Serine Protease Inhibitor SERPINE2 Reversibly Modulates Murine Sperm Capacitation
Abstract
1. Introduction
2. Results
2.1. SERPINE2 Binds to Both Capacitated and Uncapacitated Sperm
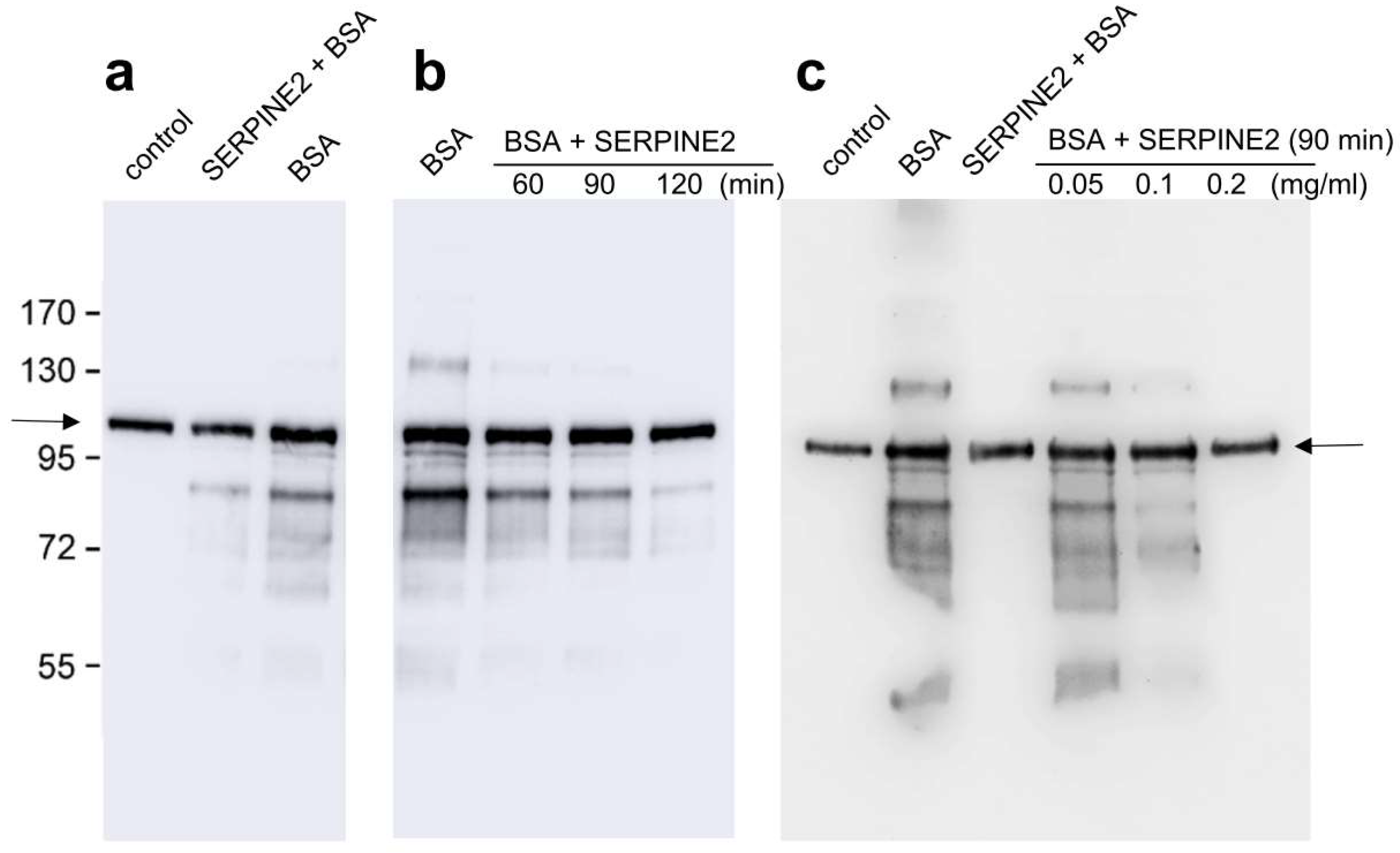
2.2. SERPINE2 Reverses BSA-Induced Protein Tyrosine Phosphorylation in Sperm
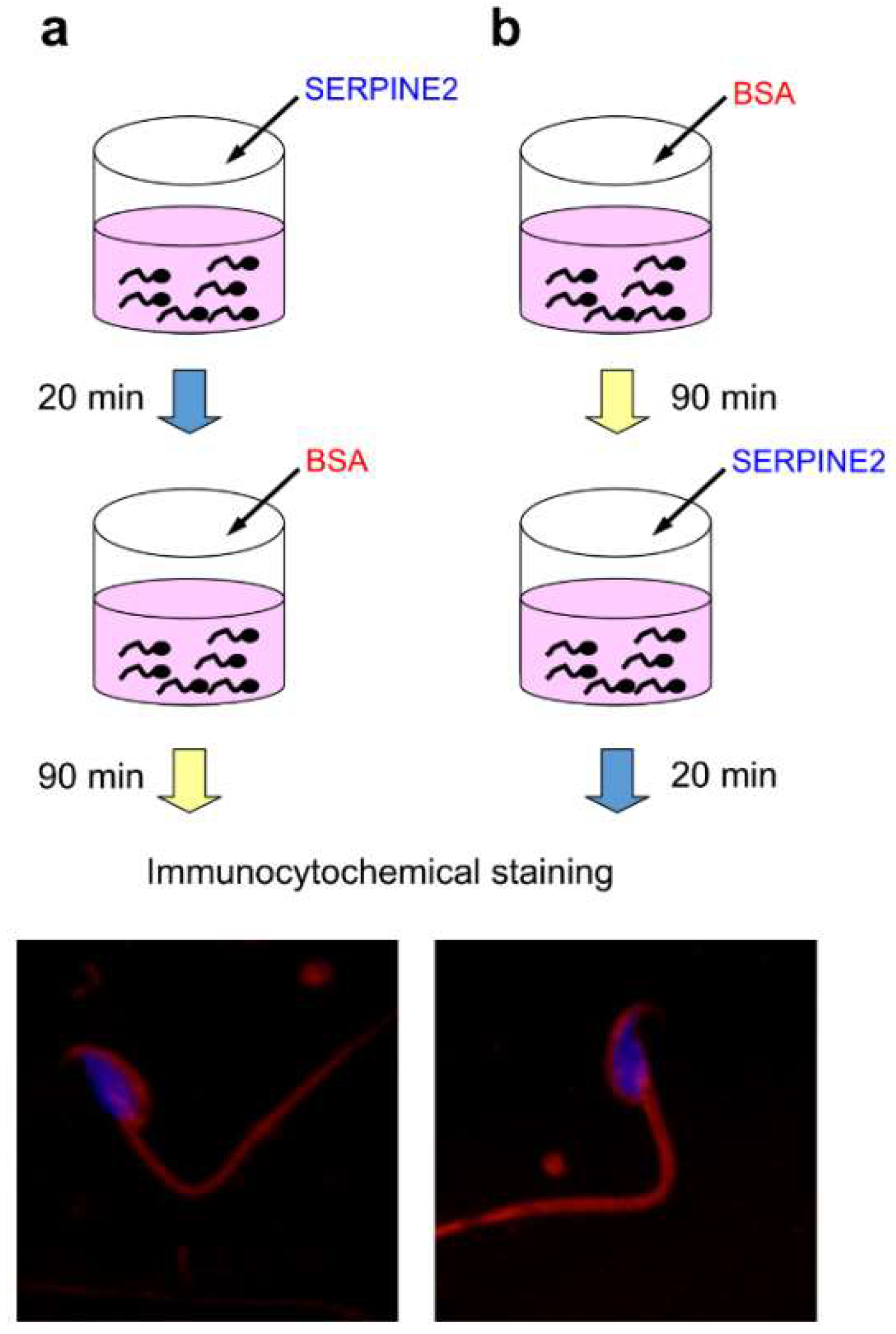
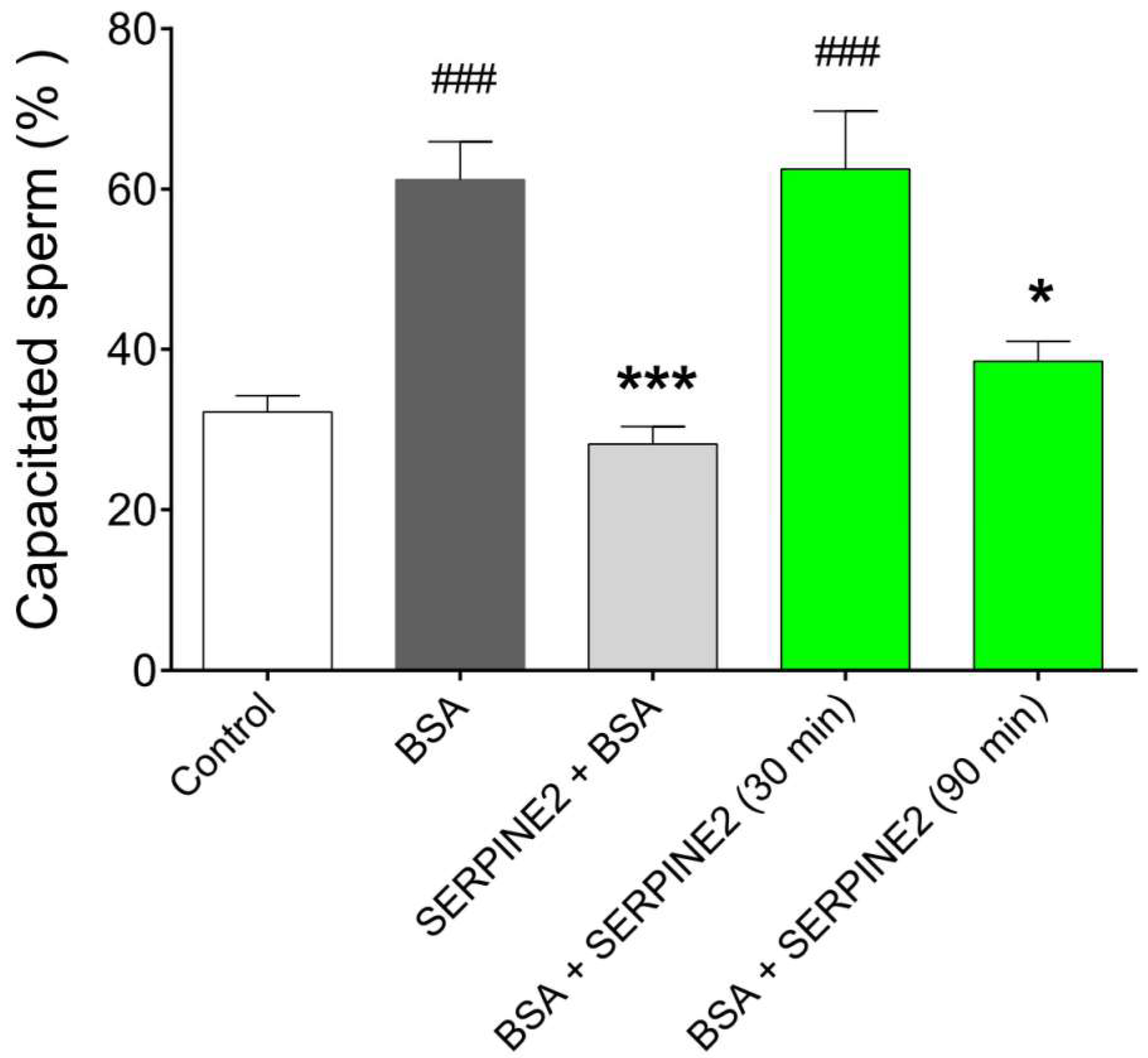
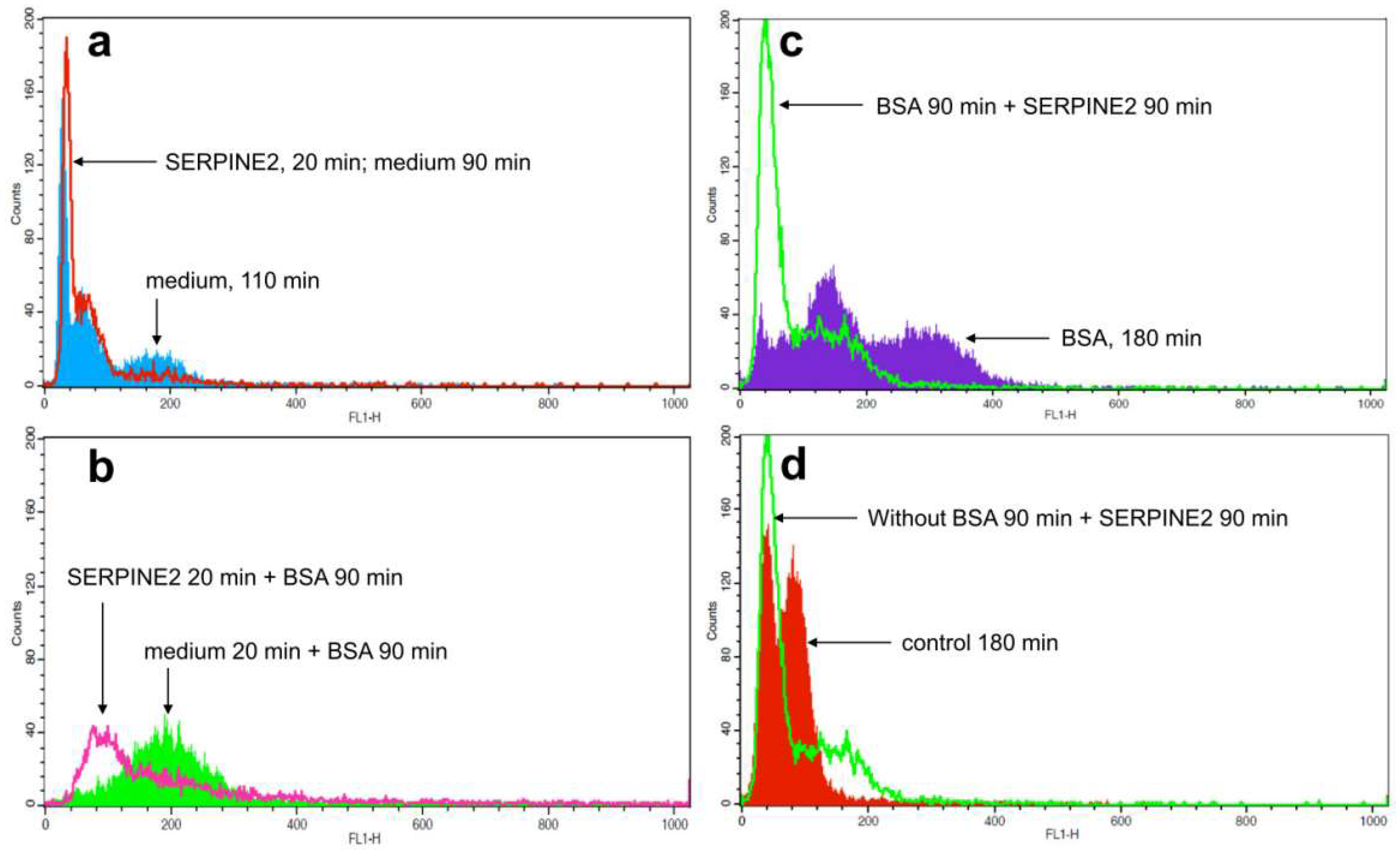
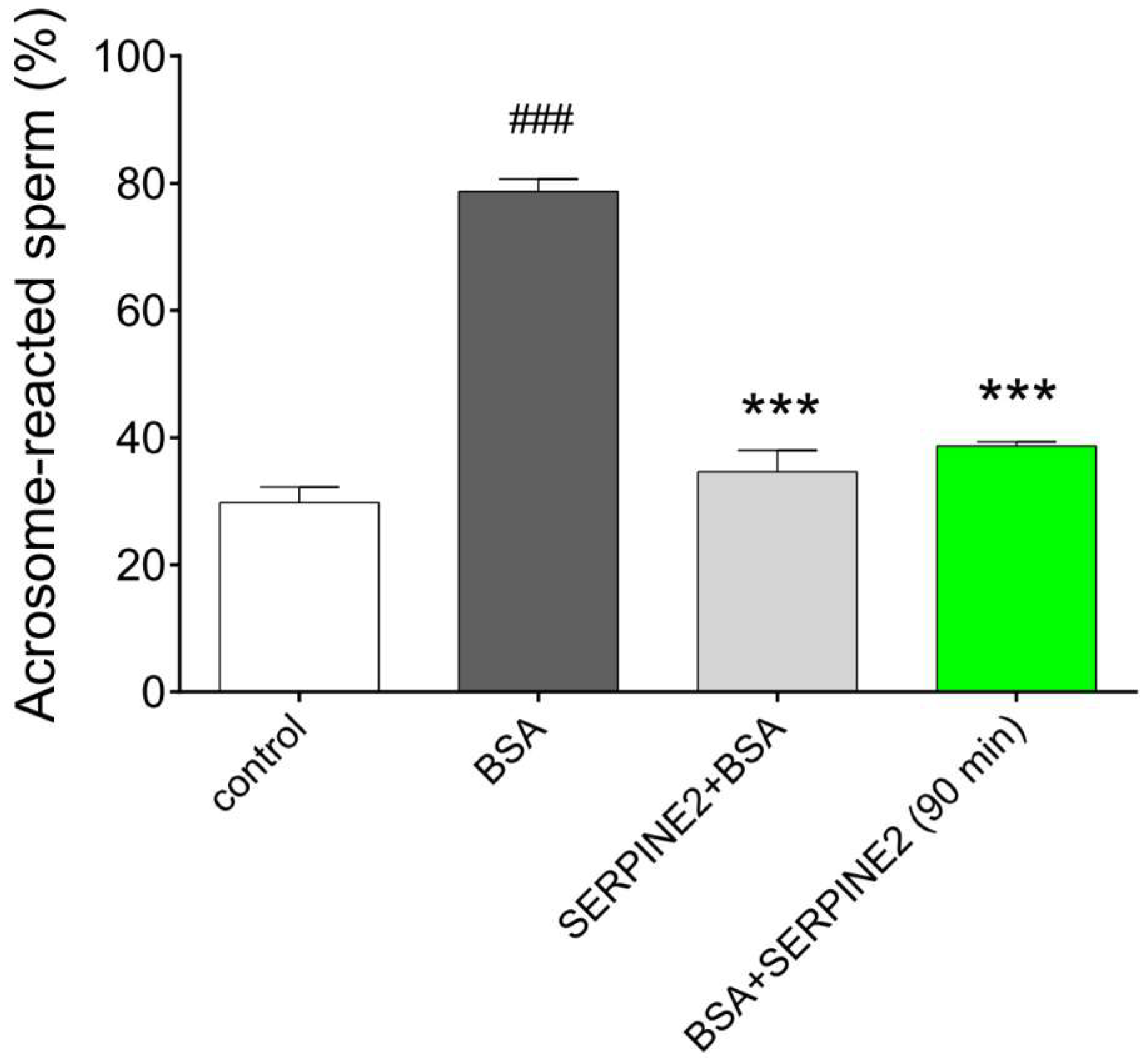
2.3. SERPINE2 Reversibly Modulates Sperm Capacitation
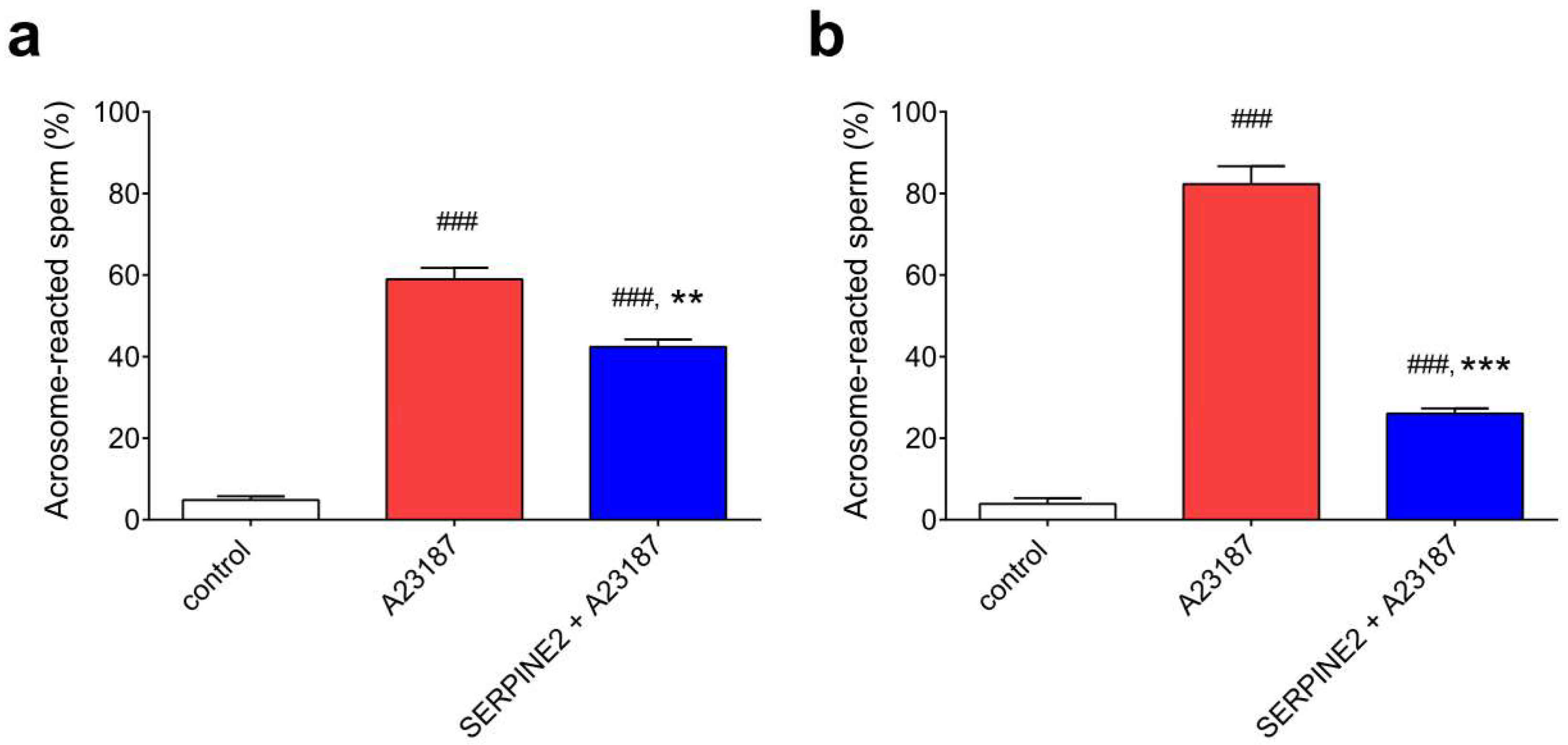
2.4. SERPINE2 Suppresses the Acrosome Reaction of Sperm Isolated from the Oviduct
3. Discussion
4. Materials and Methods
4.1. Animals and Sperm Preparation
4.2. Preparation of the SERPINE2 Protein and Its Antiserum
4.3. Immunolocalization of SERPINE2 on Murine Sperm
4.4. Sperm Protein Tyrosine Phosphorylation
4.5. CTC Fluorescence Assay for Sperm Capacitation
4.6. Measurement of Sperm [Ca2+]i
4.7. Evaluation of the Acrosome Reaction
4.8. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| BSA | bovine serum albumin |
| BWW | biggers, whitten, and whittingham media |
| [Ca2+]i | sperm intracellular calcium ion |
| CTC | chlortetracycline |
| DF | decapacitation factor |
| mKRB | modified Krebs-Ringer bicarbonate medium |
| PBS | phosphate-buffered saline |
| PNA | peanut agglutinin lectin |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| SVS | seminal vesicle secretion |
| SERPINE2 | serpin peptidase inhibitor, clade E, member 2 |
| TRITC | tetramethylrhodamine isothiocyanate |
References
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.R. The capacitation of the mammalian sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Toyoda, Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol. Reprod. 1998, 59, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.L. Role of cholesterol in sperm capacitation. Biol. Reprod. 1998, 59, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Go, K.J.; Wolf, D.P. Albumin-mediated changes in sperm sterol content during capacitation. Biol. Reprod. 1985, 32, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Shadan, S.; James, P.S.; Howes, E.A.; Jones, R. Cholesterol efflux alters lipid raft stability and distribution during capacitation of boar spermatozoa. Biol. Reprod. 2004, 71, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Kopf, G.S. Regulation of protein phosphorylation during sperm capacitation. Biol. Reprod. 1998, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C. A detrimental effect of seminal plasma on the fertilizing capacity of sperm. Nature 1957, 179, 258–259. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Fraser, L.R.; Harrison, R.A.; Herod, J.E. Characterization of a decapacitation factor associated with epididymal mouse spermatozoa. J. Reprod. Fertil. 1990, 89, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chu, S.T.; Chen, Y.H. A seminal vesicle autoantigen of mouse is able to suppress sperm capacitation-related events stimulated by serum albumin. Biol. Reprod. 2000, 63, 1562–1566. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawano, N.; Yoshida, M. Semen-coagulating protein, svs2, in mouse seminal plasma controls sperm fertility. Biol. Reprod. 2007, 76, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Lee, R.K.; Hwu, Y.M.; Chu, S.L.; Chen, Y.J.; Chang, W.C.; Lin, S.P.; Li, S.H. Serpine2, a serine protease inhibitor extensively expressed in adult male mouse reproductive tissues, may serve as a murine sperm decapacitation factor. Biol. Reprod. 2011, 84, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; MacIntyre, D.A.; Mitchell, L.A.; Gibbs, G.M.; O'Bryan, M.; Aitken, R.J. The identification of mouse sperm-surface-associated proteins and characterization of their ability to act as decapacitation factors. Biol. Reprod. 2006, 74, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.W.; Bergman, B.L.; Bajpai, A.; Hersh, R.T.; Rodriguez, H.; Jones, B.N.; Barreda, C.; Watts, S.; Baker, J.B. Protease nexin. Properties and a modified purification procedure. J. Biol. Chem. 1985, 260, 7029–7034. [Google Scholar] [PubMed]
- Bedard, J.; Brule, S.; Price, C.A.; Silversides, D.W.; Lussier, J.G. Serine protease inhibitor-e2 (serpine2) is differentially expressed in granulosa cells of dominant follicle in cattle. Mol. Reprod. Dev. 2003, 64, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Chern, S.R.; Li, S.H.; Lu, C.H.; Chen, E.I. Spatiotemporal expression of the serine protease inhibitor, serpine2, in the mouse placenta and uterus during the estrous cycle, pregnancy, and lactation. Reprod. Biol. Endocrinol. RB&E 2010, 8, 127. [Google Scholar]
- Lee, R.K.; Fan, C.C.; Hwu, Y.M.; Lu, C.H.; Lin, M.H.; Chen, Y.J.; Li, S.H. Serpine2, an inhibitor of plasminogen activators, is highly expressed in the human endometrium during the secretory phase. Reprod. Biol. Endocrinol. RB&E 2011, 9, 38. [Google Scholar]
- Lu, C.H.; Lee, R.K.; Hwu, Y.M.; Lin, M.H.; Yeh, L.Y.; Chen, Y.J.; Lin, S.P.; Li, S.H. Involvement of the serine protease inhibitor, serpine2, and the urokinase plasminogen activator in cumulus expansion and oocyte maturation. PLoS ONE 2013, 8, e74602. [Google Scholar] [CrossRef] [PubMed]
- Vassalli, J.D.; Huarte, J.; Bosco, D.; Sappino, A.P.; Sappino, N.; Velardi, A.; Wohlwend, A.; Erno, H.; Monard, D.; Belin, D. Protease-nexin i as an androgen-dependent secretory product of the murine seminal vesicle. EMBO J. 1993, 12, 1871–1878. [Google Scholar] [PubMed]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Leclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a camp-dependent pathway. Development 1995, 121, 1139–1150. [Google Scholar] [PubMed]
- Xia, J.; Ren, D. The bsa-induced ca2+ influx during sperm capacitation is catsper channel-dependent. Reprod. Biol. Endocrinol. RB&E 2009, 7, 119. [Google Scholar]
- Bedford, J.M.; Chang, M.C. Removal of decapacitation factor from seminal plasma by high-speed centrifugation. Am. J. Physiol. 1962, 202, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, H.N.; Dukelow, W.R. Decapacitation factor purification with lipid solvents. J. Reprod. Fertil. 1969, 18, 141–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliphant, G.; Reynolds, A.B.; Thomas, T.S. Sperm surface components involved in the control of the acrosome reaction. Am. J. Anat. 1985, 174, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Mangels, R.; Dean, M.D. The molecular basis and reproductive function(s) of copulatory plugs. Mol. Reprod. Dev. 2016, 83, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Kawano, N.; Araki, N.; Yoshida, K.; Hibino, T.; Ohnami, N.; Makino, M.; Kanai, S.; Hasuwa, H.; Yoshida, M.; Miyado, K.; et al. Seminal vesicle protein svs2 is required for sperm survival in the uterus. Proc. Natl. Acad. Sci. USA 2014, 111, 4145–4150. [Google Scholar] [CrossRef] [PubMed]
- Araki, N.; Trencsenyi, G.; Krasznai, Z.T.; Nizsaloczki, E.; Sakamoto, A.; Kawano, N.; Miyado, K.; Yoshida, K.; Yoshida, M. Seminal vesicle secretion 2 acts as a protectant of sperm sterols and prevents ectopic sperm capacitation in mice. Biol. Reprod. 2015, 92, 8. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Storey, B.T. Evidence for plasma membrane impermeability to small ions in acrosome-intact mouse spermatozoa bound to mouse zonae pellucidae, using an aminoacridine fluorescent ph probe: Time course of the zona-induced acrosome reaction monitored by both chlortetracycline and pH probe fluorescence. Biol. Reprod. 1985, 33, 235–246. [Google Scholar] [PubMed]
- Ward, C.R.; Storey, B.T. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev. Biol. 1984, 104, 287–296. [Google Scholar] [CrossRef]
- Ryu, D.Y.; Kim, Y.J.; Lee, J.S.; Rahman, M.S.; Kwon, W.S.; Yoon, S.J.; Pang, M.G. Capacitation and acrosome reaction differences of bovine, mouse and porcine spermatozoa in responsiveness to estrogenic compounds. J. Anim. Sci. Technol. 2014, 56, 26. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; You, Y.A.; Pang, M.G. Improving litter size by boar spermatozoa: Application of combined h33258/ctc staining in field trial with artificial insemination. Andrology 2015, 3, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.S.; Shin, D.H.; Ryu, D.Y.; Khatun, A.; Rahman, M.S.; Pang, M.G. Applications of capacitation status for litter size enhancement in various pig breeds. Asian-Aust. J. Anim. Sci. 2018, 31, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Wennemuth, G.; Babcock, D.F.; Hille, B. Calcium clearance mechanisms of mouse sperm. J. Gen. Physiol. 2003, 122, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar] [PubMed]
- Kwon, W.S.; Rahman, M.S.; Pang, M.G. Diagnosis and prognosis of male infertility in mammal: The focusing of tyrosine phosphorylation and phosphotyrosine proteins. J. Proteome Res. 2014, 13, 4505–4517. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; de Mateo, S.; Estanyol, J.M. Sperm cell proteomics. Proteomics 2009, 9, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Rahman, M.S.; Kwon, W.S.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Proteomic identification of cryostress in epididymal spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Bleil, J.D.; Wassarman, P.M. Sperm-egg interactions in the mouse: Sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev. Biol. 1983, 95, 317–324. [Google Scholar] [CrossRef]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Lee, R.K.; Hwu, Y.M.; Lu, C.H.; Chu, S.L.; Chen, Y.J.; Chang, W.C.; Li, S.H. Spinkl, a kazal-type serine protease inhibitor-like protein purified from mouse seminal vesicle fluid, is able to inhibit sperm capacitation. Reproduction 2008, 136, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.C.; Lee, R.K.; Hwu, Y.M.; Lu, C.H.; Lin, M.H.; Li, S.H. Mechanisms underlying the inhibition of murine sperm capacitation by the seminal protein, spinkl. J. Cell. Biochem. 2013, 114, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, M.; Shi, Q.; Zhang, L.; Zhen, W.; Chen, W.; Zhang, Y. An epididymis-specific secretory protein hongres1 critically regulates sperm capacitation and male fertility. PLoS ONE 2008, 3, e4106. [Google Scholar] [CrossRef] [PubMed]
- De Lamirande, E.; Yoshida, K.; Yoshiike, T.M.; Iwamoto, T.; Gagnon, C. Semenogelin, the main protein of semen coagulum, inhibits human sperm capacitation by interfering with the superoxide anion generated during this process. J. Androl. 2001, 22, 672–679. [Google Scholar] [PubMed]
- Lee, R.K.; Tseng, H.C.; Hwu, Y.M.; Fan, C.C.; Lin, M.H.; Yu, J.J.; Yeh, L.Y.; Li, S.H. Expression of cystatin c in the female reproductive tract and its effect on human sperm capacitation. Reprod. Biol. Endocrinol. RB&E 2018, 16, 8. [Google Scholar]
- Martins, S.G.; Miranda, P.V.; Brandelli, A. Acrosome reaction inhibitor released during in vitro sperm capacitation. Int. J. Androl. 2003, 26, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.C.; Chung, M.K.; Tsang, H.Y.; Koistinen, R.; Koistinen, H.; Seppala, M.; Lee, K.F.; Yeung, W.S. Glycodelin-s in human seminal plasma reduces cholesterol efflux and inhibits capacitation of spermatozoa. J. Biol. Chem. 2005, 280, 25580–25589. [Google Scholar] [CrossRef] [PubMed]
- Carballada, R.; Esponda, P. Fate and distribution of seminal plasma proteins in the genital tract of the female rat after natural mating. J. Reprod. Fertil. 1997, 109, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Jappel, D. Rapid transport of seminal immunoglobulin to distal parts of the female reproductive tract in rabbits. J. Reprod. Fertil. 1992, 96, 135–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brannigan, R.; Lipshultz, L. Sperm transport and capacitation. In Women’s Medicine; The Global Library of Women’s Medicine: London, UK, 2008. [Google Scholar]
- Suarez, S.S. Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 2008, 52, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Biggers, J.D.; Whitten, W.K.; Whittingham, D.G. The culture of mouse embryos in vitro. In Methods in Mammalian Embryology; Daniels, J.C., Ed.; Freeman Press: San Francisco, CA, USA, 1971; pp. 86–116. [Google Scholar]
- Lee, M.A.; Storey, B.T. Bicarbonate is essential for fertilization of mouse eggs: Mouse sperm require it to undergo the acrosome reaction. Biol. Reprod. 1986, 34, 349–356. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.-H.; Hwu, Y.-M.; Lu, C.-H.; Lin, M.-H.; Yeh, L.-Y.; Lee, R.K.-K. Serine Protease Inhibitor SERPINE2 Reversibly Modulates Murine Sperm Capacitation. Int. J. Mol. Sci. 2018, 19, 1520. https://doi.org/10.3390/ijms19051520
Li S-H, Hwu Y-M, Lu C-H, Lin M-H, Yeh L-Y, Lee RK-K. Serine Protease Inhibitor SERPINE2 Reversibly Modulates Murine Sperm Capacitation. International Journal of Molecular Sciences. 2018; 19(5):1520. https://doi.org/10.3390/ijms19051520
Chicago/Turabian StyleLi, Sheng-Hsiang, Yuh-Ming Hwu, Chung-Hao Lu, Ming-Huei Lin, Ling-Yu Yeh, and Robert Kuo-Kuang Lee. 2018. "Serine Protease Inhibitor SERPINE2 Reversibly Modulates Murine Sperm Capacitation" International Journal of Molecular Sciences 19, no. 5: 1520. https://doi.org/10.3390/ijms19051520
APA StyleLi, S.-H., Hwu, Y.-M., Lu, C.-H., Lin, M.-H., Yeh, L.-Y., & Lee, R. K.-K. (2018). Serine Protease Inhibitor SERPINE2 Reversibly Modulates Murine Sperm Capacitation. International Journal of Molecular Sciences, 19(5), 1520. https://doi.org/10.3390/ijms19051520




