Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues
Abstract
1. Introduction
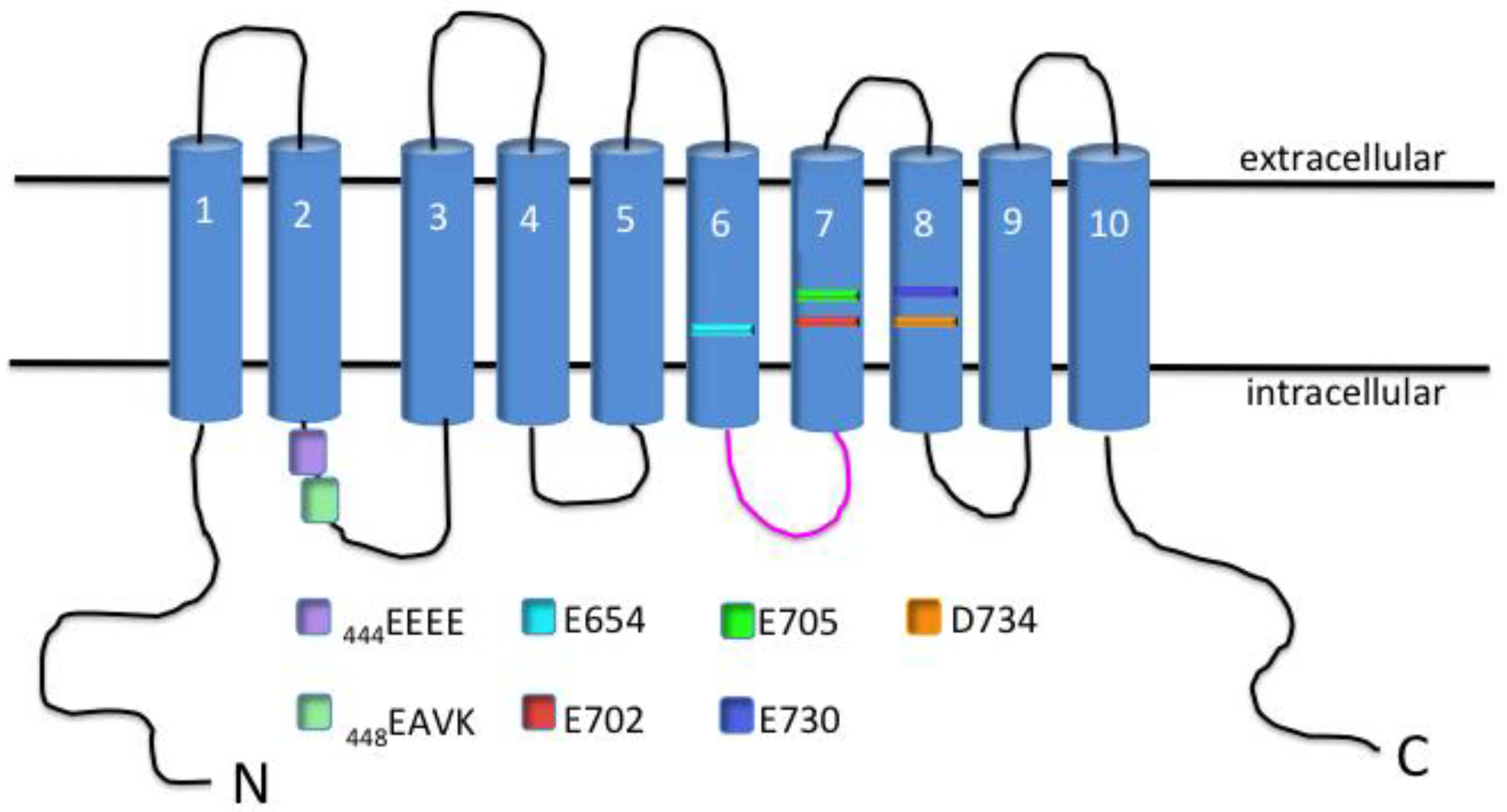
2. TMEM16A Characterization
3. TMEM16A in Epithelial Tissues
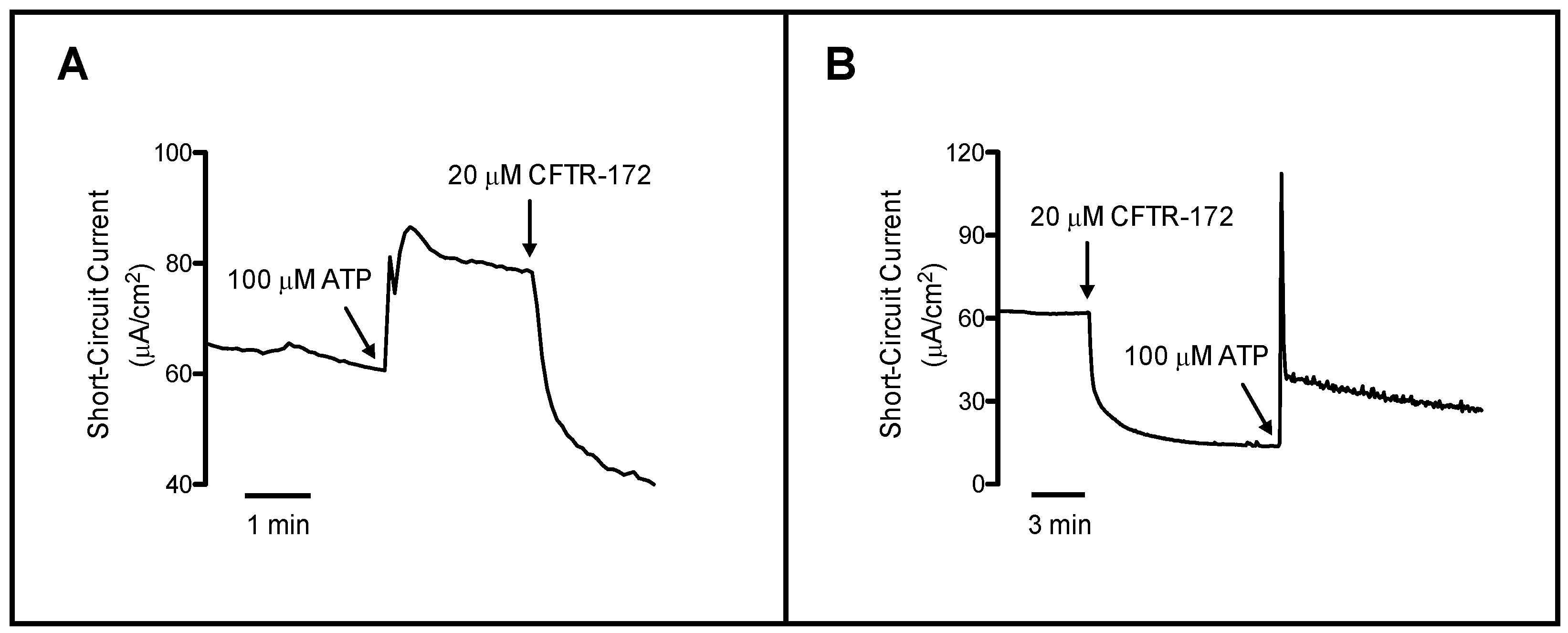
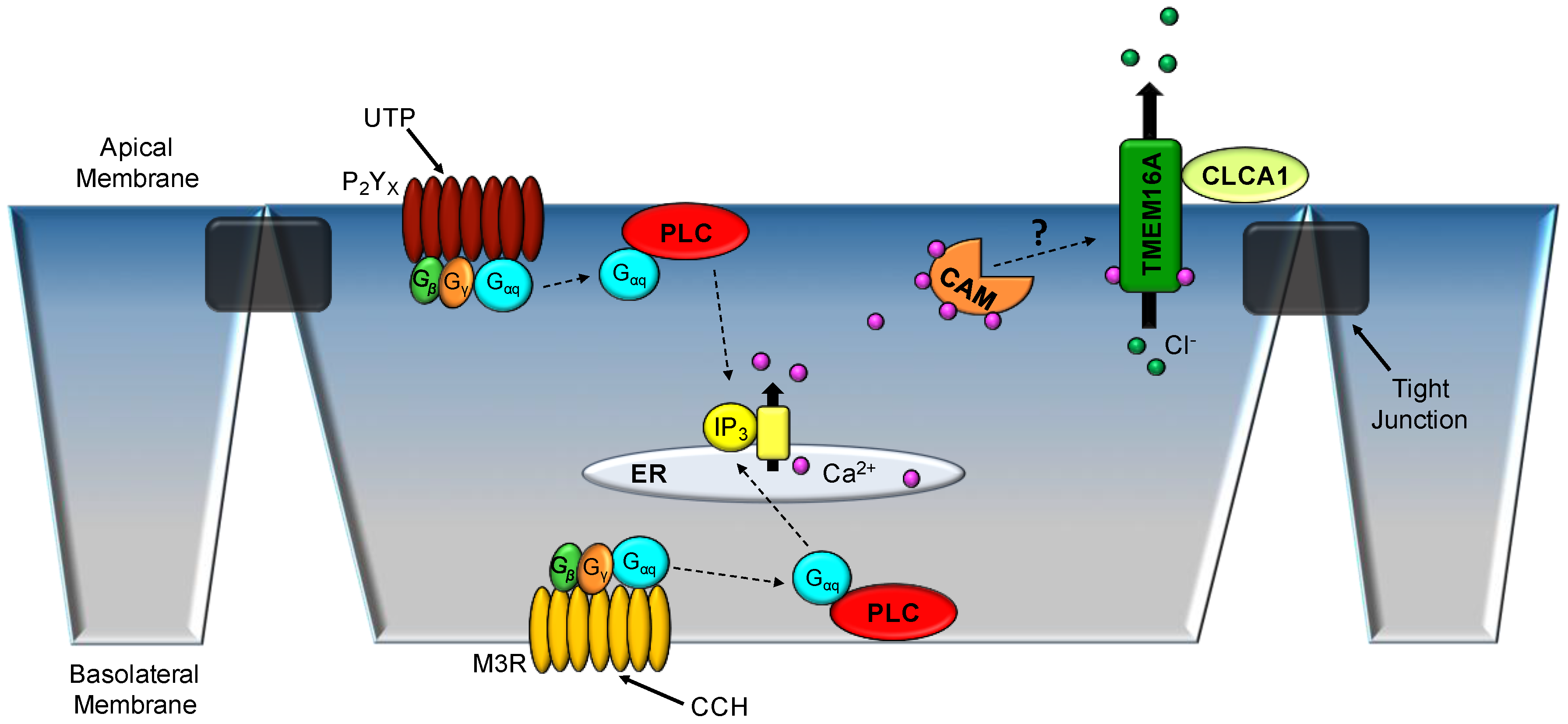
3.1. Respiratory Epithelium
3.2. Colonic Epithelium
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| CaCC | calcium-activated chloride channel |
| CaM | calmodulin |
| cAMP | cyclic adenosine monophosphate |
| CCH | carbachol |
| CF | Cystic Fibrosis |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| CLCA | Cl− channel accessory |
| cRNA | complementary ribonucleic acid |
| DTT | dithiothreitol |
| EC50 | half-maximal effective concentration |
| FRET | fluorescence resonance energy transfer |
| GFP | green fluorescent protein |
| HEK 293 | human embryonic kidney 293 cells |
| IL-4 | interleukin 4 |
| ISC | short-circuit current |
| kDa | kilodalton |
| mM | millimolar |
| mRNA | messenger ribonucleic acid |
| mV | millivolts |
| µM | micromolar |
| siRNA | small interfering ribonucleic acid |
| TMEM16A | transmembrane member 16A |
| UTP | uridine triphosphate |
| VTE | trans-epithelial voltage |
| WT | wild type |
References
- Browner, M.; Ferkany, J.W.; Enna, S.J. Biochemical identification of pharmacologically and functionally distinct gaba receptors in rat brain. J. Neurosci. 1981, 1, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Cozens, A.L.; Yezzi, M.J.; Kunzelmann, K.; Ohrui, T.; Chin, L.; Eng, K.; Finkbeiner, W.E.; Widdicombe, J.H.; Gruenert, D.C. Cftr expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 1994, 10, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Devor, D.C.; Singh, A.K.; Lambert, L.C.; DeLuca, A.; Frizzell, R.A.; Bridges, R.J. Bicarbonate and chloride secretion in calu-3 human airway epithelial cells. J. Gen. Physiol. 1999, 113, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Gallos, G.; Remy, K.E.; Danielsson, J.; Funayama, H.; Fu, X.W.; Chang, H.Y.; Yim, P.; Xu, D.; Emala, C.W., Sr. Functional expression of the TMEM16 family of calcium-activated chloride channels in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L625–L634. [Google Scholar] [CrossRef] [PubMed]
- Manoury, B.; Tamuleviciute, A.; Tammaro, P. TMEM16A/anoctamin 1 protein mediates calcium-activated chloride currents in pulmonary arterial smooth muscle cells. J. Physiol. 2010, 588, 2305–2314. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.A.; Winpenny, J.P.; Porteous, D.J.; Dorin, J.R.; Argent, B.E. CFTR and calcium-activated chloride currents in pancreatic duct cells of a transgenic cf mouse. Am. J. Physiol. 1994, 266, C213–C221. [Google Scholar] [CrossRef] [PubMed]
- Willumsen, N.J.; Boucher, R.C. Activation of an apical Cl- conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am. J. Physiol. 1989, 256, C226–C233. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Clarke, L.L.; Boucher, R.C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N. Engl. J. Med. 1991, 325, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Cozens, A.L.; Schulman, H.; Gruenert, D.C.; Stryer, L.; Gardner, P. Activation of chloride channels in normal and cystic fibrosis airway epithelial cells by multifunctional calcium/calmodulin-dependent protein kinase. Nature 1991, 349, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.L.; Grubb, B.R.; Yankaskas, J.R.; Cotton, C.U.; McKenzie, A.; Boucher, R.C. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in CFTR(-/-) mice. Proc. Natl. Acad. Sci. USA 1994, 91, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Kartner, N.; Hanrahan, J.W.; Jensen, T.J.; Naismith, A.L.; Sun, S.Z.; Ackerley, C.A.; Reyes, E.F.; Tsui, L.C.; Rommens, J.M.; Bear, C.E.; et al. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell 1991, 64, 681–691. [Google Scholar] [CrossRef]
- Cunningham, S.A.; Awayda, M.S.; Bubien, J.K.; Ismailov, I.I.; Pia Arrate, M.P.; Berdiev, B.K.; Benos, D.J.; Fuller, C.M. Cloning of an epithelial chloride channel from bovine trachea. J. Biol. Chem. 1995, 270, 31016–31026. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.D.; Pauli, B.U. Molecular cloning and biochemical characterization of a truncated, secreted member of the human family of Ca2+-activated Cl- channels. Biochim. Biophys. Acta 1999, 1444, 418–423. [Google Scholar] [CrossRef]
- Leverkoehne, I.; Gruber, A.D. The murine MCLCA3 (alias Gob-5) protein is located in the mucin granule membranes of intestinal, respiratory, and uterine goblet cells. J. Histochem. Cytochem. 2002, 50, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Elble, R.C.; Gruber, A.D.; Schreur, K.D.; Ji, H.L.; Fuller, C.M.; Pauli, B.U. Molecular and functional characterization of a calcium-sensitive chloride channel from mouse lung. J. Biol. Chem. 1998, 273, 32096–32101. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.D.; Elble, R.C.; Ji, H.L.; Schreur, K.D.; Fuller, C.M.; Pauli, B.U. Genomic cloning, molecular characterization, and functional analysis of human clca1, the first human member of the family of Ca2+-activated Cl- channel proteins. Genomics 1998, 54, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Neher, E.; Sakmann, B. Rat brain serotonin receptors in xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc. Natl. Acad. Sci. USA 1987, 84, 5063–5067. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.D.; Schreur, K.D.; Ji, H.L.; Fuller, C.M.; Pauli, B.U. Molecular cloning and transmembrane structure of HClCa2 from human lung, trachea, and mammary gland. Am. J. Physiol. 1999, 276, C1261–C1270. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression cloning of tmem16a as a calcium-activated chloride channel subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.D.; Cho, H.; Koo, J.Y.; Tak, M.H.; Cho, Y.; Shim, W.S.; Park, S.P.; Lee, J.; Lee, B.; Kim, B.M.; et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008, 455, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Jeng, G.; Aggarwal, M.; Yu, W.P.; Chen, T.Y. Independent activation of distinct pores in dimeric tmem16a channels. J. Gen. Physiol. 2016, 148, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Lim, N.K.; Lam, A.K.; Dutzler, R. Independent activation of ion conduction pores in the double-barreled calcium-activated chloride channel TMEM16A. J. Gen. Physiol. 2016, 148, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Paulino, C.; Neldner, Y.; Lam, A.K.; Kalienkova, V.; Brunner, J.D.; Schenck, S.; Dutzler, R. Structural basis for anion conduction in the calcium-activated chloride channel TMEM16A. eLife 2017, 6, e26232. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.T.; Worthington, E.N.; Yu, K.; Gabriel, S.E.; Hartzell, H.C.; Tarran, R. Characterization of the oligomeric structure of the Ca(2+)-activated Cl- channel ANO1/TMEM16A. J. Biol. Chem. 2011, 286, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Tien, J.; Lee, H.Y.; Minor, D.L., Jr.; Jan, Y.N.; Jan, L.Y. Identification of a dimerization domain in the TMEM16A calcium-activated chloride channel (CACC). Proc. Natl. Acad. Sci. USA 2013, 110, 6352–6357. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Yu, K.; Perez-Cornejo, P.; Cui, Y.; Arreola, J.; Hartzell, H.C. Voltage- and calcium-dependent gating of TMEM16A/ANO1 chloride channels are physically coupled by the first intracellular loop. Proc. Natl. Acad. Sci. USA 2011, 108, 8891–8896. [Google Scholar] [CrossRef] [PubMed]
- Tien, J.; Peters, C.J.; Wong, X.M.; Cheng, T.; Jan, Y.N.; Jan, L.Y.; Yang, H.H. A comprehensive search for calcium binding sites critical for TMEM16A calcium-activated chloride channel activity. eLife 2014, 3, e02772. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, S.; Ren, S.; Chen, Y.; Yuan, H.; Chai, R.; Yu, H.; Zhang, H.; Zhan, Y.; An, H. Two Ca(2+)-binding sites cooperatively couple together in TMEM16A channel. J. Membr. Biol. 2016, 249, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Scudieri, P.; Musante, I.; Gianotti, A.; Moran, O.; Galietta, L.J. Intermolecular interactions in the TMEM16A dimer controlling channel activity. Sci. Rep. 2016, 6, 38788. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.J.; Yu, H.B.; Tien, J.; Jan, Y.N.; Li, M.; Jan, L.Y. Four basic residues critical for the ion selectivity and pore blocker sensitivity of TMEM16A calcium-activated chloride channels. Proc. Natl. Acad. Sci. USA 2015, 112, 3547–3552. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.D.; Lim, N.K.; Schenck, S.; Duerst, A.; Dutzler, R. X-ray structure of a calcium-activated TMEM16 lipid scramblase. Nature 2014, 516, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.; Feng, S.; Tien, J.; Peters, C.J.; Bulkley, D.; Lolicato, M.; Zhao, J.; Zuberbuhler, K.; Ye, W.; Qi, L.; et al. Cryo-em structures of the TMEM16A calcium-activated chloride channel. Nature 2017, 552, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Paulino, C.; Kalienkova, V.; Lam, A.K.M.; Neldner, Y.; Dutzler, R. Activation mechanism of the calcium-activated chloride channel tmem16a revealed by cryo-em. Nature 2017, 552, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Barro-Soria, R.; Rebolledo, S.; Liin, S.I.; Perez, M.E.; Sampson, K.J.; Kass, R.S.; Larsson, H.P. Kcne1 divides the voltage sensor movement in KCNQ1/KCNE1 channels into two steps. Nat. Commun. 2014, 5, 3750. [Google Scholar] [CrossRef] [PubMed]
- Hullin, R.; Khan, I.F.; Wirtz, S.; Mohacsi, P.; Varadi, G.; Schwartz, A.; Herzig, S. Cardiac l-type calcium channel beta-subunits expressed in human heart have differential effects on single channel characteristics. J. Biol. Chem. 2003, 278, 21623–21630. [Google Scholar] [CrossRef] [PubMed]
- Orio, P.; Latorre, R. Differential effects of beta 1 and beta 2 subunits on BK channel activity. J. Gen. Physiol. 2005, 125, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Kongsuphol, P.; Hug, M.; Ousingsawat, J.; Witzgall, R.; Schreiber, R.; Kunzelmann, K. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 2011, 25, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, P.S.; Warner, B.B.; Zhou, Y.; Weinstock, G.M.; Sodergren, E.; Hall-Moore, C.M.; Stevens, H.J.; Bennett, W.E., Jr.; Shaikh, N.; Linneman, L.A.; et al. Patterned progression of bacterial populations in the premature infant gut. Proc. Natl. Acad. Sci. USA 2014, 111, 12522–12527. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhu, J.; Qu, Z.; Cui, Y.Y.; Hartzell, H.C. Activation of the ano1 (TMEM16A) chloride channel by calcium is not mediated by calmodulin. J. Gen. Physiol. 2014, 143, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Kuan, A.S.; Chen, T.Y. Calcium-calmodulin does not alter the anion permeability of the mouse tmem16a calcium-activated chloride channel. J. Gen. Physiol. 2014, 144, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Terashima, H.; Picollo, A.; Accardi, A. Purified TMEM16A is sufficient to form Ca2+-activated Cl-channels. Proc. Natl. Acad. Sci. USA 2013, 110, 19354–19359. [Google Scholar] [CrossRef] [PubMed]
- Mura, C.V.; Delgado, R.; Delgado, M.G.; Restrepo, D.; Bacigalupo, J. A clca regulatory protein present in the chemosensory cilia of olfactory sensory neurons induces a Ca(2+)-activated Cl(−) current when transfected into HEK293. BMC Neurosci. 2017, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Yurtsever, Z.; Berry, K.N.; Nichols, C.G.; Brett, T.J. Modulation of TMEM16A channel activity by the von willebrand factor type a (VWA) domain of the calcium-activated chloride channel regulator 1 (CLCA1). J. Biol. Chem. 2017, 292, 9164–9174. [Google Scholar] [CrossRef] [PubMed]
- Sala-Rabanal, M.; Yurtsever, Z.; Nichols, C.G.; Brett, T.J. Secreted clca1 modulates tmem16a to activate ca(2+)-dependent chloride currents in human cells. eLife 2015, 4, e05875. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Khimji, A.K.; Kresge, C.; Bugde, A.; Dougherty, M.; Esser, V.; Ueno, Y.; Glaser, S.S.; Alpini, G.; Rockey, D.C.; et al. Identification and functional characterization of TMEM16A, a Ca2+-activated Cl- channel activated by extracellular nucleotides, in biliary epithelium. J. Biol. Chem. 2011, 286, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Rock, J.R.; Harfe, B.D.; Cheng, T.; Huang, X.; Jan, Y.N.; Jan, L.Y. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc. Natl. Acad. Sci. USA 2009, 106, 21413–21418. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; Futtner, C.R.; Harfe, B.D. The transmembrane protein tmem16a is required for normal development of the murine trachea. Dev. Biol. 2008, 321, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ousingsawat, J.; Martins, J.R.; Schreiber, R.; Rock, J.R.; Harfe, B.D.; Kunzelmann, K. Loss of TMEM16A causes a defect in epithelial Ca2+-dependent chloride transport. J. Biol. Chem. 2009, 284, 28698–28703. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, R.; Ousingsawat, J.; Wanitchakool, P.; Zhang, Y.; Holtzman, M.J.; Amaral, M.; Rock, J.R.; Schreiber, R.; Kunzelmann, K. Epithelial chloride transport by CFTR requires TMEM16A. Sci. Rep. 2017, 7, 12397. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, W.; Guan, L.; Lu, M.; Wang, K. Inhibition of calcium-activated chloride channel ANO1/TMEM16A suppresses tumor growth and invasion in human lung cancer. PLoS ONE 2015, 10, e0136584. [Google Scholar] [CrossRef] [PubMed]
- Lerias, J.; Pinto, M.; Benedetto, R.; Schreiber, R.; Amaral, M.; Aureli, M.; Kunzelmann, K. Compartmentalized crosstalk of CFTR and TMEM16A (ANO1) through EPAC1 and ADCY1. Cell. Signal. 2018, 44, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Galietta, L.J.; Pagesy, P.; Folli, C.; Caci, E.; Romio, L.; Costes, B.; Nicolis, E.; Cabrini, G.; Goossens, M.; Ravazzolo, R.; et al. IL-4 is a potent modulator of ion transport in the human bronchial epithelium in vitro. J. Immunol. 2002, 168, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.X.; Lu, L.W.; Liu, W.J.; Huang, M. Plasma inflammatory cytokine IL-4, IL-8, IL-10, and tnf-alpha levels correlate with pulmonary function in patients with asthma-chronic obstructive pulmonary disease (copd) overlap syndrome. Med. Sci. Monit. 2016, 22, 2800–2808. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Qiu, Y.; Valobra, M.; Qiu, S.; Majumdar, S.; Matin, D.; De Rose, V.; Jeffery, P.K. Plasma cells and IL-4 in chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2007, 175, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhang, H.; Wu, M.; Yang, H.; Kudo, M.; Peters, C.J.; Woodruff, P.G.; Solberg, O.D.; Donne, M.L.; Huang, X.; et al. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airway smooth muscle contraction. Proc. Natl. Acad. Sci. USA 2012, 109, 16354–16359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Wang, H.; Jiao, J.; Li, Y.; Fan, E.; Zhang, L.; Bachert, C. TMEM16A-mediated mucin secretion in IL-13-induced nasal epithelial cells from chronic rhinosinusitis patients. Allergy Asthma Immunol. Res. 2015, 7, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Sonneville, F.; Ruffin, M.; Coraux, C.; Rousselet, N.; Le Rouzic, P.; Blouquit-Laye, S.; Corvol, H.; Tabary, O. Microrna-9 downregulates the ano1 chloride channel and contributes to cystic fibrosis lung pathology. Nat. Commun. 2017, 8, 710. [Google Scholar] [CrossRef] [PubMed]
- Rock, J.R.; O’Neal, W.K.; Gabriel, S.E.; Randell, S.H.; Harfe, B.D.; Boucher, R.C.; Grubb, B.R. Transmembrane protein 16a (TMEM16A) is a Ca2+-regulated Cl-secretory channel in mouse airways. J. Biol. Chem. 2009, 284, 14875–14880. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Harris, W.T.; Kortyka, S.; Kotha, K.; Ostmann, A.J.; Rezayat, A.; Sridharan, A.; Sanders, Y.; Naren, A.P.; Clancy, J.P. Tgf-beta downregulation of distinct chloride channels in cystic fibrosis-affected epithelia. PLoS ONE 2014, 9, e106842. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Schreiber, R.; Wanitchakool, P.; Kongsuphol, P.; Sousa, M.; Uliyakina, I.; Palma, M.; Faria, D.; Traynor-Kaplan, A.E.; Fragata, J.I.; et al. Control of tmem16a by ino-4995 and other inositolphosphates. Br. J. Pharmacol. 2013, 168, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Namkung, W.; Phuan, P.W.; Verkman, A.S. Tmem16a inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J. Biol. Chem. 2011, 286, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Stutts, M.J.; Lazarowski, E.R.; Paradiso, A.M.; Boucher, R.C. Activation of CFTR Cl- conductance in polarized T84 cells by luminal extracellular atp. Am. J. Physiol. 1995, 268, C425–C433. [Google Scholar] [CrossRef] [PubMed]
- Ball, J.M.; Tian, P.; Zeng, C.Q.; Morris, A.P.; Estes, M.K. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 1996, 272, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.P.; Scott, J.K.; Ball, J.M.; Zeng, C.Q.; O’Neal, W.K.; Estes, M.K. Nsp4 elicits age-dependent diarrhea and Ca(2+)mediated i(-) influx into intestinal crypts of cf mice. Am. J. Physiol. 1999, 277, G431–G444. [Google Scholar] [PubMed]
- Ousingsawat, J.; Mirza, M.; Tian, Y.; Roussa, E.; Schreiber, R.; Cook, D.I.; Kunzelmann, K. Rotavirus toxin NSP4 induces diarrhea by activation of tmem16a and inhibition of na+ absorption. Pflugers Arch. 2011, 461, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Sun, M.; Wu, F.; Yang, L.; Di, W.; Zhang, G.; Zhong, L.; Ma, Z.; Zheng, J.; Fang, X.; et al. Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. PLoS ONE 2014, 9, e115443. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Wu, F.; Lv, J.; Li, H.; Li, X.; Du, Z.; Sun, M.; Zheng, Y.; Yang, L.; Zhong, L.; et al. Identification of the novel TMEM16A inhibitor dehydroandrographolide and its anticancer activity on SW620 cells. PLoS ONE 2015, 10, e0144715. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Wee, J.; Jung, J.; Jang, Y.; Lee, B.; Hong, G.S.; Chang, B.C.; Choi, Y.L.; Shin, Y.K.; Min, H.Y.; et al. Anoctamin 1 (TMEM16A) is essential for testosterone-induced prostate hyperplasia. Proc. Natl. Acad. Sci. USA 2015, 112, 9722–9727. [Google Scholar] [CrossRef] [PubMed]
- Godse, N.R.; Khan, N.; Yochum, Z.A.; Gomez-Casal, R.; Kemp, C.; Shiwarski, D.J.; Seethala, R.S.; Kulich, S.; Seshadri, M.; Burns, T.F.; et al. TMEM16A/ANO1 inhibits apoptosis via downregulation of bim expression. Clin. Cancer Res. 2017, 23, 7324–7332. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, M.; Liu, B.; Huang, Y.; Wang, K. Inhibition of Ca(2+)-activated Cl(−) channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012, 326, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Rottgen, T.S.; Nickerson, A.J.; Minor, E.A.; Stewart, A.B.; Harold, A.D.; Rajendran, V.M. Dextran sulfate sodium (DSS)-induced chronic colitis attenuates Ca(2+)-activated Cl(−) secretion in murine colon by down-regulating TMEM16A. Am. J. Physiol. Cell Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Almaca, J.; Tian, Y.; Aldehni, F.; Ousingsawat, J.; Kongsuphol, P.; Rock, J.R.; Harfe, B.D.; Schreiber, R.; Kunzelmann, K. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J. Biol. Chem. 2009, 284, 28571–28578. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dutta, A.; Kresge, C.; Bugde, A.; Feranchak, A.P. Bile acids stimulate cholangiocyte fluid secretion by activation of membraneTMEM16A Cl(-) channels. Hepatology 2018. [Google Scholar] [CrossRef]
- Sauter, D.R.P.; Novak, I.; Pedersen, S.F.; Larsen, E.H.; Hoffmann, E.K. Ano1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC). Pflugers Arch. 2015, 467, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rottgen, T.S.; Nickerson, A.J.; Rajendran, V.M. Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues. Int. J. Mol. Sci. 2018, 19, 1432. https://doi.org/10.3390/ijms19051432
Rottgen TS, Nickerson AJ, Rajendran VM. Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues. International Journal of Molecular Sciences. 2018; 19(5):1432. https://doi.org/10.3390/ijms19051432
Chicago/Turabian StyleRottgen, Trey S., Andrew J. Nickerson, and Vazhaikkurichi M. Rajendran. 2018. "Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues" International Journal of Molecular Sciences 19, no. 5: 1432. https://doi.org/10.3390/ijms19051432
APA StyleRottgen, T. S., Nickerson, A. J., & Rajendran, V. M. (2018). Calcium-Activated Cl− Channel: Insights on the Molecular Identity in Epithelial Tissues. International Journal of Molecular Sciences, 19(5), 1432. https://doi.org/10.3390/ijms19051432



