Doxycycline Impairs Mitochondrial Function and Protects Human Glioma Cells from Hypoxia-Induced Cell Death: Implications of Using Tet-Inducible Systems
Abstract
1. Introduction
2. Results
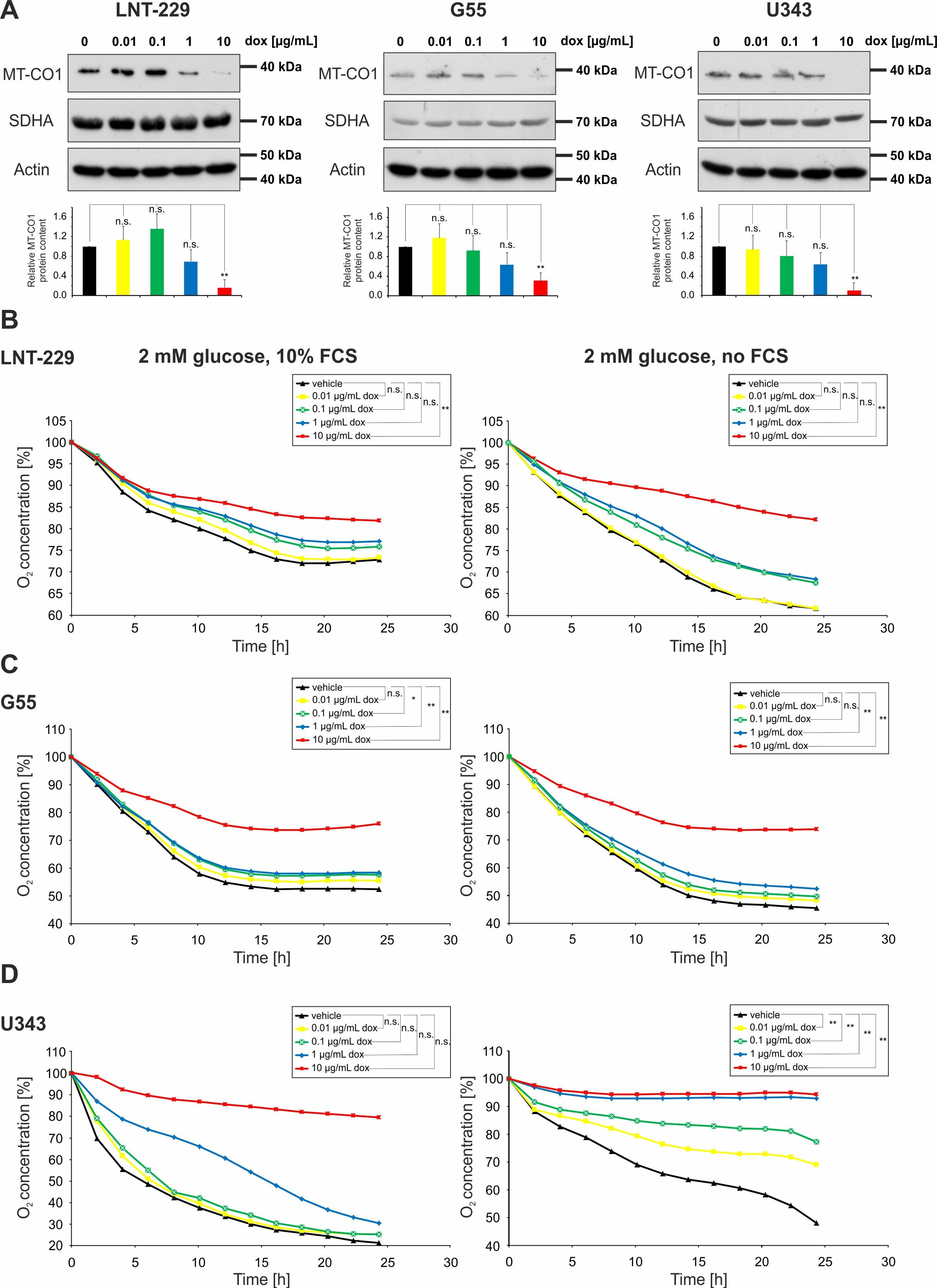
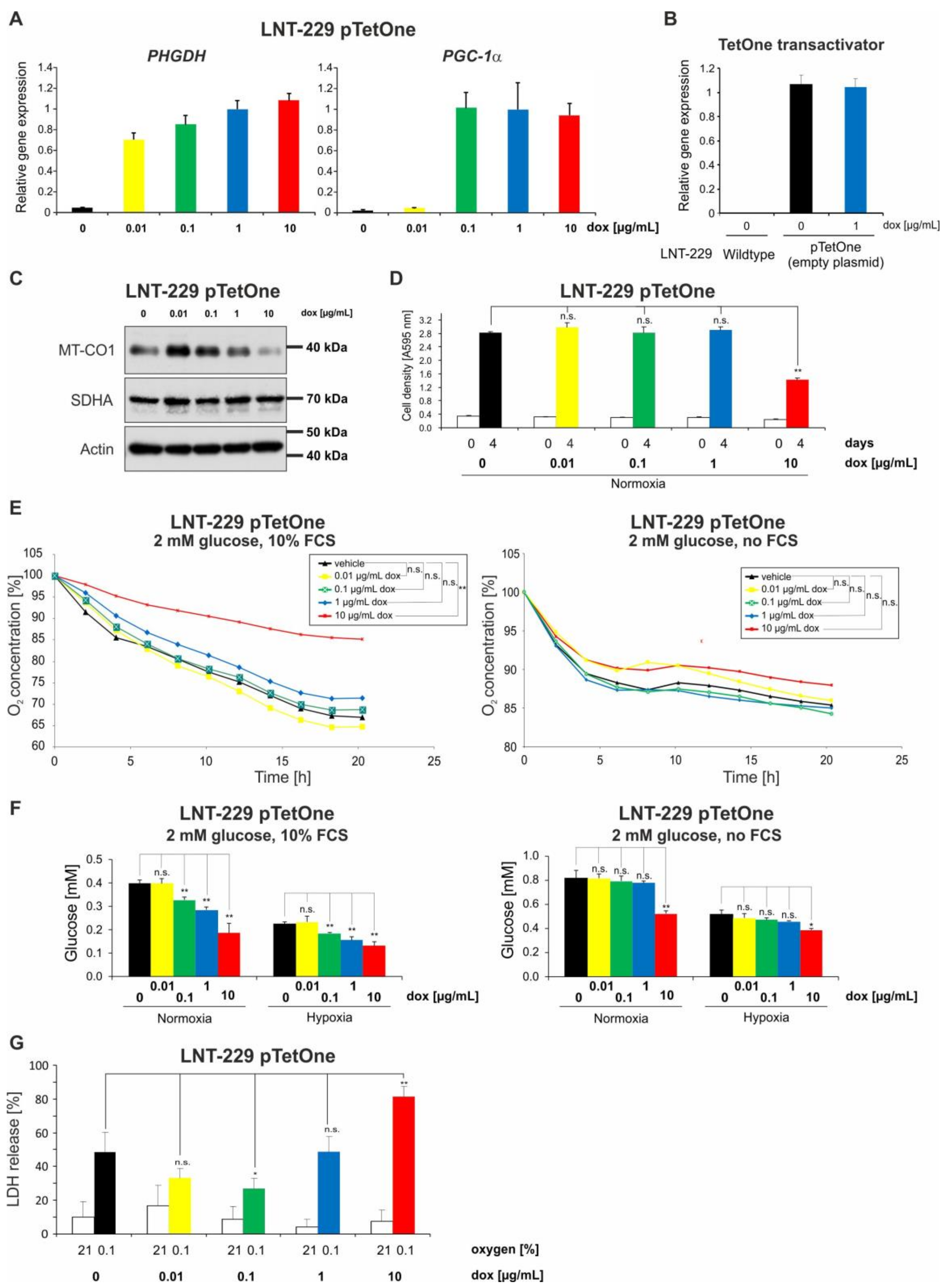
2.1. Doxycycline Impairs Mitochondrial Function in Glioma Cells
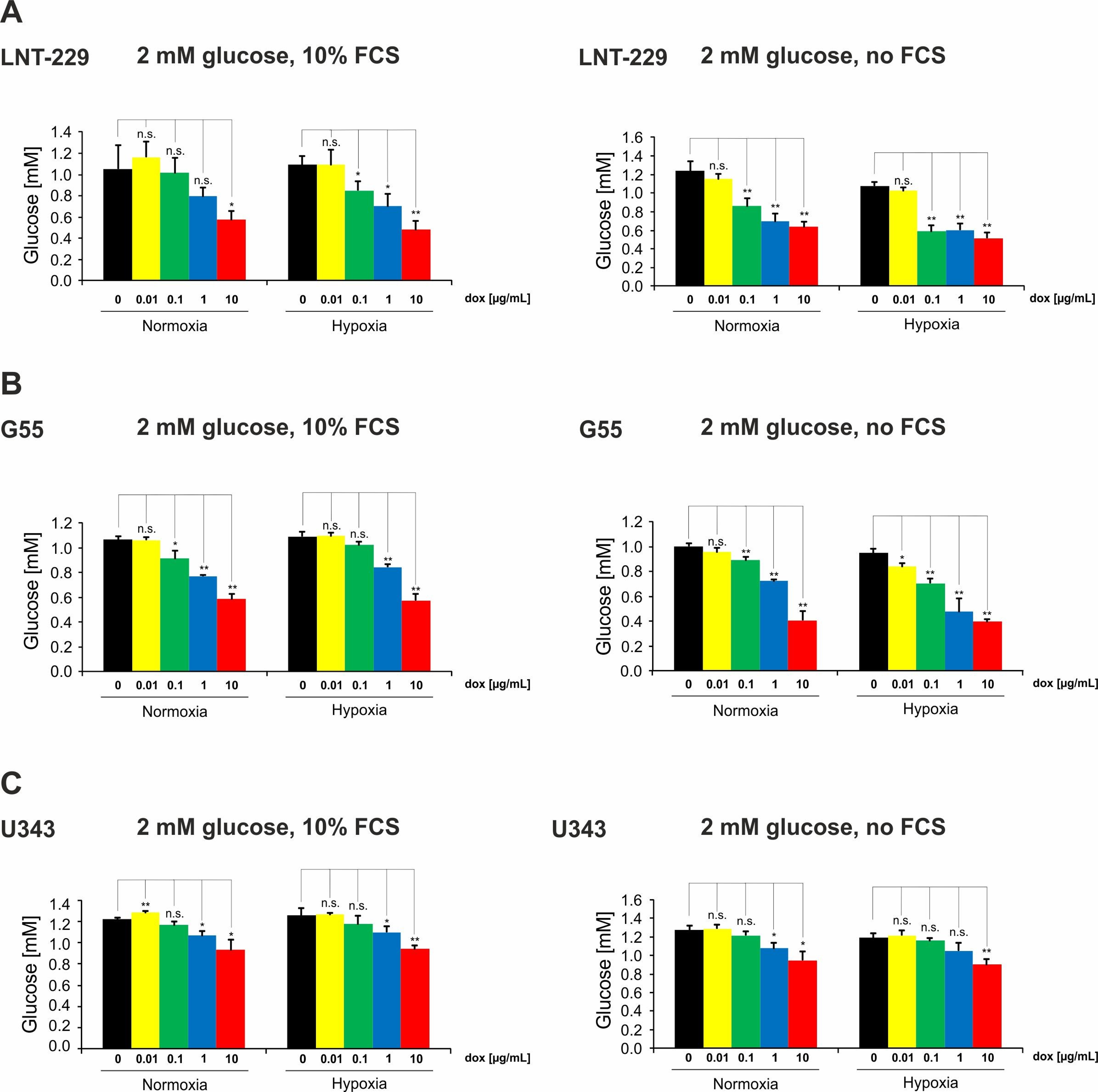
2.2. Doxycycline Induces Glucose Consumption in Glioma Cells
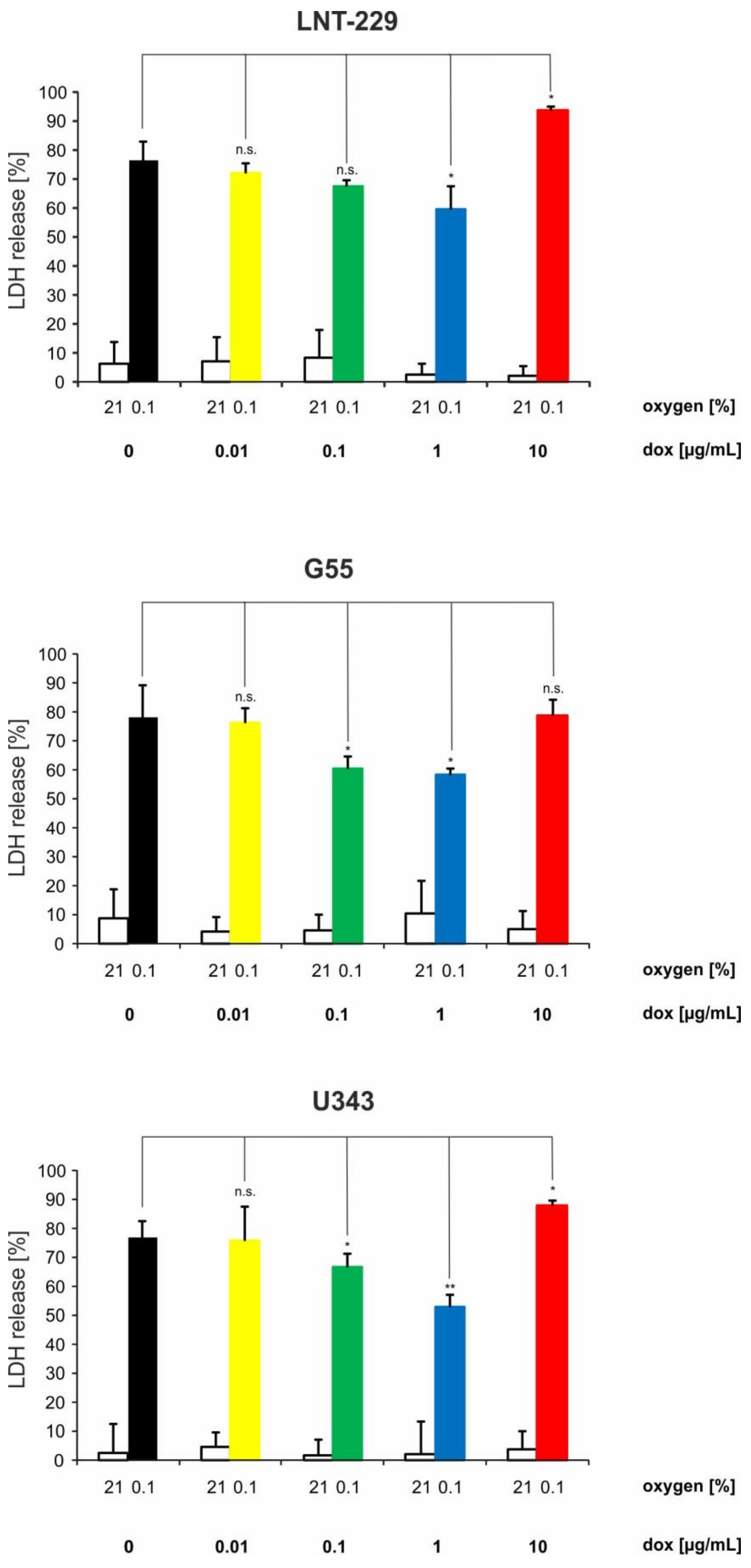
2.3. Doxycycline Can Have Converse Effects on Hypoxia-Induced Cell Death in Glioma Cells
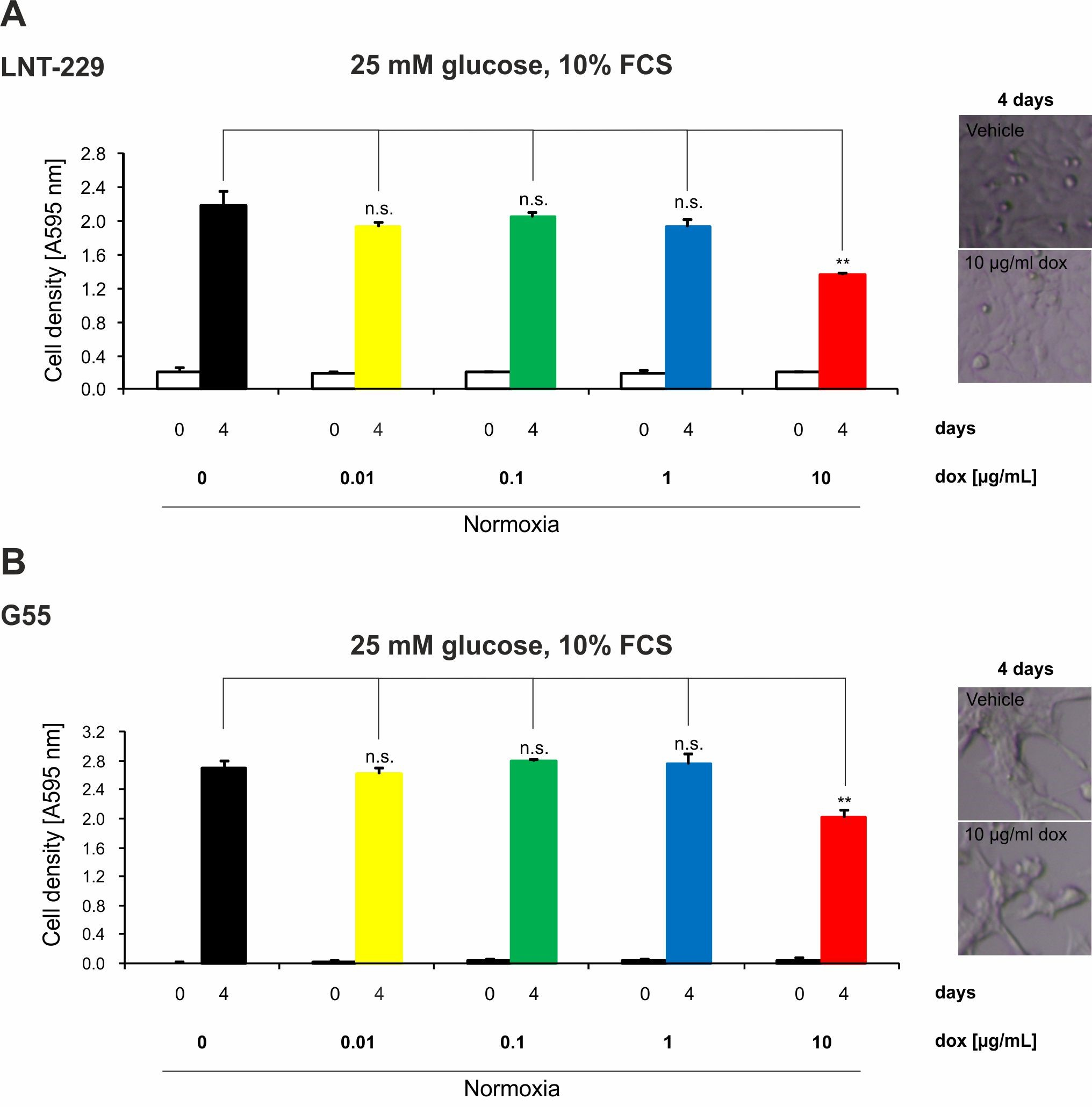
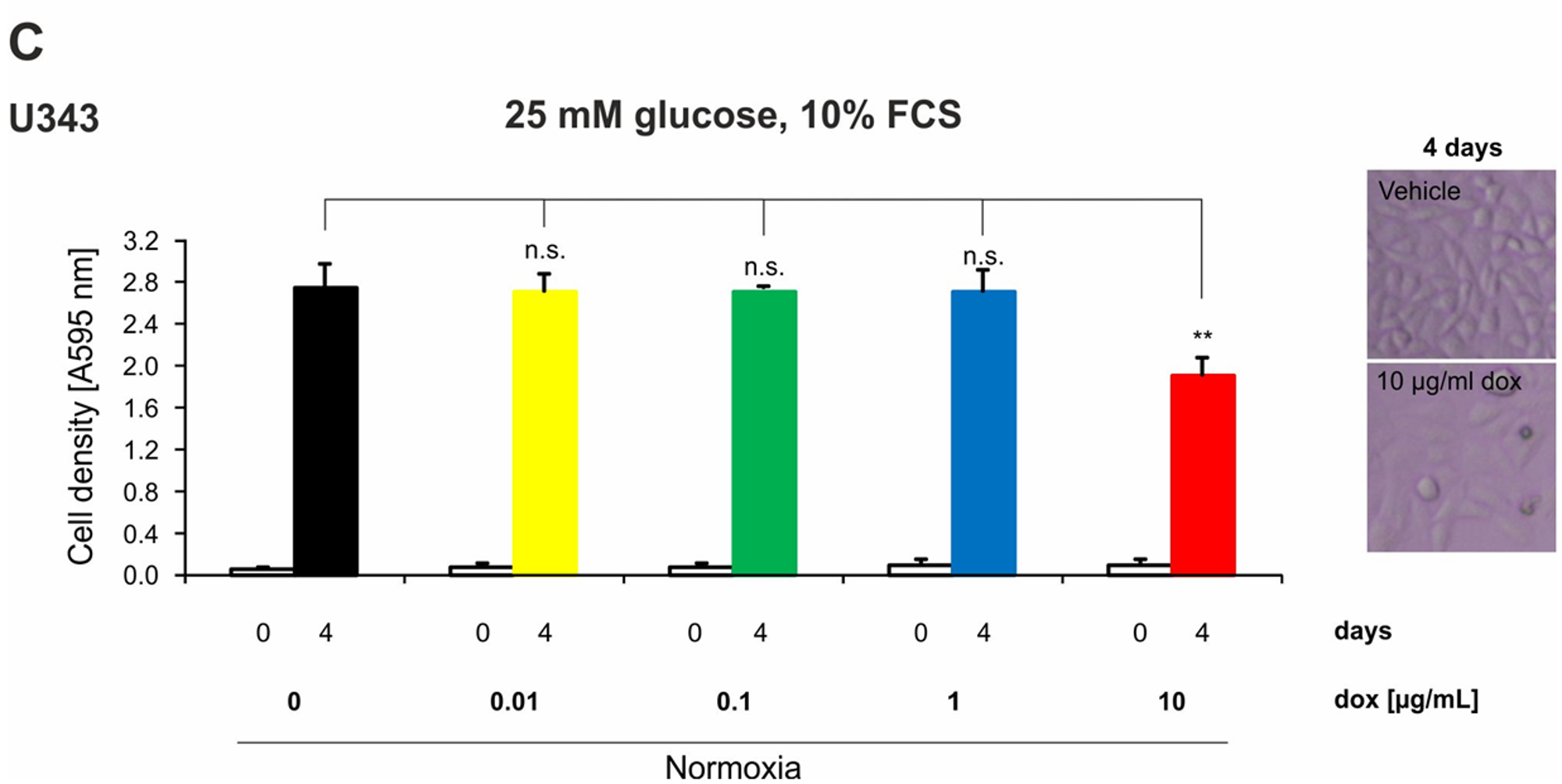
2.4. Doxycycline Inhibits Growth of Glioma Cells Only under High Concentrations
2.5. Doxycycline Concentrations of 0.1 and 1 µg/mL Are Sufficient for Full Induction of a Tet-System and Doxycycline-Mediated Effects on Metabolism in Tet-System-Transfected Glioma Cells Correspond to Those in Wild-Type Cells
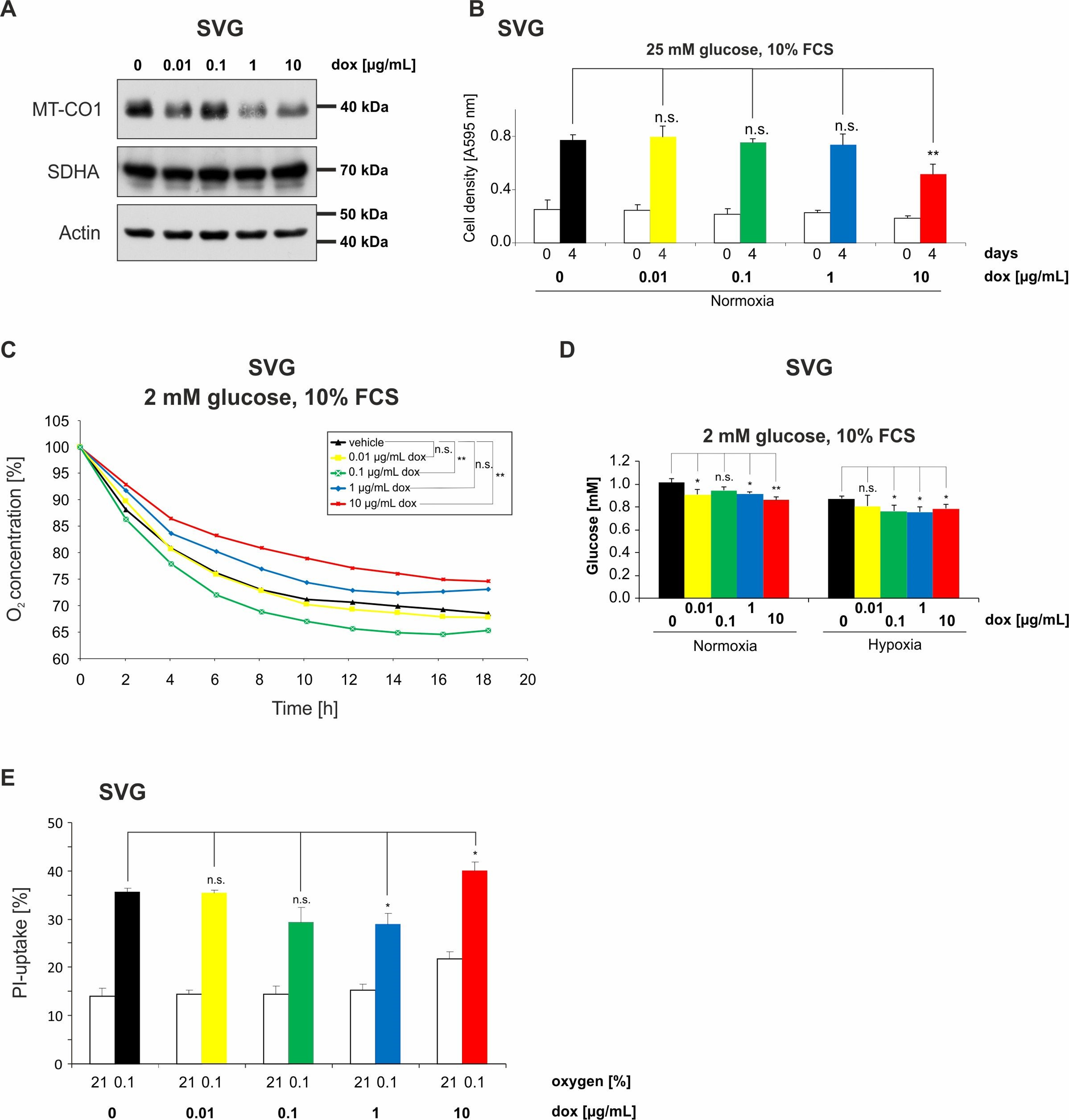
2.6. Doxycycline Impairs Mitochondrial Function and Alters Metabolism of Astroglial SVG Cells
3. Discussion
4. Materials and Methods
4.1. Reagents, Cell Lines, and Culture Conditions
4.2. Generation of pTetOne Cells
4.3. Induction of Hypoxia
4.4. RNA Extraction and Quantitative Reverse Transcription-PCR (qPCR) Analysis
4.5. Lysate Preparation and Immunoblot Analysis
4.6. Cell Density and Cell Viability Assays
4.7. Measurement of Glucose
4.8. Oxygen Consumption
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CV | Crystal violet |
| FCS | Fetal calf serum |
| LDH | Lactate dehydrogenase |
| MT-CO1 | Mitochondrially-encoded cytochrome c oxidase I |
| PHGDH | Phosphoglycerate-dehydrogenase |
| PPARGC1A/PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| SDHA | Succinate dehydrogenase complex, subunit A |
| Tet-inducible systems | Tetracycline-inducible systems |
References
- Gossen, M.; Freundlieb, S.; Bender, G.; Muller, G.; Hillen, W.; Bujard, H. Transcriptional activation by tetracyclines in mammalian cells. Science 1995, 268, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Gossen, M.; Bujard, H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 1992, 89, 5547–5551. [Google Scholar] [CrossRef] [PubMed]
- Heinz, N.; Schambach, A.; Galla, M.; Maetzig, T.; Baum, C.; Loew, R.; Schiedlmeier, B. Retroviral and transposon-based Tet-regulated all-in-one vectors with reduced background expression and improved dynamic range. Hum. Gene Ther. 2011, 22, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Connamacher, R.H.; Mandel, H.G. Binding of tetracycline to the 30S ribosomes and to polyuridylic acid. Biochem. Biophys. Res. Commun. 1965, 20, 98–103. [Google Scholar] [CrossRef]
- Maxwell, I.H. Studies of the binding of tetracycline to ribosomes in vitro. Mol. Pharmacol. 1968, 4, 25–37. [Google Scholar] [PubMed]
- Geigenmuller, U.; Nierhaus, K.H. Tetracycline can inhibit tRNA binding to the ribosomal P site as well as to the A site. Eur. J. Biochem. 1986, 161, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Moullan, N.; Mouchiroud, L.; Wang, X.; Ryu, D.; Williams, E.G.; Mottis, A.; Jovaisaite, V.; Frochaux, M.V.; Quiros, P.M.; Deplancke, B.; et al. Tetracyclines disturb mitochondrial function across eukaryotic models: A call for caution in biomedical research. Cell Rep. 2015, 10, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.W.; Burger, G.; Lang, B.F. Mitochondrial evolution. Science 1999, 283, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; de Bruijn, M.H.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Mai, N.; Chrzanowska-Lightowlers, Z.M.; Lightowlers, R.N. The process of mammalian mitochondrial protein synthesis. Cell Tissue. Res. 2017, 367, 5–20. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.W. The general occurrence of 55 S ribosomes in mammalian liver mitochondria. J. Biol. Chem. 1971, 246, 3409–3417. [Google Scholar] [PubMed]
- Greber, B.J.; Ban, N. Structure and function of the mitochondrial ribosome. Annu. Rev. Biochem. 2016, 85, 103–132. [Google Scholar] [CrossRef] [PubMed]
- Bread, N.S., Jr.; Armentrout, S.A.; Weisberger, A.S. Inhibition of mammalian protein synthesis by antibiotics. Pharmacol. Rev. 1969, 21, 213–245. [Google Scholar] [PubMed]
- Ahler, E.; Sullivan, W.J.; Cass, A.; Braas, D.; York, A.G.; Bensinger, S.J.; Graeber, T.G.; Christofk, H.R. Doxycycline alters metabolism and proliferation of human cell lines. PLoS ONE 2013, 8, e64561. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Houtkooper, R.H.; Mouchiroud, L.; Ryu, D.; Moullan, N.; Katsyuba, E.; Knott, G.; Williams, R.W.; Auwerx, J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 2013, 497, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Thiepold, A.L.; Lorenz, N.I.; Foltyn, M.; Engel, A.L.; Dive, I.; Urban, H.; Heller, S.; Bruns, I.; Hofmann, U.; Drose, S.; et al. Mammalian target of rapamycin complex 1 activation sensitizes human glioma cells to hypoxia-induced cell death. Brain 2017, 140, 2623–2638. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Ayala, M.; Thiede, B.R.; Zhang, S.C. In vitro- and in vivo-induced transgene expression in human embryonic stem cells and derivatives. Stem Cells 2008, 26, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Randolph, L.N.; Bao, X.; Zhou, C.; Lian, X. An all-in-one, Tet-On 3G inducible PiggyBac system for human pluripotent stem cells and derivatives. Sci. Rep. 2017, 7, 1549. [Google Scholar] [CrossRef] [PubMed]
- Lamb, R.; Ozsvari, B.; Lisanti, C.L.; Tanowitz, H.B.; Howell, A.; Martinez-Outschoorn, U.E.; Sotgia, F.; Lisanti, M.P. Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: Treating cancer like an infectious disease. Oncotarget 2015, 6, 4569–4584. [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, W.C.; Popovic, S.V.; Lhotak, S.; Seidlitz, E.; Hirte, H.W.; Tozer, R.G.; Singh, G. Doxycycline decreases tumor burden in a bone metastasis model of human breast cancer. Cancer Res. 2002, 62, 1588–1591. [Google Scholar] [PubMed]
- Foroodi, F.; Duivenvoorden, W.C.; Singh, G. Interactions of doxycycline with chemotherapeutic agents in human breast adenocarcinoma MDA-MB-231 cells. Anti-Cancer Drugs 2009, 20, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.; Zhang, F.; Vlashi, E. Doxycycline inhibits the cancer stem cell phenotype and epithelial-to-mesenchymal transition in breast cancer. Cell Cycle 2017, 16, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Ronellenfitsch, M.W.; Brucker, D.P.; Burger, M.C.; Wolking, S.; Tritschler, F.; Rieger, J.; Wick, W.; Weller, M.; Steinbach, J.P. Antagonism of the mammalian target of rapamycin selectively mediates metabolic effects of epidermal growth factor receptor inhibition and protects human malignant glioma cells from hypoxia-induced cell death. Brain 2009, 132, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Naumann, U.; Ohgaki, H.; Rastinejad, F.; Weller, M. CP-31398, a novel p53-stabilizing agent, induces p53-dependent and p53-independent glioma cell death. Oncogene 2003, 22, 8233–8245. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Hansel, M.; Hamel, W.; Kunzmann, R.; Holzel, F. Karyotype analyses of 20 human glioma cell lines. Acta Neurochir. 1994, 126, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Grady, J.E.; Lummis, W.L.; Smith, C.G. An improved tissue culture assay. III. Alternate methods for measuring cell growth. Cancer Res. 1960, 20, 1114–1117. [Google Scholar] [PubMed]
- Steinbach, J.P.; Klumpp, A.; Wolburg, H.; Weller, M. Inhibition of epidermal growth factor receptor signaling protects human malignant glioma cells from hypoxia-induced cell death. Cancer Res. 2004, 64, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Wanka, C.; Brucker, D.P.; Bahr, O.; Ronellenfitsch, M.; Weller, M.; Steinbach, J.P.; Rieger, J. Synthesis of cytochrome C oxidase 2: A p53-dependent metabolic regulator that promotes respiratory function and protects glioma and colon cancer cells from hypoxia-induced cell death. Oncogene 2012, 31, 3764–3776. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.; Fontana, A.; Trepel, M.; Reed, J.C.; Dichgans, J.; Weller, M. Immunochemotherapy of malignant glioma: Synergistic activity of CD95 ligand and chemotherapeutics. Cancer Immunol. Immunother. 1997, 44, 55–63. [Google Scholar] [CrossRef] [PubMed]
| Gene | Fwd | Rev |
|---|---|---|
| 18S | 5′-CGGCTACCACATCCAAGGAA-3′ | 5′-GCTGGAATTACCGCGGCT-3′ |
| SDHA | 5′-TGGGAACAAGAGGGCATCTG-3′ | 5′-CCACCACTGCATCAAATTCATG-3′ |
| PGC-1α | 5′-TCTGAGTCTGTATGGAGTGACAT-3′ | 5′-CCAAGTCGTTCACATCTAGTTCA-3′ |
| PHGDH | 5′-CTGCGGAAAGTGCTCATCAGT-3′ | 5′-TGGCAGAGCGAACAATAAGGC-3′ |
| TetOne transactivator | 5′-CTATGCCCCCACTTCTGAAA-3′ | 5′-GTCAGCAGGCAGCATATCAA-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luger, A.-L.; Sauer, B.; Lorenz, N.I.; Engel, A.L.; Braun, Y.; Voss, M.; Harter, P.N.; Steinbach, J.P.; Ronellenfitsch, M.W. Doxycycline Impairs Mitochondrial Function and Protects Human Glioma Cells from Hypoxia-Induced Cell Death: Implications of Using Tet-Inducible Systems. Int. J. Mol. Sci. 2018, 19, 1504. https://doi.org/10.3390/ijms19051504
Luger A-L, Sauer B, Lorenz NI, Engel AL, Braun Y, Voss M, Harter PN, Steinbach JP, Ronellenfitsch MW. Doxycycline Impairs Mitochondrial Function and Protects Human Glioma Cells from Hypoxia-Induced Cell Death: Implications of Using Tet-Inducible Systems. International Journal of Molecular Sciences. 2018; 19(5):1504. https://doi.org/10.3390/ijms19051504
Chicago/Turabian StyleLuger, Anna-Luisa, Benedikt Sauer, Nadja I. Lorenz, Anna L. Engel, Yannick Braun, Martin Voss, Patrick N. Harter, Joachim P. Steinbach, and Michael W. Ronellenfitsch. 2018. "Doxycycline Impairs Mitochondrial Function and Protects Human Glioma Cells from Hypoxia-Induced Cell Death: Implications of Using Tet-Inducible Systems" International Journal of Molecular Sciences 19, no. 5: 1504. https://doi.org/10.3390/ijms19051504
APA StyleLuger, A.-L., Sauer, B., Lorenz, N. I., Engel, A. L., Braun, Y., Voss, M., Harter, P. N., Steinbach, J. P., & Ronellenfitsch, M. W. (2018). Doxycycline Impairs Mitochondrial Function and Protects Human Glioma Cells from Hypoxia-Induced Cell Death: Implications of Using Tet-Inducible Systems. International Journal of Molecular Sciences, 19(5), 1504. https://doi.org/10.3390/ijms19051504





