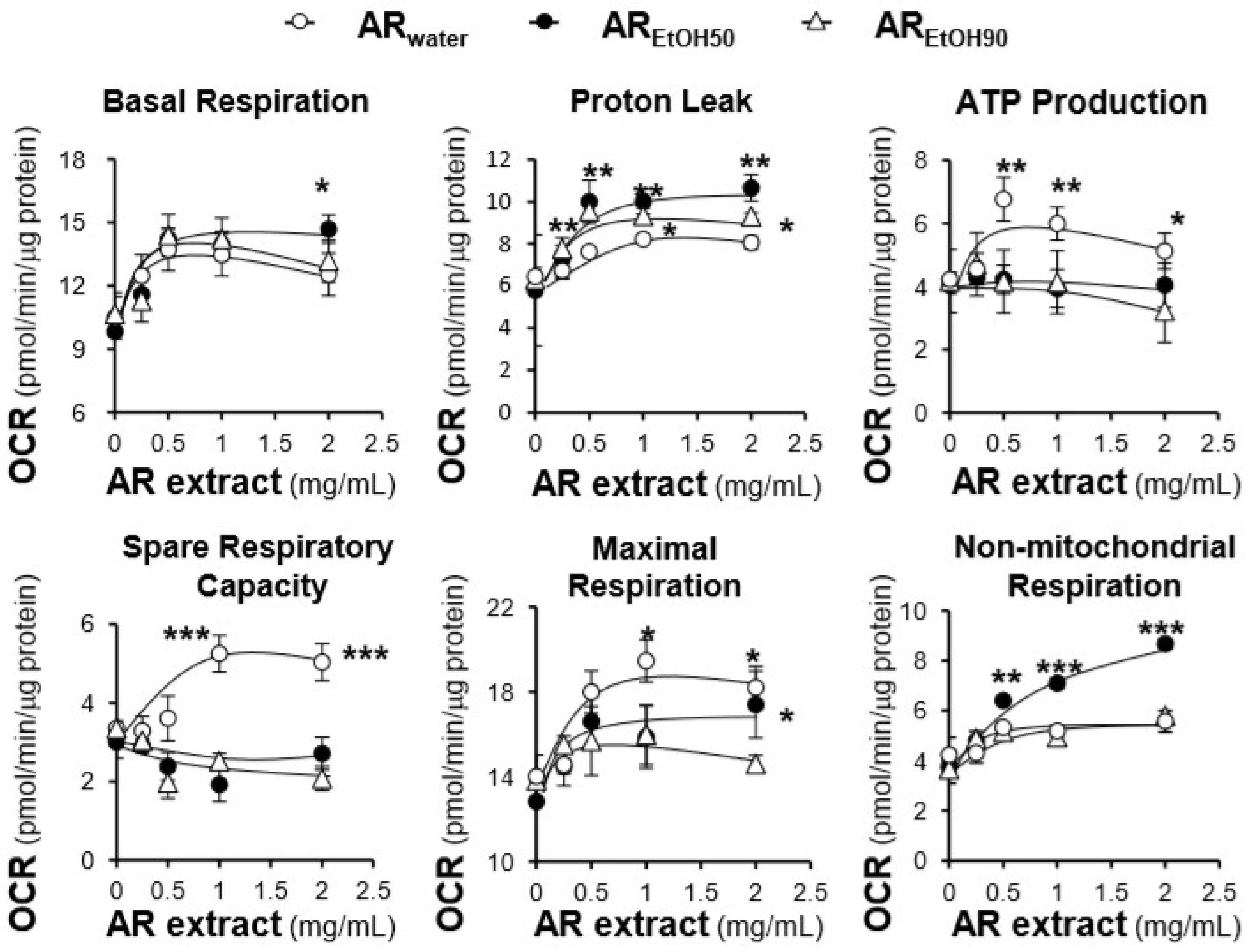
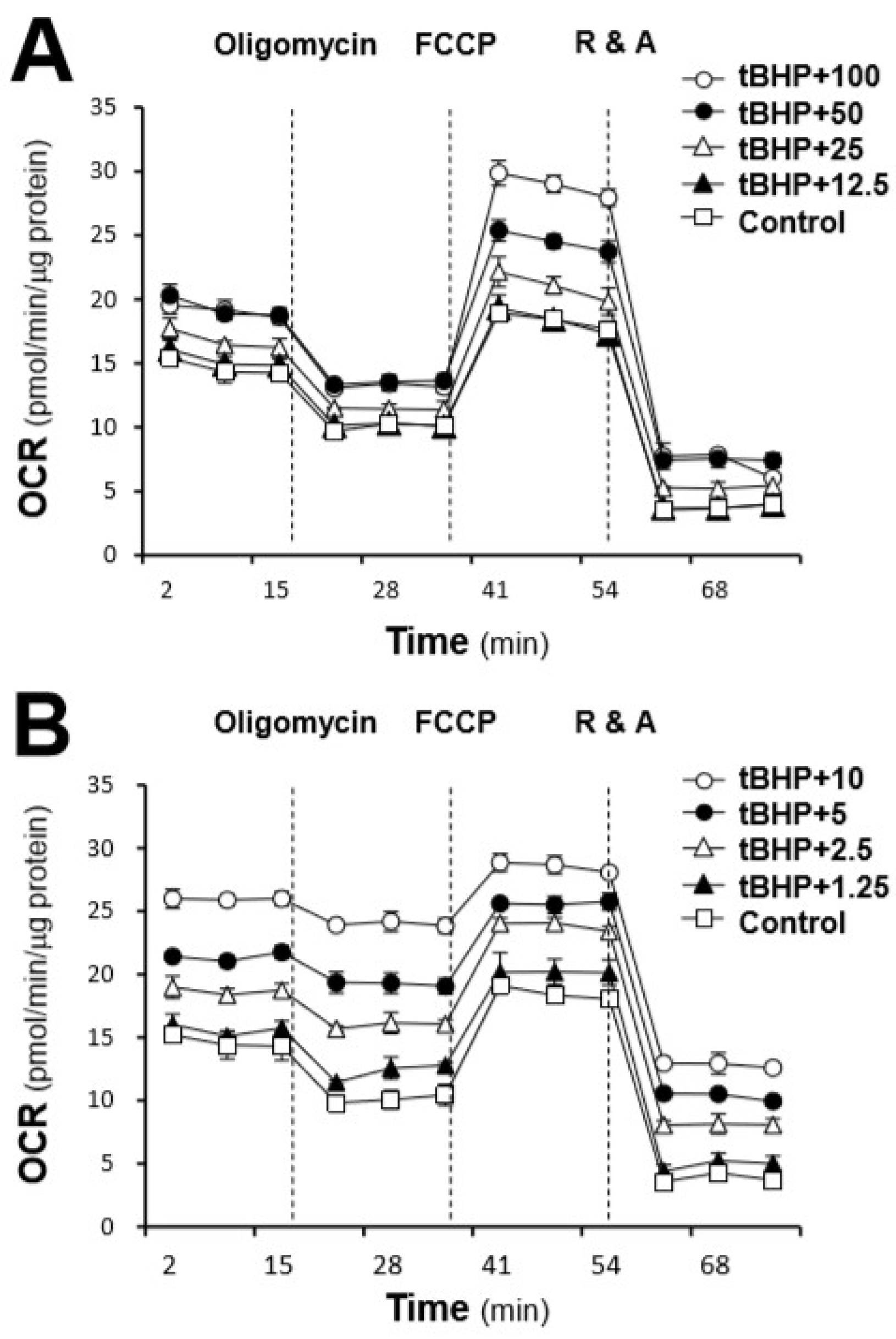
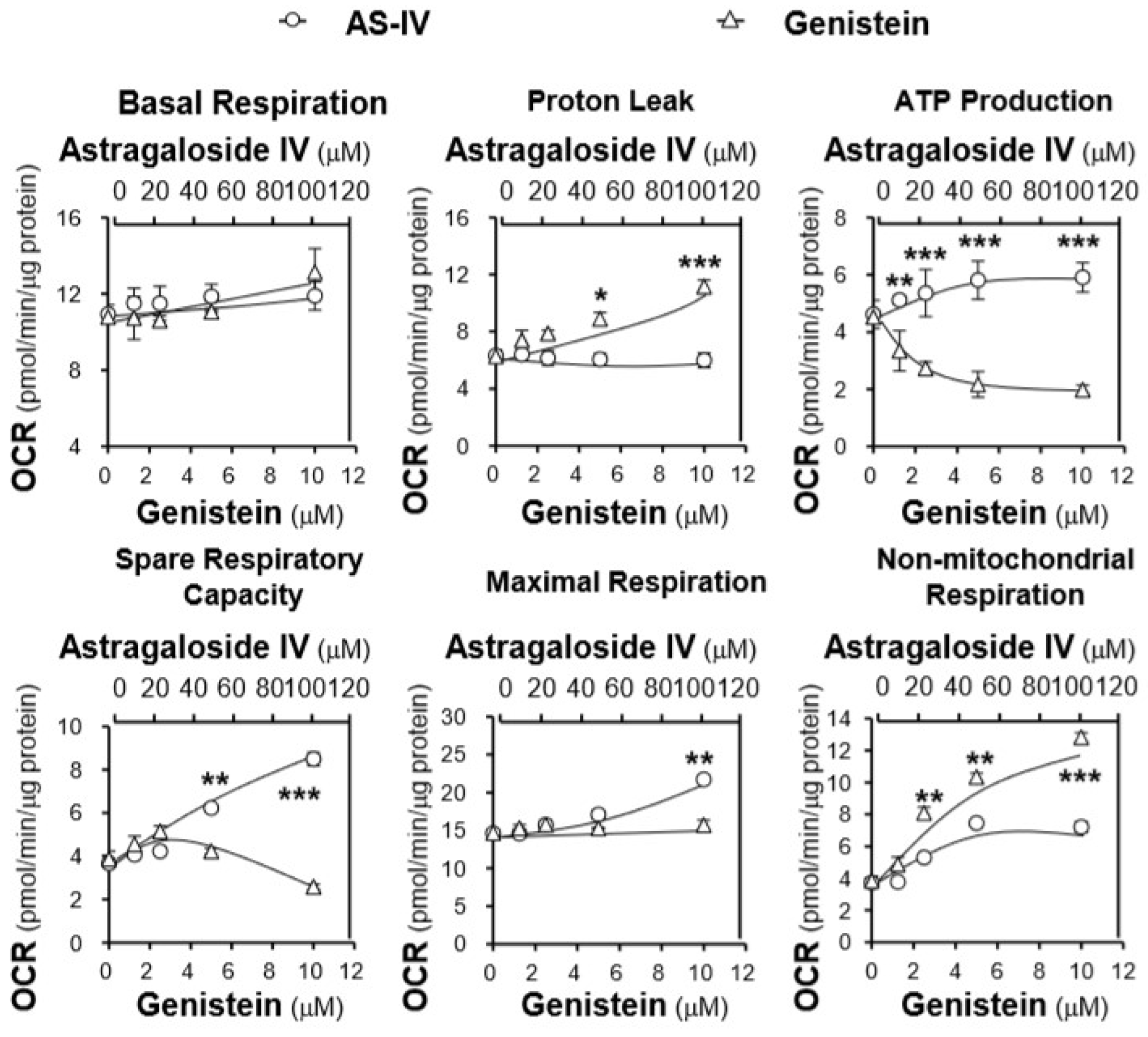
2.3. Effects of the AR Extracts and Compounds on Mitochondrial Bioenergetics
3. Discussion
4. Materials and Methods
4.1. Chemicals and Preparation of the RA Extracts
4.2. Standardization of Herbal Extracts
4.3. Cell Culture
4.4. Cell Viability
4.5. Folin–Ciocalteu Assay
4.6. DPPH Radical Scavenging Assay
4.7. tBHP-Induced Oxidative Stress Assay
4.8. ROS Formation Assay
4.9. Measurement of the Mitochondrial Membrane Potential
4.10. Mitochondrial Bioenergetic Analysis
4.11. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lin, H.Q.; Gong, A.G.W.; Wang, H.Y.; Duan, R.; Dong, T.T.X.; Zhao, K.J.; Tsim, K.W.K. Danggui Buxue Tang (Astragali Radix and Angelicae Sinensis Radix) for menopausal symptoms: A review. J. Ethnopharmacol. 2017, 199, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Leong, P.K.; Wong, H.S.; Chen, J.H.; Ko, K.M. Yang/Qi invigoration: An herbal therapy for chronic fatigue syndrome with Yang deficiency? Evid. Based Complement. Altern. 2015, 2015, 945901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.P.; Zhang, X.P.; Ruan, Y.F.; Ye, S.Y.; Zhao, H.C.; Cheng, Q.H.; Wu, D.J. Protective effect of Radix Astragali injection on immune organs of rats with obstructive jaundice and its mechanism. World J. Gastroenterol. 2009, 15, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, Z.; Ma, X.; Huang, Y.; Liu, X.; Tu, P.; Tong, T. HDTIC-1 and HDTIC-2, two compounds extracted from Astragali Radix, delay replicative senescence of human diploid fibroblasts. Mech. Ageing Dev. 2003, 124, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Qi, L.W.; Liu, E.H.; Li, B.; Gao, W.; Li, P. Radix Astragali (Astragalus): Latest advancements and trends in chemistry, analysis, pharmacology and pharmacokinetics. Curr. Org. Chem. 2010, 14, 1792–1807. [Google Scholar] [CrossRef]
- Luo, Z.; Zhong, L.; Han, X.; Wang, H.; Zhong, J.; Xuan, Z. Astragalus membranaceus prevents daunorubicin-induced apoptosis of cultured neonatal cardiomyocytes: Role of free radical effect of Astragalus membranaceus on daunorubicin cardiotoxicity. Phytother. Res. 2009, 23, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Song, F.; Liu, Z.; Liu, S. Studies on principal components and antioxidant activity of different Radix Astragali samples using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta 2009, 78, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Sadiq, A.; Abbas, A.; Mughal, E.; Khan, K.M.; Ali, M. Isolation and synthesis of flavonols and comparison of their antioxidant activity. Nat. Prod. Res. 2010, 24, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Liu, H.K.; Hsiao, P.C.; Yang Kuo, L.M.; Lee, I.J.; Wu, T.S.; Chiou, W.F.; Kuo, Y.H. New isoflavonoid glycosides and related constituents from Astragali Radix (Astragalus membranaceus) and their inhibitory activity on nitric oxide production. J. Agric. Food Chem. 2011, 59, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, Z.H.; Huang, L.F.; Zheng, S.H.; Wang, D.M.; Chen, S.L.; Zhang, H.T.; Yang, S.H. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hirotani, M.; Lui, K.H.; Furuya, T. Two triglycosidic triterpene astragalosides from hairy root cultures of Astragalus membranaceus. Phytochemistry 1994, 38, 1407–1410. [Google Scholar] [CrossRef]
- Dong, Z.W.; Zhang, C.; Chen, Y.J.; Chen, Y.; Yuan, Z.Q.; Peng, Y.Z.; Cao, T.T. Astragaloside-IV protects against heat-induced apoptosis by inhibiting excessive activation of mitochondrial Ca2+ uniporter. Cell. Physiol. Biochem. 2017, 42, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.Z.; Liu, W.H.; Liu, N.N.; Fu, X.Q.; Kwan, H.Y.; Liu, S.J.; Liu, B.R.; Zhang, S.W.; Yu, Z.L.; et al. Calycosin inhibits oxidative stress-induced cardiomyocyte apoptosis via activating estrogen receptor-α/β. Bioorg. Med. Chem. Lett. 2016, 26, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Xia, Z.Y.; Han, Y.F.; Rong, J.H. Plant natural product formononetin protects rat cardiomyocyte H9c2 cells against oxygen glucose deprivation and reoxygenation via inhibiting ROS formation and promoting GSK-3β phosphorylation. Oxid. Med. Cell. Longev. 2016, 2016, 2060874. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Bi, L.Y.; Li, Z.Z.; Zhang, X.; Ye, Y. Formononetin induces the mitochondrial apoptosis pathway in prostate cancer cells via downregulation of the IGF-1/IGF-1R signaling pathway. Pharm. Biol. 2014, 52, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Russo, G.L.; Daglia, M.; Kasi, P.D.; Ravi, S.; Nabavi, S.F.; Nabavi, S.M. Understanding genistein in cancer: The “good” and the “bad” effects: A review. Food Chem. 2016, 196, 589–600. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R. Evidence for genistein as a mitochondriotropic molecule. Mitochondrion 2016, 29, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.G.; Yun, C.K.; Lee, H.S. Screening and comparison of antioxidant activity of solvent extracts of herbal medicines used in Korea. J. Ethnopharmacol. 2003, 87, 231–236. [Google Scholar] [CrossRef]
- Navarro, A.; Boveris, A. The mitochondrial energy transduction system and the aging process. Am. J. Physiol. Cell Physiol. 2007, 292, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S. Mitochondrial H+ leak and ROS generation: An odd couple. Free Radic. Biol. Med. 2005, 38, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Dott, W.; Mistry, P.; Wright, J.; Cain, K.; Herbert, K.E. Modulation of mitochondrial bioenergetics in a skeletal muscle cell line model of mitochondrial toxicity. Redox Biol. 2014, 2, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Neilson, A.; Swift, A.L.; Moran, R.; Tamagnine, J.; Parslow, D.; Armistead, S.; Lemire, K.; Orrell, J.; Teich, J.; et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 2007, 292, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Han, S.Y.; Ma, X.; Zhang, K.; Wang, L.; Ma, Z.Z.; Tu, P.F. The saponin of red ginseng protects the cardiacmyocytes against ischemic injury in vitro and in vivo. Phytomedicine 2012, 19, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W.; Fang, J.; Tang, L.Y.; Lu, P.; Xu, H.Y.; Zhao, Y.; Li, D.F.; Zhang, Y.; Fu, M.H.; Yang, H.J. Quality evaluation of Astragali Radix based on DPPH radical scavenging activity and chemical analysis. Chin. Herb. Med. 2014, 6, 282–289. [Google Scholar] [CrossRef]
- Zheng, K.Y.Z.; Choi, R.C.Y.; Guo, A.J.Y.; Bi, C.W.C.; Zhu, K.Y.; Du, C.Y.Q.; Zhang, Z.X.; Lau, D.T.W.; Dong, T.T.X.; Tsim, K.W.K. The membrane permeability of Astragali Radix-derived formononetin and calycosin is increased by Angelicae Sinensis Radix in Caco-2 cells: A synergistic action of an ancient herbal decoction Danggui Buxue Tang. J. Pharm. Biomed. 2012, 70, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kwan, K.K.L.; Leung, K.W.; Yao, P.; Wang, H.Y.; Dong, T.T.X.; Tsim, K.W.K. Ginseng extracts modulate mitochondrial bioenergetics of live cardiomyoblasts: A functional comparison of different extraction solvents. J. Ginseng Res. 2018. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zu, Y.G.; Fu, Y.J.; Luo, M.; Zhao, C.J.; Wang, W.; Zhao, B.S.; Li, J.; Efferth, T. Preparation and antioxidant activity of Radix Astragali residues extracts rich in calycosin and formononetin. Biochem. Eng. J. 2011, 56, 84–93. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, Z.Z.; Yan, R.Q.; Olaleye, O.; Zhang, X.L.; Chai, X.; Wang, Y.F. Quality evaluation of Astragali Radix products by quantitative analysis of multi-components by single marker. Chin. Herb. Med. 2013, 5, 272–279. [Google Scholar] [CrossRef]
- Yoon, H.S.; Moon, S.C.; Kim, N.D.; Park, B.S.; Jeong, M.H.; Yoo, Y.H. Genistein induces apoptosis of RPE-J cells by opening mitochondrial PTP. Biochem. Biophys. Res. Commun. 2000, 276, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.M.; Johannsen, D.L.; Ravussin, E. Skeletal muscle mitochondria and aging: A review. J. Aging Res. 2012, 2012, 194821. [Google Scholar] [CrossRef] [PubMed]
- Li, A.E.; Ito, H.; Rovira, I.I.; Kim, K.S.; Takeda, K.; Yu, Z.Y.; Ferrans, V.J.; Finkel, T. A role for reactive oxygen species in endothelial cell anoikis. Circ. Res. 1999, 85, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Salabei, J.K.; Gibb, A.A.; Hill, B.G. Comprehensive measurement of respiratory activity in permeabilized cells using extracellular flux analysis. Nat. Protoc. 2014, 9, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, S.; Wu, H.F.; Bian, Z.P.; Xu, J.D.; Gu, C.R.; Chen, X.J.; Yang, D. Beneficial effects of astragaloside IV against angiotensin II-induced mitochondrial dysfunction in rat vascular smooth muscle cells. Int. J. Mol. Med. 2015, 36, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.W.; Zhao, P.; Xu, M.; Zhang, C.; Guo, W.; Chen, H.H.; Tian, J.; Wei, H.C.; Iu, R.; Cao, T.T. Astragaloside IV alleviates heart failure via activating PPARα to switch glycolysis to fatty acid β-oxidation. Sci. Rep. 2017, 7, 2691. [Google Scholar] [CrossRef] [PubMed]
- Rasbach, K.A.; Schnellmann, R.G. Isoflavones promote mitochondrial biogenesis. J. Pharmacol. Exp. Ther. 2008, 325, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ramirez, V.D. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br. J. Pharmacol. 2000, 130, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
| Standards | AR-1 1 | AR-2 | AR-3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| W 2 | E50 | E90 | W | E50 | E90 | W | E50 | E90 | |
| Calycosin | 522.3 ± 6.32 3 | 491.56 ± 6.12 | 468.26 ± 2.92 | 467.23 ± 5.86 | 399.45 ± 7.42 | 333.22 ± 1.32 | 498.22 ± 5.89 | 387.22 ± 5.49 | 318.98 ± 8.19 |
| Astragaloside IV | 686.67 ± 4.23 | 579.23 ± 3.12 | 543.98 ± 2.39 | 537.28 ± 2.89 | 469.02 ± 2.19 | 450.22 ± 1.29 | 543.33 ± 5.67 | 582.11 ± 5.92 | 512.45 ± 4.89 |
| Genistein | 6.58 ± 0.13 | 9.14 ± 0.21 | 7.03 ± 0.22 | 5.43 ± 0.11 | 8.99 ± 0.32 | 7.93 ± 0.12 | 4.89 ± 0.21 | 5.89 ± 0.11 | 5.83 ± 0.12 |
| Formononetin | 139.34 ± 7.23 | 192.29 ± 1.91 | 179.34 ± 1.21 | 169.18 ± 5.38 | 187.29 ± 2.99 | 166.98 ± 1.11 | 169.29 ± 4.89 | 188.27 ± 3.22 | 138.39 ± 8.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Kwan, K.K.L.; Leung, K.W.; Wang, H.; Kong, X.P.; Dong, T.T.X.; Tsim, K.W.K. The Extracts and Major Compounds Derived from Astragali Radix Alter Mitochondrial Bioenergetics in Cultured Cardiomyocytes: Comparison of Various Polar Solvents and Compounds. Int. J. Mol. Sci. 2018, 19, 1574. https://doi.org/10.3390/ijms19061574
Huang Y, Kwan KKL, Leung KW, Wang H, Kong XP, Dong TTX, Tsim KWK. The Extracts and Major Compounds Derived from Astragali Radix Alter Mitochondrial Bioenergetics in Cultured Cardiomyocytes: Comparison of Various Polar Solvents and Compounds. International Journal of Molecular Sciences. 2018; 19(6):1574. https://doi.org/10.3390/ijms19061574
Chicago/Turabian StyleHuang, Yun, Kenneth Kin Leung Kwan, Ka Wing Leung, Huaiyou Wang, Xiang Peng Kong, Tina Ting Xia Dong, and Karl Wah Keung Tsim. 2018. "The Extracts and Major Compounds Derived from Astragali Radix Alter Mitochondrial Bioenergetics in Cultured Cardiomyocytes: Comparison of Various Polar Solvents and Compounds" International Journal of Molecular Sciences 19, no. 6: 1574. https://doi.org/10.3390/ijms19061574
APA StyleHuang, Y., Kwan, K. K. L., Leung, K. W., Wang, H., Kong, X. P., Dong, T. T. X., & Tsim, K. W. K. (2018). The Extracts and Major Compounds Derived from Astragali Radix Alter Mitochondrial Bioenergetics in Cultured Cardiomyocytes: Comparison of Various Polar Solvents and Compounds. International Journal of Molecular Sciences, 19(6), 1574. https://doi.org/10.3390/ijms19061574







