Rho A Regulates Epidermal Growth Factor-Induced Human Osteosarcoma MG63 Cell Migration
Abstract
1. Introduction
2. Results
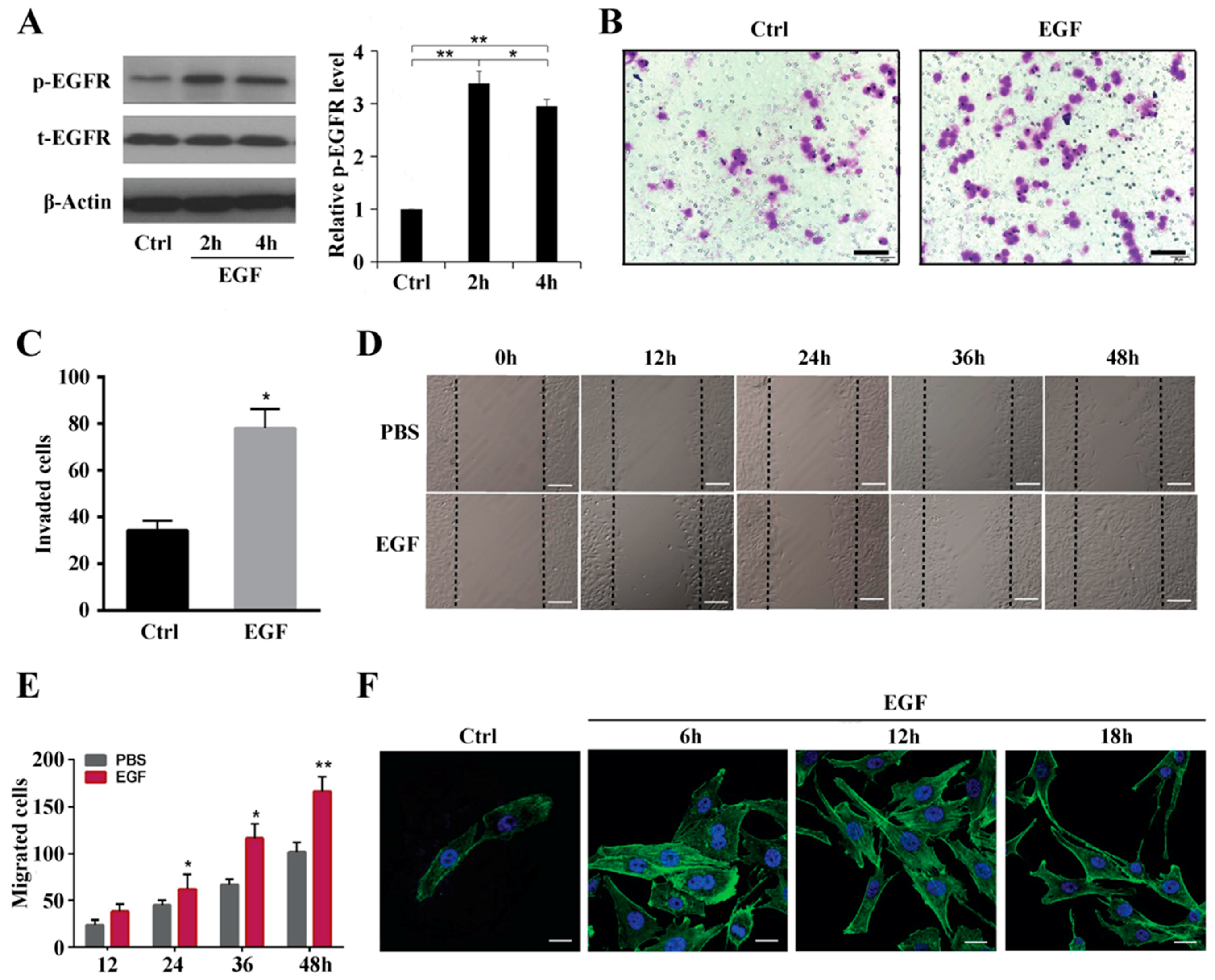
2.1. EGF Activates EGFR Expression in MG63 Cells and Promotes Cell Migration by Increasing Actin Stress Fiber Formation
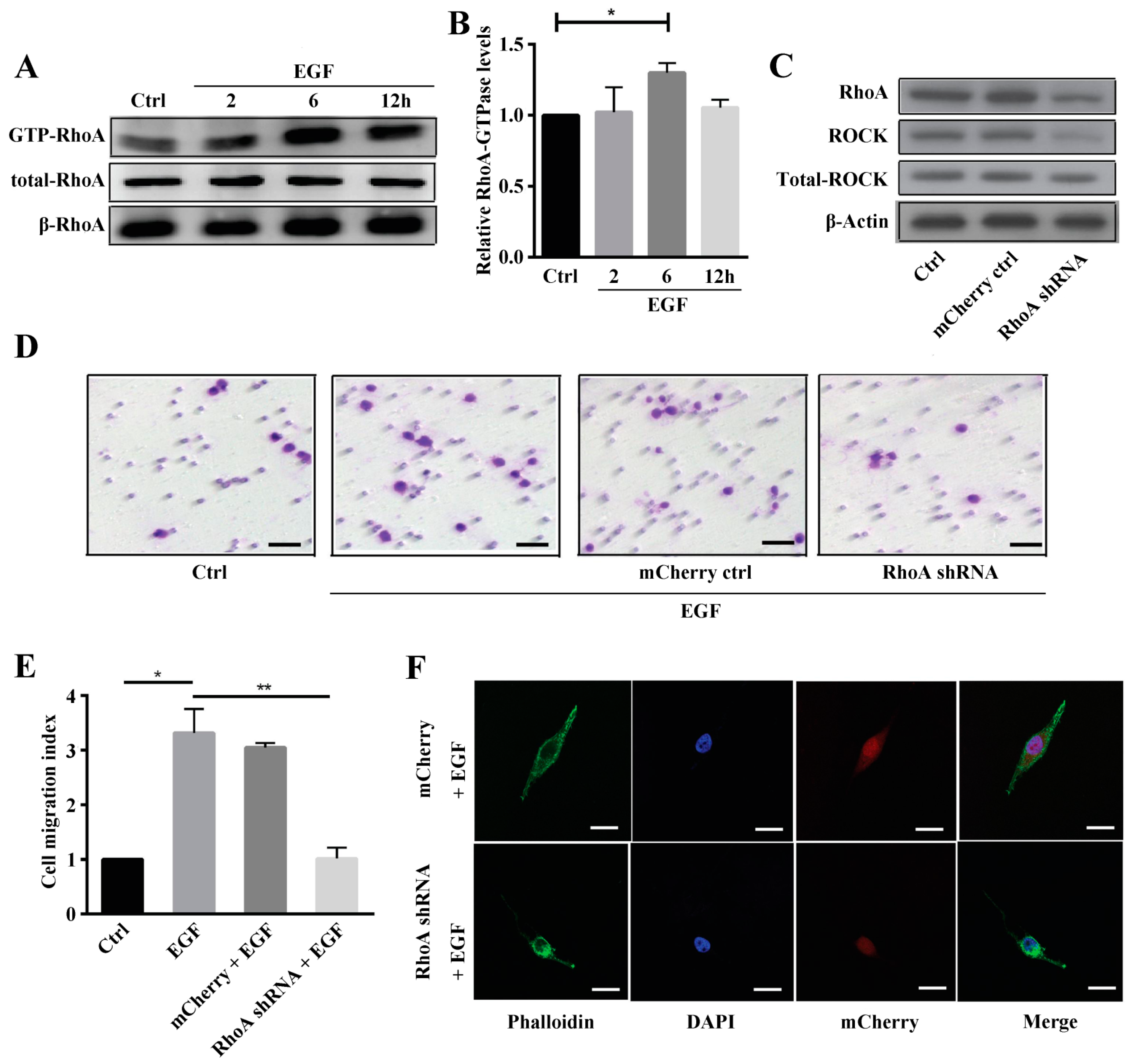
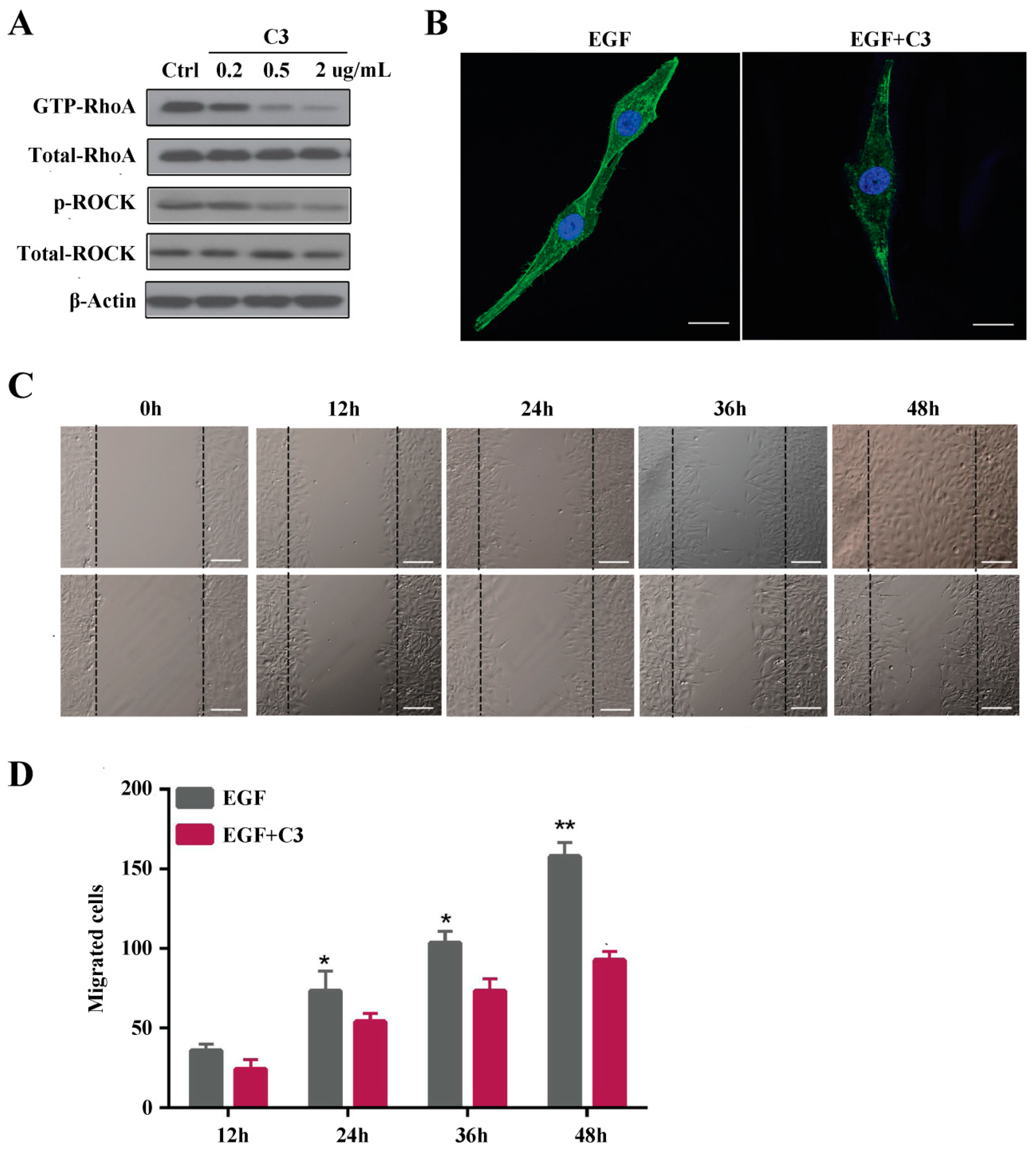
2.2. Rho A Is Involved in EGF-Induced Migration of MG63 Cells
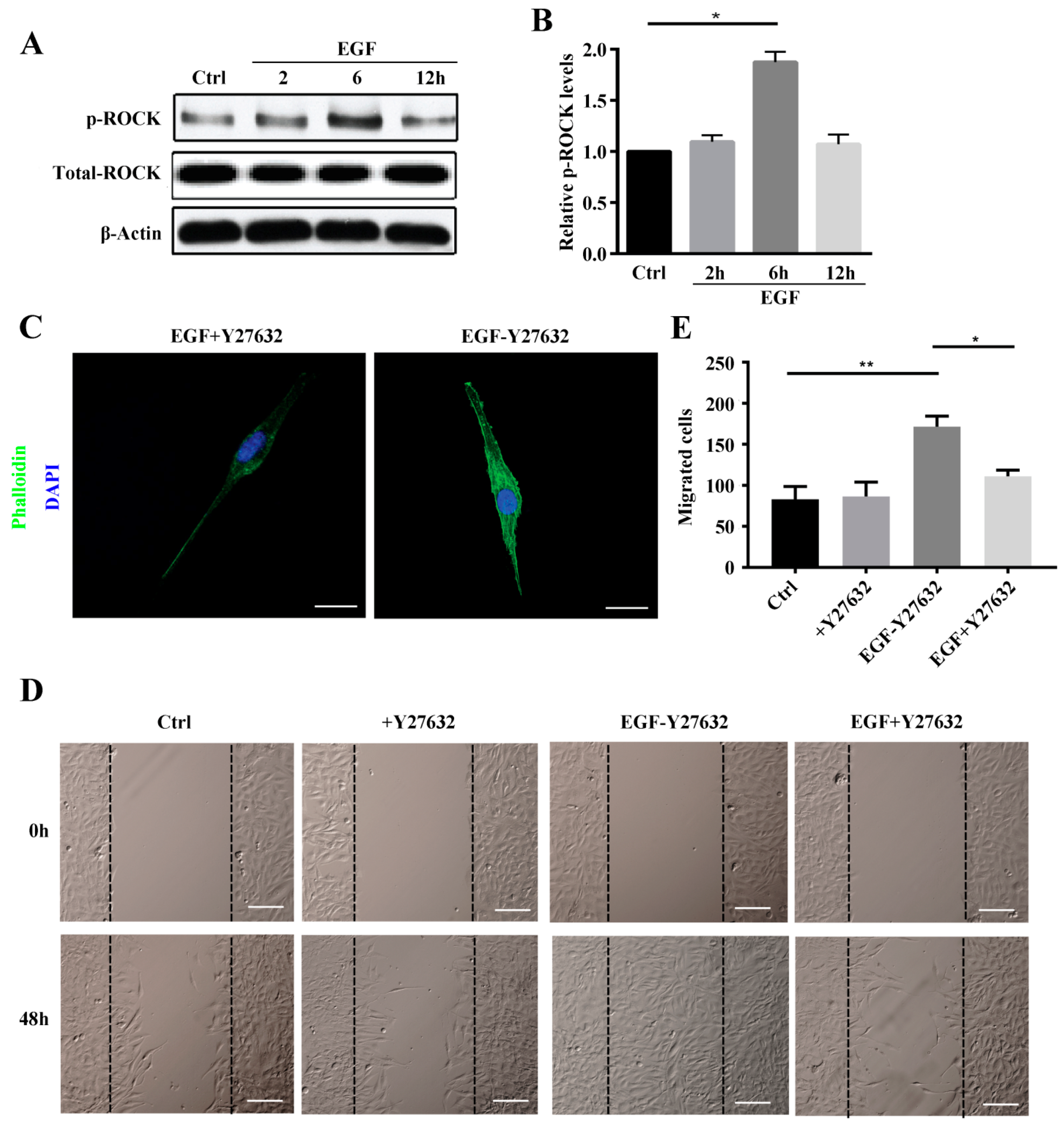
2.3. ROCK Promotes MG63 Cell Migration and Stress Fiber Reorganization
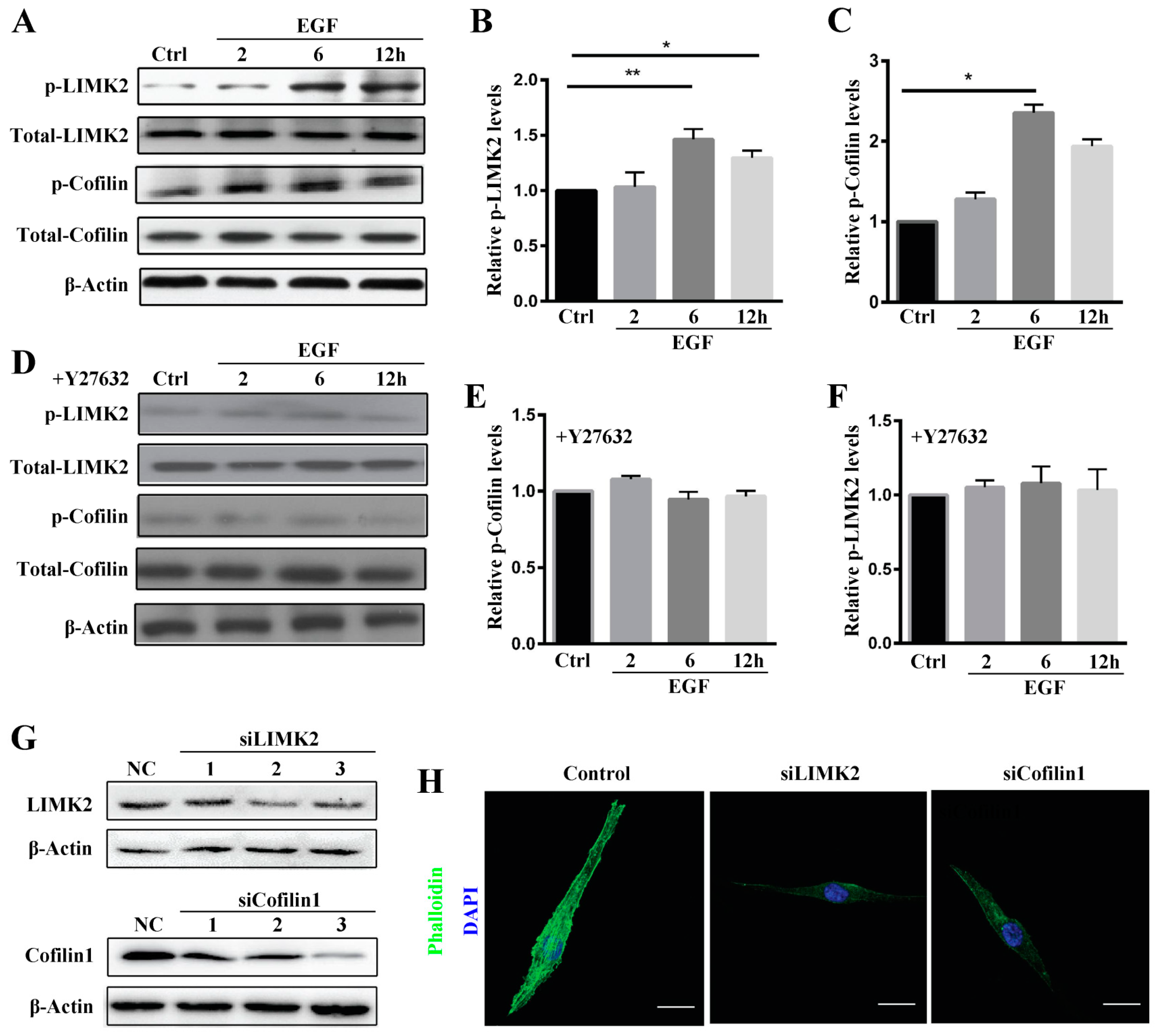
2.4. ROCK Mediates MG63 Cell Migration via Activation of LIMK2/Cofilin1 Signaling
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture
4.3. Pull Down Assay
4.4. Lentiviral shRNA and Transduction In Vitro
4.5. Real-Time Quantitative PCR
4.6. siRNA and Transfection In Vitro
4.7. Wound-Healing Assay
4.8. Transwell Migration Assay
4.9. Fluorescent Staining of the Cytoskeleton
4.10. Western Blotting
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Marina, N.; Gebhardt, M.; Teot, L.; Gorlick, R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist 2004, 9, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Bacci, G.; Forni, C.; Ferrari, S.; Longhi, A.; Bertoni, F.; Mercuri, M.; Donati, D.; Capanna, R.; Bernini, G.; Briccoli, A.; et al. Neoadjuvant chemotherapy for osteosarcoma of the extremity: Intensification of preoperative treatment does not increase the rate of good histologic response to the primary tumor or improve the final outcome. J. Pediatr. Hematol. Oncol. 2003, 25, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Kager, L.; Zoubek, A.; Potschger, U.; Kastner, U.; Flege, S.; Kempf-Bielack, B.; Branscheid, D.; Kotz, R.; Salzer-Kuntschik, M.; Winkelmann, W.; et al. Primary metastatic osteosarcoma: Presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J. Clin. Oncol. 2003, 21, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Gao, F.; Sun, X.; Bi, H.; Zhu, Y. Paris saponin VII suppresses osteosarcoma cell migration and invasion by inhibiting MMP2/9 production via the p38 MAPK signaling pathway. Mol. Med. Rep. 2016, 14, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.B.; Liang, Y.C.; Wang, C.Y.; Chang, T.C.; Lee, W.S. Lovastatin suppresses invasiveness of anaplastic thyroid cancer cells by inhibiting Rho geranylgeranylation and RhoA/ROCK signaling. Endocr. Relat. Cancer 2005, 12, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Dobashi, Y.; Suzuki, S.; Sugawara, H.; Ooi, A. Involvement of epidermal growth factor receptor and downstream molecules in bone and soft tissue tumors. Hum. Pathol. 2007, 38, 914–925. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Wehrmann, B.; Radig, K.; Walter, H.; Rose, I.; Neumann, W.; Roessner, A. Expression of growth factors and their receptors in human osteosarcomas. Immunohistochemical detection of epidermal growth factor, platelet-derived growth factor and their receptors: Its correlation with proliferating activities and p53 expression. Gen. Diagn. Pathol. 1995, 141, 97–103. [Google Scholar] [PubMed]
- Dobashi, Y.; Takei, N.; Suzuki, S.; Yoneyama, H.; Hanawa, M.; Ooi, A. Aberration of epidermal growth factor receptor expression in bone and soft-tissue tumors: Protein overexpression, gene amplification and activation of downstream molecules. Mod. Pathol. 2004, 17, 1497–1505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wen, Y.H.; Koeppen, H.; Garcia, R.; Chiriboga, L.; Tarlow, B.D.; Peters, B.A.; Eigenbrot, C.; Yee, H.; Steiner, G.; Greco, M.A. Epidermal growth factor receptor in osteosarcoma: Expression and mutational analysis. Hum. Pathol. 2007, 38, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Do, S.I.; Jung, W.W.; Kim, H.S.; Park, Y.K. The expression of epidermal growth factor receptor and its downstream signaling molecules in osteosarcoma. Int. J. Oncol. 2009, 34, 797–803. [Google Scholar] [PubMed]
- Freeman, S.S.; Allen, S.W.; Ganti, R.; Wu, J.; Ma, J.; Su, X.; Neale, G.; Dome, J.S.; Daw, N.C.; Khoury, J.D. Copy number gains in EGFR and copy number losses in PTEN are common events in osteosarcoma tumors. Cancer 2008, 113, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Sevelda, F.; Mayr, L.; Kubista, B.; Lotsch, D.; van Schoonhoven, S.; Windhager, R.; Pirker, C.; Micksche, M.; Berger, W. EGFR is not a major driver for osteosarcoma cell growth in vitro but contributes to starvation and chemotherapy resistance. J. Exp. Clin. Cancer Res. 2015, 34, 134. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.H.; Ruslan, S.E.N.; Buddingh, E.P.; Santos, S.J.; Szuhai, K.; Serra, M.; Gelderblom, H.; Hogendoorn, P.C.; Egeler, R.M.; Schilham, M.W.; et al. Anti-EGFR antibody cetuximab enhances the cytolytic activity of natural killer cells toward osteosarcoma. Clin. Cancer Res. 2012, 18, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Yarden, Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 2001, 37 (Suppl. 4), S3–S8. [Google Scholar] [CrossRef]
- Olayioye, M.A.; Neve, R.M.; Lane, H.A.; Hynes, N.E. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000, 19, 3159–3167. [Google Scholar] [CrossRef] [PubMed]
- Benitah, S.A.; Valeron, P.F.; van Aelst, L.; Marshall, C.J.; Lacal, J.C. Rho GTPases in human cancer: An unresolved link to upstream and downstream transcriptional regulation. Biochim. Biophys. Acta 2004, 1705, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Nobes, C.D.; Hall, A. Rho, rac and cdc42 GTPases: Regulators of actin structures, cell adhesion and motility. Biochem. Soc. Trans. 1995, 23, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Parri, M.; Chiarugi, P. Rac and Rho GTPases in cancer cell motility control. Cell Commun. Signal. 2010, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Bustelo, X.R.; Sauzeau, V.; Berenjeno, I.M. GTP-binding proteins of the Rho/Rac family: Regulation, effectors and functions in vivo. Bioessays 2007, 29, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.Y.; Bailly, M.; Zebda, N.; Segall, J.E.; Condeelis, J.S. Role of cofilin in epidermal growth factor-stimulated actin polymerization and lamellipod protrusion. J. Cell Biol. 2000, 148, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399. [Google Scholar] [CrossRef]
- Lee, J.A.; Choi, J.W.; In, J.H.; Jung, H.S.; Kim, Y.S.; Jeon, Y.S.; Kang, Y.J.; Kim, D.W.; Lim, Y.G.; Park, J.H.; et al. Hepatic ischemic preconditioning provides protection against distant renal ischemia and reperfusion injury in mice. J. Korean Med. Sci. 2012, 27, 547–552. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernard, O. Lim kinases, regulators of actin dynamics. Int. J. Biochem. Cell Biol. 2007, 39, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Paterson, H.F.; Johnston, C.L.; Diekmann, D.; Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992, 70, 401–410. [Google Scholar] [CrossRef]
- Laflamme, C.; Curt, S.; Rouabhia, M. Epidermal growth factor and bone morphogenetic proteins upregulate osteoblast proliferation and osteoblastic markers and inhibit bone nodule formation. Arch. Oral Biol. 2010, 55, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, J.M. Cytoskeletal mechanisms responsible for invasive migration of neoplastic cells. Int. J. Dev. Biol. 2004, 48, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, L.; Hu, J.; Yu, L.; Zheng, Y.; Guo, J.; Zheng, X.; Yi, P.; Zhou, Y. Epidermal growth factor stimulates human trophoblast cell migration through Rho A and Rho C activation. Endocrinology 2010, 151, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Komers, R. Rho kinase inhibition in diabetic nephropathy. Curr. Opin. Nephrol. Hypertens. 2011, 20, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Narumiya, S.; Tanji, M.; Ishizaki, T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009, 28, 65–76. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, L.; Qu, R.; Zhang, L.; Huang, W. Rho A Regulates Epidermal Growth Factor-Induced Human Osteosarcoma MG63 Cell Migration. Int. J. Mol. Sci. 2018, 19, 1437. https://doi.org/10.3390/ijms19051437
Wang J, Zhang L, Qu R, Zhang L, Huang W. Rho A Regulates Epidermal Growth Factor-Induced Human Osteosarcoma MG63 Cell Migration. International Journal of Molecular Sciences. 2018; 19(5):1437. https://doi.org/10.3390/ijms19051437
Chicago/Turabian StyleWang, Jinyang, Lei Zhang, Rongmei Qu, Lin Zhang, and Wenhua Huang. 2018. "Rho A Regulates Epidermal Growth Factor-Induced Human Osteosarcoma MG63 Cell Migration" International Journal of Molecular Sciences 19, no. 5: 1437. https://doi.org/10.3390/ijms19051437
APA StyleWang, J., Zhang, L., Qu, R., Zhang, L., & Huang, W. (2018). Rho A Regulates Epidermal Growth Factor-Induced Human Osteosarcoma MG63 Cell Migration. International Journal of Molecular Sciences, 19(5), 1437. https://doi.org/10.3390/ijms19051437




