Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering
Abstract
1. Background
2. Scaffolds in Top-Down Tissue Engineering
2.1. Cartilage
2.2. Bone
3. Developmental Tissue Engineering
3.1. Spheroids
3.2. Organogenesis and Spheroids
4. Scaffolds and Spheroids
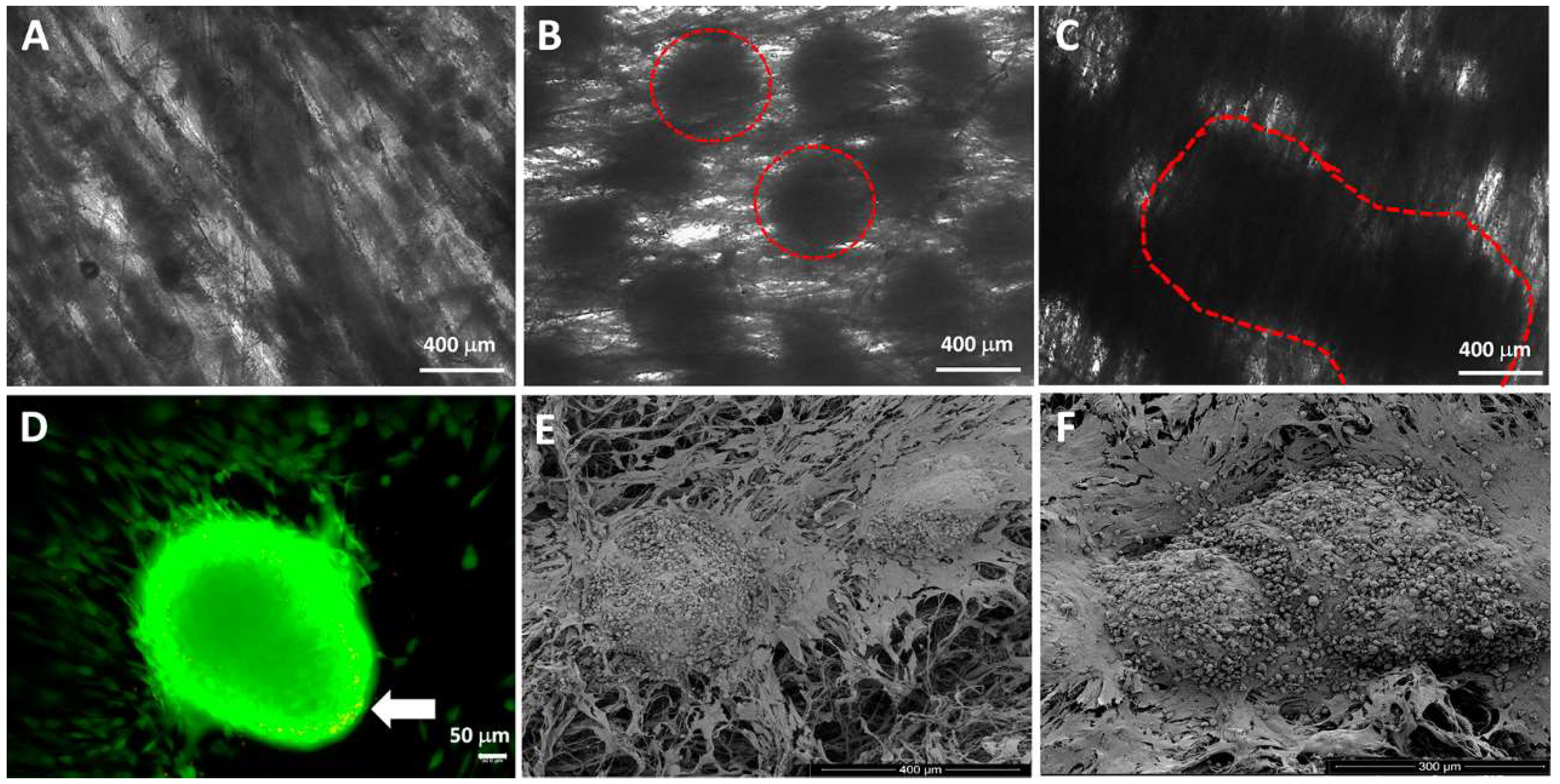
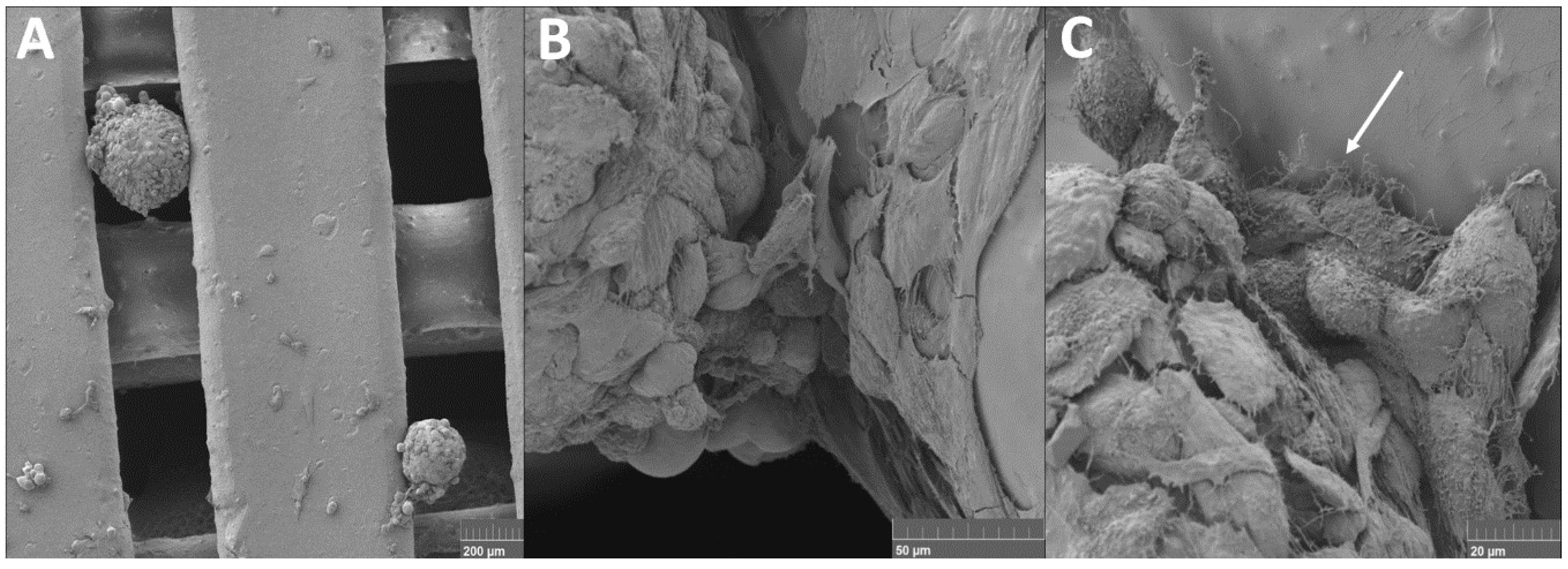
4.1. Spheroids Seeded into Nanofibers
4.2. Cell Suspension and Spheroids Seeded into 3D Printed Scaffold
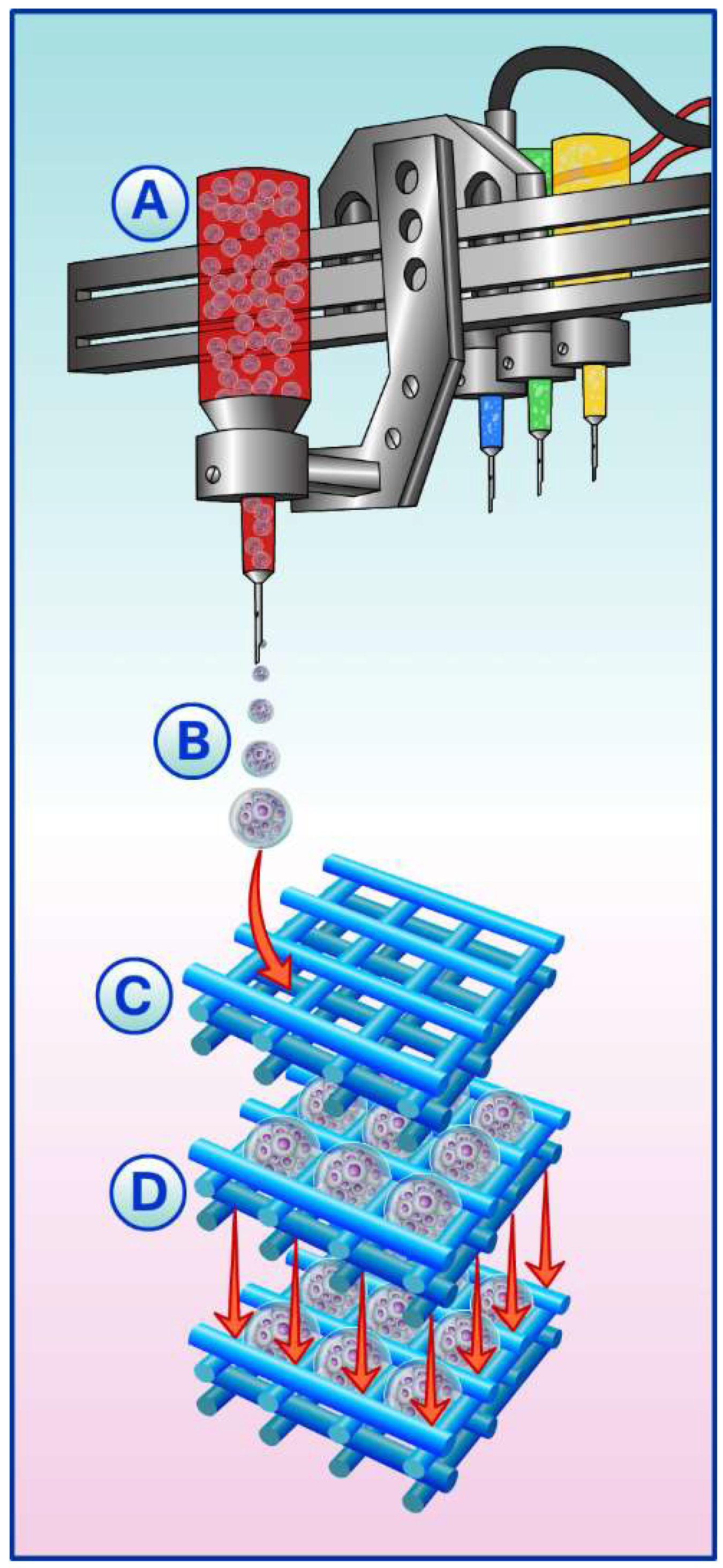
5. Spheroids, Scaffolds, and Building-Blocks
Building-Blocks in Biofabrication
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Hiew, V.V.; Simat, S.F.B.; Teoh, P.L. The advancement of biomaterials in regulating stem cell fate. Stem Cell Rev. 2018, 14, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M. Designing Smart Biomaterials for Tissue Engineering. Int. J. Mol. Sci. 2017, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, H.; Lee, J.; Na, K.; Lee, E. pH-responsive starch microparticles for a tumor-targeting implant. Polym. Adv. Technol. 2018. [Google Scholar] [CrossRef]
- Morochnik, S.; Zhu, Y.; Duan, C.; Cai, M.; Reid, R.; He, T.; Koh, J.; Szleifer, I.; Ameer, G. A thermoresponsive, citrate-based macromolecule for bone regenerative engineering. J. Biomed. Mater. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, E.; Byun, H.; Chang, H.; Jeong, K.; Aman, Z.; Choi, Y.; Park, J.; Shin, H. Engineering spheroids potentiating cell-cell and cell-ECM interactions by self-assembly of stem cell microlayer. Biomaterials 2018, 165, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, J.; Yeong, W. Smart hydrogels for 3D bioprinting. Int. J. Bioprint. 2015, 1, 3–14. [Google Scholar] [CrossRef]
- Guven, S.; Chen, P.; Inci, F.; Tasoglu, S.; Erkmen, B.; Demirc, U.M. Multiscale assembly for tissue engineering and regenerative medicine. Trends Biotechnol. 2015, 33, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Gaspar, D.; Sorushanova, A.; Milcovich, G.; Spanoudes, K.; Mullen, A.M.; O’Brien, T.; Pandit, A.; Zeugolis, D. Scaffold and scaffold-free self-assembled systems in regenerative medicine. Biotechnol. Bioeng. 2015, 113, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Bartosh, T.J.; Ylöstalo, J.H.; Mohammadipoor, A.; Bazhanov, N.; Coble, K.; Claypool, K.; Lee, R.H.; Choi, H.; Prockop, D.J. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc. Natl. Acad. Sci. USA 2010, 107, 13724–13729. [Google Scholar] [CrossRef] [PubMed]
- Frey, O.; Misun, P.M.; Fluri, D.A.; Hengstler, J.G.; Hierlemann, A. Reconfigurable microfluidic hanging drop network for multi-tissue interaction and analysis. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, C.; Büth, H.; Thielecke, H. A scaffold-free in vitro model for osteogenesis of human mesenchymal stem cells. Tissue Cell 2011, 43, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Ohno, J.; Sato, A.; Kido, H.; Fukushima, T. Mesenchymal stem cell spheroids exhibit enhanced in-vitro and in-vivo osteoregenerative potential. BMC Biotechnol. 2014, 105, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gurumurthy, B.; Bierdeman, P.C.; Janorkar, A.V. Spheroid model for functional osteogenic evaluation of human adipose derived stem cells. J. Biomed. Mater. Res. A 2016, 105, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2015, 34, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.T.; Tansaz, S.; Zanotto, E.D.; Boccaccini, A.R. Bioactive glass fiber-reinforced PGS matrix composites for cartilage regeneration. Materials 2017, 10, 83. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, G.; Cao, Y. Recent Progress in Cartilage Tissue Engineering—Our Experience and Future Directions. Tissue Eng. 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Choi, B.; Kim, S.; Lin, B.; Wu, B.M.; Lee, W. Cartilaginous Extracellular Matrix-Modified Chitosan Hydrogels for Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2014, 6, 20110–20121. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Burdick, J.A. Engineering Cartilage Tissue. Adv. Drug Deliv. Rev. 2008, 60, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Kontturi, L.S.; Järvinen, E.; Muhonen, V.; Collin, E.C.; Pandit, A.S.; Kiviranta, I.; Yliperttula, M.; Urtti, A. An injectable, in situ forming type II collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering. Drug Deliv. Transl. Res. 2014, 4, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, Q.; Dutta, A.; Wang, Y.; Huang, Y.; Weng, H.; Tang, L.; Hong, Y. An injectable extracellular matrix derived hydrogel for meniscus repair and regeneration. Acta Biomater. 2015, 16, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugam, A.; Arun Kumar, R.; Vishnu, P.M.; Shantikumar, V.N.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Comblain, F.; Rocasalbas, G.; Gauthier, S.; Henrotin, Y. Chitosan: A promising polymer for cartilage repair and viscosupplementation. Biomed. Mater. Eng. 2017, 28, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Utomo, L.; Pleumeekers, M.M.; Nimeskern, L.; Nurnberger, S.; Stok, K.S.; Hildner, F.; Osch, G.J.V.M. Preparation and characterization of a decellularized cartilage scaffold for ear cartilage reconstruction. Biomed. Mater. 2015, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Peng, J.; Lu, S.; Liu, S.; Zhang, L.; Huang, J.; Sui, X.; Zhao, B.; Wang, A.; Xu, W.; Luo, Z.; et al. In vivo cartilage repair using adipose-derived stem cell-loaded decellularized cartilage ECM scaffolds. J. Tissue Eng. Regen. Med. 2014, 8, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elçin, A.E.; Elçin, Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ohyabua, Y.; Adegawaa, T.; Yoshioka, T.; Ikoma, T.; Uemurac, T.; Tanakaa, J. Cartilage regeneration using a porous scaffold, a collagen sponge incorporating a hydroxyapatite/chondroitinsulfate composite. Mater. Sci. Eng. B 2010, 173, 204–207. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Fang, G.; Zhou, J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: A review. Materials 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, M.; Sun, H.; Li, P.; Wang, K.; Ren, F.; Lu, X. Porous titanium scaffolds with self-assembled micro/nano-hierarchical structure for dual functions of bone regeneration and anti-infection. J. Biomed. Mater. Res. A 2017, 105, 3482–3492. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Jahan, K.; Tabrizian, M. Composite biopolymers for bone regeneration enhancement in bony defects. Biomater. Sci. 2016, 4, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for bone regenerative engineering. Adv. Healthc. Mater. 2015, 9, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Huang, X.; Wang, C.; Dang, X.; Wang, K. Repair of bone defects using a new biomimetic construction fabricated by adipose-derived stem cells, collagen I, and porous beta-tricalcium phosphate scaffolds. Soc. Exp. Biol. Med. 2013, 238, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Jakob, F.; Ebert, R.; Ignatius, A.; Matsushita, T.; Watanabe, Y.; Groll, J.; Walles, H. Bone tissue engineering in osteoporosis. Maturitas 2013, 75, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Forrestal, D.P.; Klein, T.J.; Woodruff, M.A. Challenges in engineering large customized bone constructs. Biotechnol. Bioeng. 2017, 114, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- McMillen, P.; Holley, S.A. Integration of cell-cell and cell-ECM adhesion in vertebrate morphogenesis. Curr. Opin. Cell Biol. 2015, 36, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Moscona, A.; Moscona, H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J. Anat. 1952, 86, 287–301. [Google Scholar] [PubMed]
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trend Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Ong, H.S.; Zhu, Y.; Liu, S.W.; Liu, L.M.; Zhou, K.H.; Xu, Z.Q.; Gao, J.; Zhang, Y.; Ye, J.H.; et al. In situ release of VEGF enhances osteogenesis in 3D porous scaffolds engineered with osterix-modified adipose-derived stem cells. Tissue Eng. Part A 2017, 23, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, I.; Barnhoorn, M.C.; de Jonge-Muller, E.S.; Mieremet-Ooms, M.A.; van der Reijden, J.J.; van der Helm, D.; Hommes, D.W.; van der Meulen-de Jong, A.E.; Verspaget, H.W. Intraluminal Injection of mesenchymal stromal cells in spheroids attenuates experimental colitis. J. Crohns Colitis 2016, 10, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.; Syková, E.; Kubinová, Š. The therapeutic potential of three-dimensional multipotent mesenchymal stromal cell spheroids. Stem Cell Res. Ther. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marga, F.; Adrian, N.; Kosztin, I.; Forgacs, G. Developmental Biology and Tissue Engineering. Birth Defects Res. (Part C) 2007, 81, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Koo, B.K. Modeling mouse and human development using organoid cultures. Development 2015, 142, 3113–3125. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Mead, B.E.; Safaee, H.; Langer, R.; Karp, J.M.; Levy, O. Engineering Stem Cell Organoids. Cell Stem Cell 2016, 18, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Kale, S.; Biermann, S.; Edwards, C.; Tamowski, C.; Morris, M.; Long, M.W. Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat Biotechnol. 2000, 18, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.H.; Werner, B.C.; Liang, H.; Shang, H.; Yang, N.; Li, X.; Shimer, A.L.; Balian, G.; Katz, A.J. Implications of adipose-derived stromal cells in a 3D culture system for osteogenic differentiation: An in vitro and in vivo investigation. Spine J. 2013, 13, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Breit, S.; Parsch, D.; Benz, K.; Steck, E.; Hauner, H.; Weber, R.M.; Ewerbeck, V.; Richter, W. Cartilage-like gene expression in differentiated human stem cell spheroids: A comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheumatol. 2003, 48, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.P.; Matsui, R.A.M.; Santos, M.F.S.; Côrtes, I.; Azevedo, M.S.; Silva, K.R.; Beatrici, A.; Leite, P.E.C.; Falagan-Lotsch, P.; Granjeiro, J.M.; Mironov, V.; Baptista, L.S. Successful Low-Cost Scaffold-Free Cartilage Tissue Engineering Using Human Cartilage Progenitor Cell Spheroids Formed by Micromolded Nonadhesive Hydrogel. Stem Cells Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Khoruzhenko, A.I. 2D- and 3D-cell culture. Biopolym. Cell 2011, 27, 17–24. [Google Scholar] [CrossRef]
- Breslin, S.; O’Driscoll, L. The relevance of using 3D cell cultures, in addition to 2D monolayer cultures, when evaluating breast cancer drug sensitivity and resistance. Oncontarget 2016, 7, 45745–45756. [Google Scholar] [CrossRef] [PubMed]
- Creaney, J.; Robinson, B.W.S. Malignant Mesothelioma Biomarkers. Chest 2017, 152, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.M.; Camões, S.P.; Filipe, E.; Cipriano, M.; Barcia, R.N.; Filipe, M.; Teixeira, M.; Simões, S.; Gaspar, M.; Mosqueira, D.; Nascimento, D.S.; Pinto-do-Ó, P.; Cruz, P.; et al. Three-dimensional spheroid cell culture of umbilical cord tissue-derived mesenchymal stromal cells leads to enhanced paracrine induction of wound healing. Stem Cell Res. Ther. 2015, 6, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jakab, K.; Neagu, A.; Mironov, V.; Markwald, R.R.; Forgacs, G. Engineering biological structures of prescribed shape using self-assembling multicellular systems. Proc. Natl. Acad. Sci. USA 2004, 101, 2864–2869. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Pai-Shan, H.; Tseng, C.; Hsu, S. The substrate-dependent regeneration capacity of mesenchymal stem cell spheroids derived on various biomaterial surfaces. Biomater. Sci. 2014, 11, 1–9. [Google Scholar] [CrossRef]
- Ho, W.J.; Pham, E.A.; Kim, J.W.; Ng, C.W.; Kim, J.H.; Kamei, D.T.; Wu, B.M. Incorporation of multicellular spheroids into 3-D polymeric scaffolds provides an improved tumor model for screening anticancer drugs. Cancer Sci. 2010, 101, 2637–2643. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Schank, T.E.; Scheuer, C.; Kleer, S.; Schuler, S.; Metzger, W.; Eglin, D.; Alini, M.; Menger, M.D. Three-dimensional spheroids of adipose-derived mesenchymal stem cells are potent initiators of blood vessel formation in porous polyurethane scaffolds. Acta Biomater. 2013, 9, 6876–6884. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Murphy, K.C.; Binder, B.Y.K.; Vissers, C.B.; Leach, J.K. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cell Transl. Med. 2016, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tutak, W.; Sarkar, S.; Lin-Gibson, S.; Farooque, T.M.; Jyotsnendu, G.; Wang, D.; Kohn, J.; Bolikal, D.; Simon, C.G. The support of bone marrow stromal cell differentiation by airbrushed nanofiber scaffolds. Biomaterials 2013, 34, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Popielarczyk, T.L.; Nain, A.S.; Barrett, J.G. Aligned Nanofiber Topography Directs the Tenogenic Differentiation of Mesenchymal Stem Cells. Appl. Sci. 2017, 7, 59. [Google Scholar] [CrossRef]
- Zamani1, R.; Aval1, S.F.; Pilehvar-Soltanahmadi1, Y.; Nejati-Koshki1, K.; Zarghami, N. Recent Advances in Cell Electrospining of Natural and Synthetic Nanofibers for Regenerative Medicine. Drug Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sohier, J.; Corre, P.; Perret, C.; Pilet, P.; Weiss, P. Novel and Simple Alternative to Create Nanofibrillar Matrices of Interest for Tissue Engineering. Tissue Eng. Part C Methods 2014, 4, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Côrtes, I. Biofabricação de Cartilagem a Partir de Esferoides de Células-Tronco de Tecido Adiposo Humano. Master’s Dissertation, Post-graduation Program of Translational Biomedicine (Biotrans). Unigranrio, Campus I, Duque de Caxias, Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Chua, K.N.; Lim, W.S.; Zhang, P.; Lu, H.; Wen, J.; Ramakrishna, S.; Leong, K.W.; Mao, H.Q. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials 2005, 26, 2537–2547. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Ma, L.; Zhang, B.; Sun, J.; Sun, Y.; Fan, Y.; Gou, Z.; Zhou, C.; Zhang, X. Creating hierarchical porosity hydroxyapatite scaffolds with osteoinduction by three-dimensional printing and microwave sintering. Biofabrication 2017, 9, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.; Diez-Escudero, A.; Maazouz, Y.; Rappe, K.; Espanol, M.; Montufar, E.B.; Bonany, M.; Sadowska, J.M.; Guillem-Marti, J.; Öhman-Mägi, C.; et al. Osteoinduction by foamed and 3D-printed calcium phosphate scaffolds: Effect of nanostructure and pore architecture. ACS Appl. Mater. Interfaces 2017, 9, 41722–41736. [Google Scholar] [CrossRef] [PubMed]
- Della Bella, E.; Parrilli, A.; Bigi, A.; Panzavolta, S.; Amadori, S.; Giavaresi, G.; Martini, L.; Borsari, V.; Fini, M. Osteoinductivity of nanostructured hydroxyapatite-functionalized gelatin modulated by human and endogenous mesenchymal stromal cells. J. Biomed. Mater. Res. A 2017, 106, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Shichang, Z.; Chen, X.; Yufang, Z.; Changqing, Z. 3D-printed dimethyloxallyl glycine delivery scaffolds to improve angiogenesis and osteogenesis. Biomater. Sci. 2015, 3, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.J.; O’Brien, J.; Zhang, L.G. Integrating biologically inspired nanomaterials and table-top stereolithography for 3D printed biomimetic osteochondral scaffolds. Nanoscale 2015, 7, 14010–14022. [Google Scholar] [CrossRef] [PubMed]
- Weinand, C.; Neville, C.M.; Weinberg, E.; Tabata, Y.; Vacanti, J.P. Optimizing biomaterials for tissue engineering human bone using mesenchymal stem cells. Plast. Reconstr. Surg. 2016, 137, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Mao, X.; Du, Z.; Jiang, W.; Han, X.; Zhao, D.; Han, D.; Li, Q. Three dimensional printed macroporous polylactic acid/hydroxyapatite composite scaffolds for promoting bone formation in a critical-size rat calvarial defect model. Sci. Technol. Adv. Mater. 2016, 17, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive hydrogel and 3D printed polycaprolactone system for bone tissue engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Szivek, J.A.; Gonzales, D.A.; Wojtanowski, A.M.; Martinez, M.A.; Smith, J.L. Mesenchymal stem cell seeded, biomimetic 3D printed scaffolds induce complete bridging of femoral critical sized defects. J. Biomed. Mater. Res. B Appl. Biomater. 2018. [Google Scholar] [CrossRef] [PubMed]
- Grémare, A.; Guduric, V.; Bareille, R.; Heroguez, V.; Latour, S.; L’heureux, N.; Fricain, J.C.; Catros, S.; Le Nihouannen, D. Characterization of printed PLA scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; Malhotra, M.; Curtin, C.M.; O’ Brien, F.J.; Driscoll, C.M. Life in 3D is never flat: 3D models to optimise drug delivery. J Control. Release 2015, 215, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Rezende, R.; Pereira, F.; Kasyanov, V.; Ovsianikov, A.; Torgensen, J.; Gruber, P.; Stampfl, J.; Brakkle, K.M.; Nogeuria, J.; Mironov, V.; et al. Design, physical prototyping and initial characterisation of ‘lockyballs’. Virtual Phys. Prototyp. 2012, 7, 287–301. [Google Scholar] [CrossRef]
- Silva, K.; Rezende, R.; Pereira, F.; Gruber, P.; Stuart, M.; Ovsianikov, A.; Brakke, K.; Kasyanov, V.; Silva, J.; Granjeiro, J.; et al. Delivery of Human Adipose Stem Cells Spheroids into Lockyballs. PLoS ONE 2016, 11, e0166073. [Google Scholar] [CrossRef] [PubMed]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Fini, M.; Di Primio, R.; Mattioli-Belmonte, M. Biofabrication and Bone tissue regeneration: Cell source, approaches, and challenges. Front. Bioeng. Biotechnol. 2017, 5, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pedde, R.D.; Mirani, B.; Navaei, A.; Styan, T.; Wong, S.; Mehrali, M.; Thakur, A.; Mohtaram, N.K.; Bayati, A.; Dolatshahi-Pirouz, A.; et al. Emerging biofabrication strategies for engineering complex tissue constructs. Adv. Mater. 2017, 29, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Mekhileri, N.; Lim, K.; Brown, G.; Mutreja, I.; Schon, B.; Hooper, G.; Woodfield, T. Automated 3D bioassembly of micro-tissues for biofabrication of hybrid tissue engineered constructs. Biofabrication 2018, 10, 024103. [Google Scholar] [CrossRef] [PubMed]
- Bhise, N.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Shrik Zhang, Y.; Shin, S.; Calzone, G.; et al. A liver-on-a-chip platform with bi printed hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baptista, L.S.; Kronemberger, G.S.; Côrtes, I.; Charelli, L.E.; Matsui, R.A.M.; Palhares, T.N.; Sohier, J.; Rossi, A.M.; Granjeiro, J.M. Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 1285. https://doi.org/10.3390/ijms19051285
Baptista LS, Kronemberger GS, Côrtes I, Charelli LE, Matsui RAM, Palhares TN, Sohier J, Rossi AM, Granjeiro JM. Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering. International Journal of Molecular Sciences. 2018; 19(5):1285. https://doi.org/10.3390/ijms19051285
Chicago/Turabian StyleBaptista, Leandra Santos, Gabriela Soares Kronemberger, Isis Côrtes, Letícia Emiliano Charelli, Renata Akemi Morais Matsui, Thiago Nunes Palhares, Jerome Sohier, Alexandre Malta Rossi, and José Mauro Granjeiro. 2018. "Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering" International Journal of Molecular Sciences 19, no. 5: 1285. https://doi.org/10.3390/ijms19051285
APA StyleBaptista, L. S., Kronemberger, G. S., Côrtes, I., Charelli, L. E., Matsui, R. A. M., Palhares, T. N., Sohier, J., Rossi, A. M., & Granjeiro, J. M. (2018). Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering. International Journal of Molecular Sciences, 19(5), 1285. https://doi.org/10.3390/ijms19051285




