Mecasermin in Insulin Receptor-Related Severe Insulin Resistance Syndromes: Case Report and Review of the Literature
Abstract
1. Introduction
2. Materials and Methods
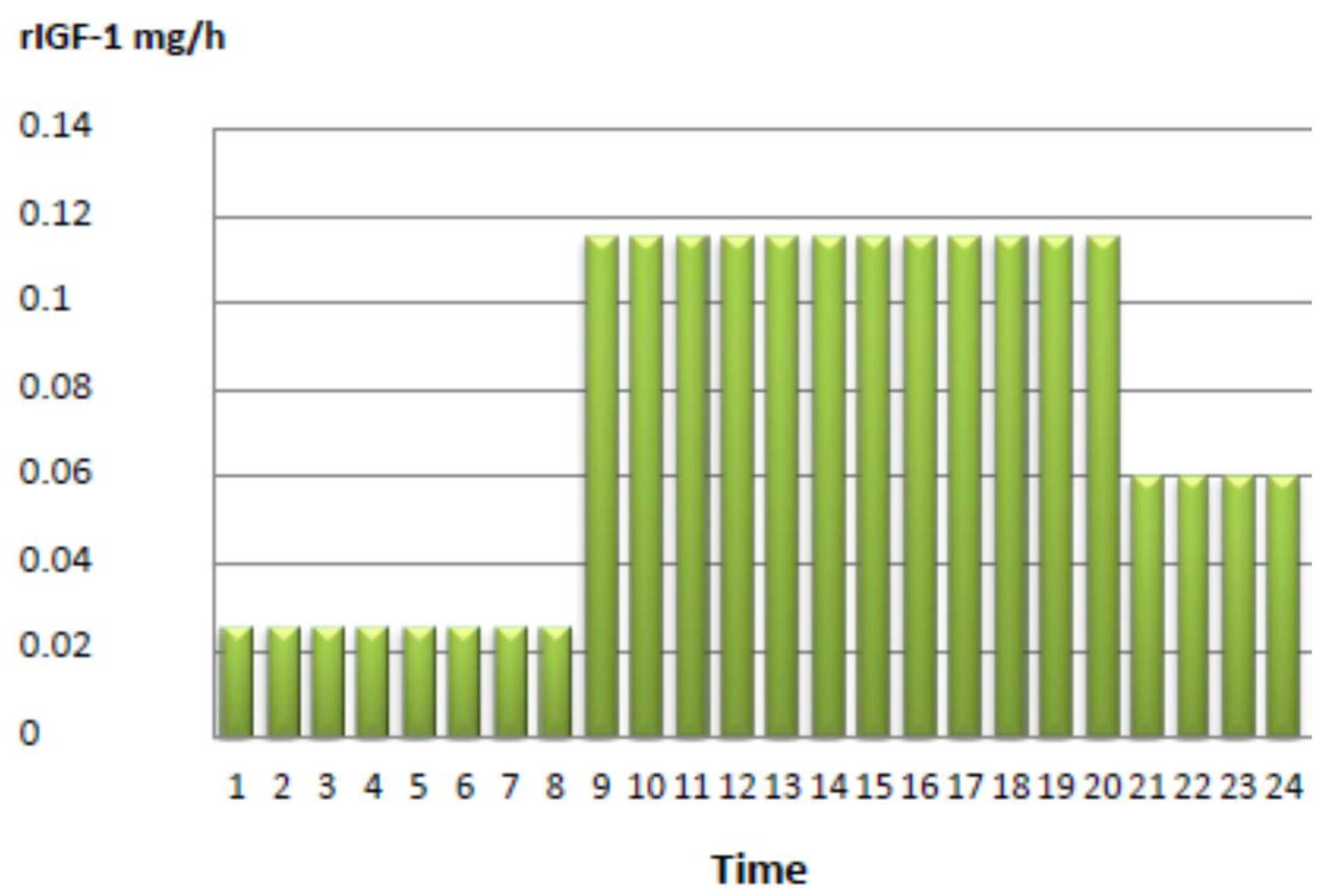
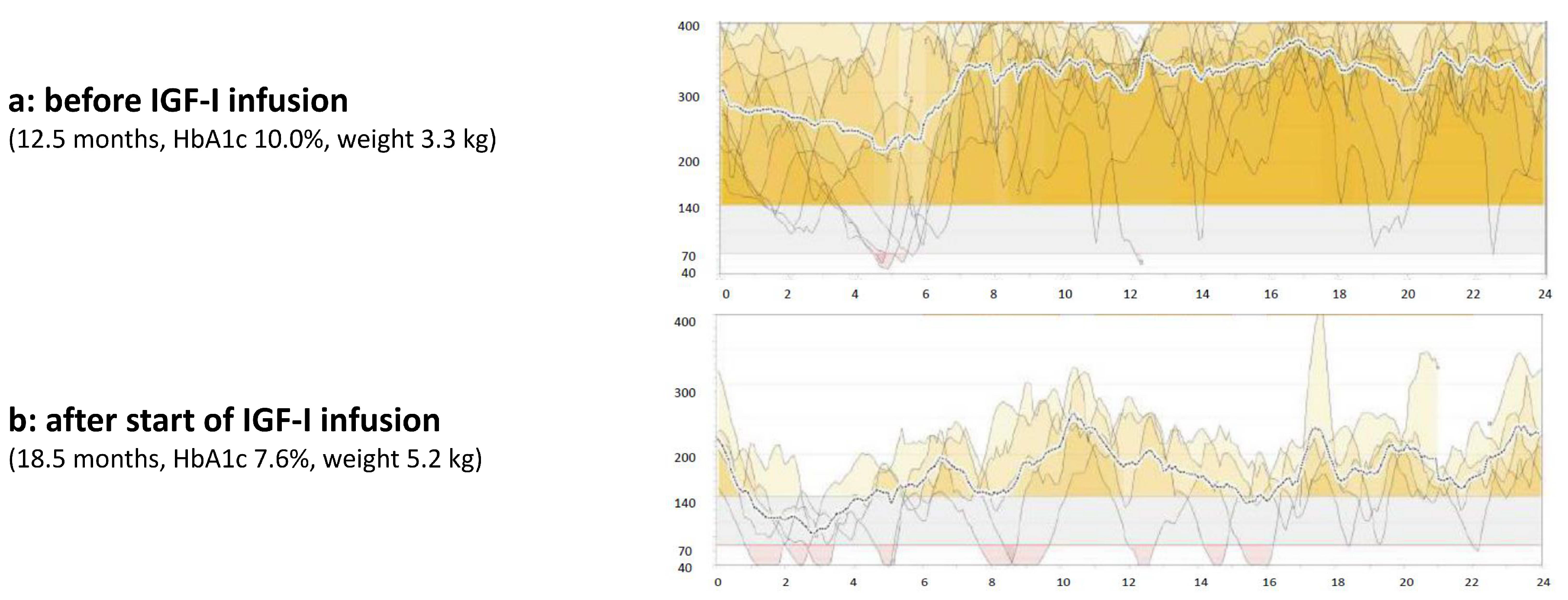
3. Case Report
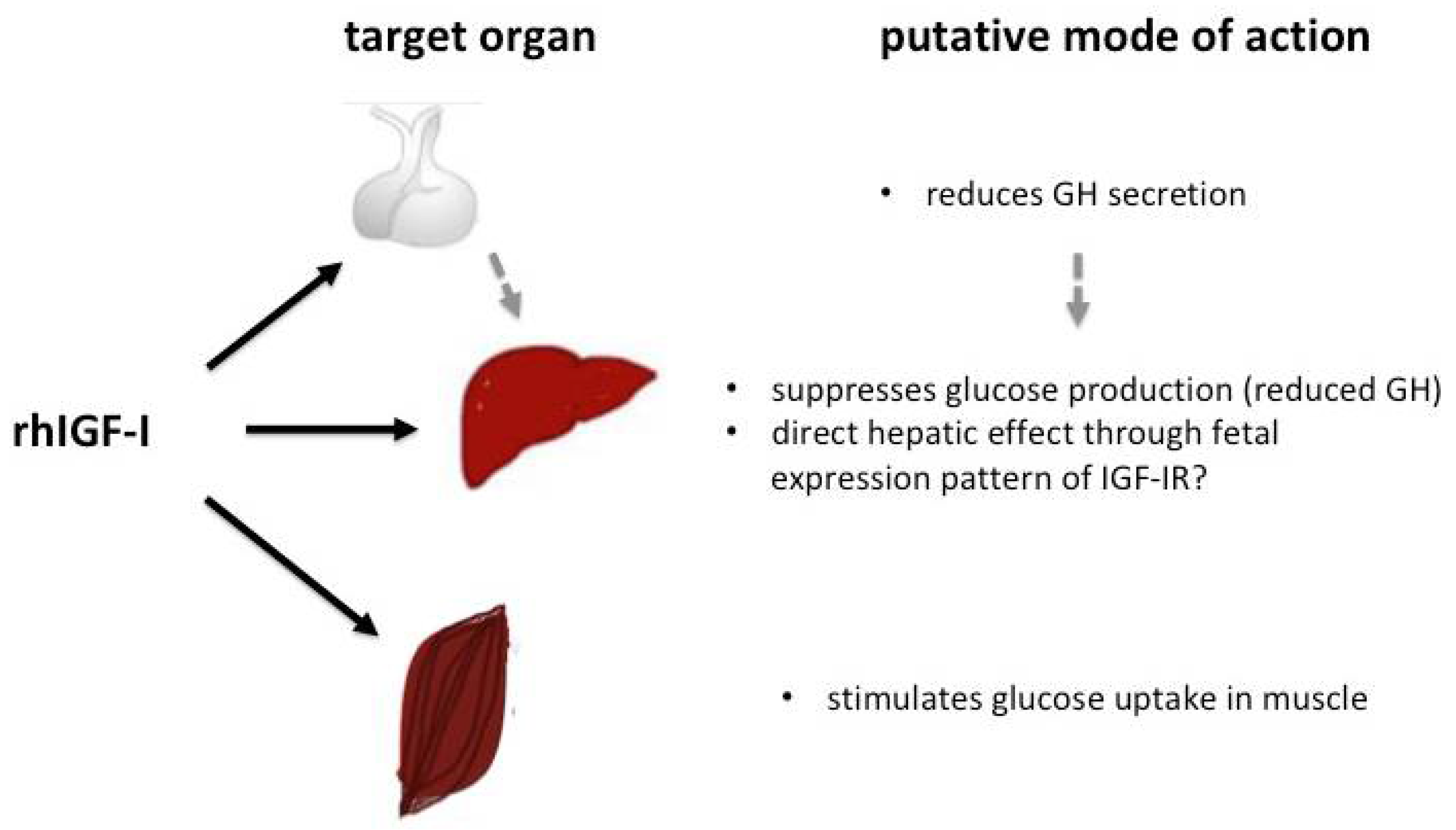
4. Overview of Current Knowledge on the Use of rhIGF-I in SIR
4.1. Clinical Picture of SIR
4.2. Treatment Options in SIR Other Than rhIGF-I
4.3. Use of rhIGF-I in Insulin Receptor-Related Severe Insulin Resistance Syndromes
5. Conclusions
Author Contributions
Conflicts of Interest
References
- McDonald, A.; Williams, R.; Regan, F.M.; Semple, R.K.; Dunger, D.B. IGF-I treatment of insulin resistance. Eur. J. Endocrinol. 2007, 157, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Parker, V.E.R.; Semple, R.K. Genetic Forms of Severe Insulin Resistance: What Endocrinologists Should Know. Eur. J. Endocrinol. 2013, 169, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ben Harouch, S.; Falik Zaccai, T.C.; Klar, A. INSR-Related Severe Syndromic Insulin Resistance. In GeneReviews® [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Stephens, K., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2018; Available online: http://www.ncbi.nlm.nih.gov/books/NBK476444/ (accessed on 25 January 2018).
- Donohue, W.L.; Uchida, I. Leprechaunism: A euphemism for a rare familial disorder. J. Pediatr. 1954, 455, 505–519. [Google Scholar] [CrossRef]
- Elders, M.J.; Schedewie, H.K.; Olefsky, J.; Givens, B.; Char, F.; Bier, D.M.; Baldwin, D.; Fiser, R.H.; Seyedabadi, S.; Rubenstein, A. Endocrine-metabolic relationships in patients with leprechaunism. J. Natl. Med. Assoc. 1982, 74, 1195–1210. [Google Scholar] [PubMed]
- De Bock, M.; Hayes, I.; Semple, R. Donohue syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 1416–1417. [Google Scholar] [CrossRef] [PubMed]
- Grasso, V.; Colombo, C.; Favalli, V.; Galderisi, A.; Rabbone, I.; Gombos, S.; Bonora, E.; Massa, O.; Meschi, F.; Cerutti, F.; et al. Six cases with severe insulin resistance (SIR) associated with mutations of insulin receptor: Is a Bartter-like syndrome a feature of congenital SIR? Acta Diabetol. 2013, 50, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Falik Zaccai, T.C.; Kalfon, L.; Klar, A.; Ben Elisha, M.; Hurvitz, H.; Weingarten, G.; Chechik, E.; Fleisher Sheffer, V.; Haj Yahya, R.; Meidan, G.; et al. Two novel mutations identified in familial cases with Donohue syndrome. Mol. Genet. Genom. Med. 2014, 2, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Desbois-Mouthon, C.; Girodon, E.; Ghanem, N.; Caron, M.; Pennerath, A.; Conteville, P.; Magre, J.; Besmond, C.; Goossens, M.; Capeau, J.; et al. Molecular analysis of the insulin receptor gene for prenatal diagnosis of leprechaunism in two families. Prenat. Diagn. 1997, 17, 657–663. [Google Scholar] [CrossRef]
- Rabson, S.M.; Mendenhall, E.N. Familial hypertrophy of pineal body, hyperplasia of adrenal cortex and diabetes mellitus. Am. J. Clin. Pathol. 1956, 26, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Wang, Y.; Pasquali, M. Progressive decline in insulin levels in Rabson-Mendenhall syndrome. J. Clin. Endocrinol. Metab. 1999, 84, 2623–2629. [Google Scholar] [CrossRef] [PubMed]
- Semple, R.; Williams, R.; Dunger, D.B. What is the best management strategy for patients with severe insulin resistance? Clin. Endocrinol. 2010, 73, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Semple, R.K.; Savage, D.B.; Cochran, E.K.; Gorden, P.; O’Rahilly, S. Genetic syndromes of severe insulin resistance. Endocr. Rev. 2011, 32, 498–514. [Google Scholar] [CrossRef] [PubMed]
- Cochran, E.; Musso, C.; Gorden, P. The use of U-500 in patients with extreme insulin resistance. Diabetes Care 2005, 28, 1240–1244. [Google Scholar] [CrossRef] [PubMed]
- Huggard, D.; Stack, T.; Satas, S.; Gorman, C.O. Donohue syndrome and use of continuous subcutaneous insulin pump therapy. BMJ Case Rep. 2015, 27, 2015. [Google Scholar] [CrossRef] [PubMed]
- Atabek, M.E.; Pirgon, O. Some effect of metformin on insulin resistance in an infant with leprechaunism. J. Pediatr. Endocrinol. Metab. 2006, 19, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Cochran, E.; Young, J.R.; Sebring, N.; DePaoli, A.; Oral, E.A.; Gorden, P. Efficacy of recombinant methionyl human leptin therapy for the extreme insulin resistance of the Rabson-Mendenhall syndrome. J. Clin. Endocrinol. Metab. 2004, 89, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.J.; Cochran, E.; Gorden, P. Metreleptin improves blood glucose in patients with insulin receptor mutations. J. Clin. Endocinol. Metab. 2013, 98, E1749–E1756. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Gray, A.; Tam, A.W.; Yanf-Feng, T.; Tsubokawa, M.; Collin, C.; Henzel, W.; Le Bon, T.; Kathuria, S.; Chen, E. Insulin-like growth factor I receptor primary structure: Comparison with insulin receptor suggests structural determinants that define functional specifity. EMBO J. 1986, 5, 2503–2512. [Google Scholar] [PubMed]
- Rosenbloom, A.L. Mecasermin (recombinant human insulin-like growth factor I). Adv. Ther. 2009, 26, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.M.; McDonald, A.; O’Savage, M.; Dunger, D.B. Mecasermin rinfabate: rhIGF-I/rhIGFBP-3 complex: iPLEX. Expert Opin. Drug Metab. Toxicol. 2008, 4, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.A.; Froesch, E.R. Treatment of type A insulin resistance with insulin-like growth factor-I. Lancet 1993, 3418859, 1536–1537. [Google Scholar] [CrossRef]
- Quin, J.D.; Fisher, B.M.; Paterson, K.R.; Inoue, A.; Beastall, G.H.; MacCuish, A.C. Acute response to recombinant insulin-like growth factor I in a patient with Mendenhall’s syndrome. N. Engl. J. Med. 1990, 323, 1425–1426. [Google Scholar] [PubMed]
- Schoenle, E.J.; Zenobi, P.D.; Torresani, T.; Werder, E.A.; Zachmann, M.; Froesch, E.R. Recombinant human insulin-like growth factor I (rhIGF I) reduces hyperglycaemia in patients with extreme insulin resistance. Diabetologia 1991, 34, 675–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zenobi, P.D.; Glatz, Y.; Keller, A.; Graf, S.; Jaeggi-Groisman, S.E.; Riesen, W.F.; Schoenle, E.J.; Froesch, E.R. Beneficial metabolic effects of insulin-like growth factor I in patients with severe insulin-resistant diabetes type A. Eur. J. Endocrinol. 1994, 131, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, H.; Rossen, M.; Urhammer, S.A.; Müller, J.; Pedersen, O. Short- and long-term metabolic effects of recombinant human IGF-I treatment in patients with severe insulin resistance and diabetes mellitus. Eur. J. Endocrinol. 1997, 136, 475–482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morrow, L.A.; O’Brien, M.B.; Moller, D.E.; Flier, J.S.; Moses, A.C. Recombinant human insulin-like growth factor-I therapy improves glycemic control and insulin action in the type A syndrome of severe insulin resistance. J. Clin. Endocrinol. Metab. 1994, 79, 205–210. [Google Scholar] [PubMed]
- Nakashima, N.; Umeda, F.; Yanase, T.; Nawata, H. Insulin resistance associated with substitution of histidine for arginine 252 in the alpha-subunit of the human insulin receptor: Trial of insulin-like growth factor I injection therapy to enhance insulin sensitivity. J. Clin. Endocrinol. Metab. 1995, 80, 3662–3667. [Google Scholar] [PubMed]
- Nakae, J.; Kato, M.; Murashita, M.; Shinohara, N.; Tajima, T.; Fujieda, K. Long-term effect of recombinant human insulin-like growth factor I on metabolic and growth control in a patient with leprechaunism. JCEM 1998, 83, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Stanescu, D.; Semple, R.; Holland, C.; Magge, S.N. Continuous, subcutaneous IGF-I therapy via insulin pump in patient with Donohue Syndrome. J. Pediatr. Endocrinol. Metab. 2014, 27, 1237–1241. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carmody, D.; Ladsaria, S.S.; Buikema, R.K.; Semple, R.K.; Greeley, S.A. Case Report: Successful rhIGF1 treatment for over 5 years in a patient with severe insulin resistance due to homozygous insulin receptor mutation. Diabet. Med. 2016, 33, e8–e12. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Kadowaki, H.; Momomura, K.; Fukushima, Y.; Orban, T.; Okai, T.; Taketani, Y.; Akanuma, Y.; Yazaki, Y.; Kadowaki, T. A homozygous kinase-defective mutation in the insulin receptor gene in a patient with leprechaunism. Diabetologia 1997, 40, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Grosse, G.; Hilger, A.; Ludwig, M.; Reutter, H.; Lorenzen, F.; Even, G.; Holterhus, P.M.; Woelfle, J.; German GHI Study Group. Targeted Resequencing of Putative Growth-Related Genes Using Whole Exome Sequencing in Patients with Severe Primary IGF-I Deficiency. Horm. Res. Paediatr. 2017, 88, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, J.; Chia, D.J.; Massart-Schlesinger, M.B.; Moyano, P.; Rotwein, P. Molecular physiology, pathology, and regulation of the growth hormone/insulin-like growth factor-I system. Pediatr. Nephrol. 2005, 20, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, J.; Chia, D.J.; Rotwein, P. Mechanisms of growth hormone (GH) action. Identification of conserved Stat5 binding sites that mediate GH-induced insulin-like growth factor-I gene activation. J. Biol. Chem. 2003, 278, 51261–51266. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, J.; Rotwein, P. In vivo regulation of growth hormone-stimulated gene transcription by STAT5b. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E393–E401. [Google Scholar] [CrossRef] [PubMed]
- Chia, D.J.; Ono, M.; Woelfle, J.; Schlesinger-Massart, M.; Jiang, H.; Rotwein, P. Characterization of distinct Stat5b binding sites that mediate growth hormone-stimulated IGF-I gene transcription. J. Biol. Chem. 2006, 281, 3190–3197. [Google Scholar] [CrossRef] [PubMed]
- Vidal, O.M.; Merino, R.; Rico-Bautista, E.; Fernandez-Perez, L.; Chia, D.J.; Woelfle, J.; Ono, M.; Lenhard, B.; Norstedt, G.; Rotwein, P.; et al. In vivo transcript profiling and phylogenetic analysis identifies suppressor of cytokine signaling 2 as a direct signal transducer and activator of transcription 5b target in liver. Mol. Endocrinol. 2007, 21, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Woelfle, J.; Billiard, J.; Rotwein, P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J. Biol. Chem. 2003, 278, 22696–22702. [Google Scholar] [CrossRef] [PubMed]
- Rotwein, P.; Billiard, J.; Woelfle, J. Molecular physiology of IGF-I expression. J. Pediatr. Endocrinol. Metab. 2002, 15 (Suppl. 5), 1455–1458. [Google Scholar] [PubMed]
- Woelfle, J. Wachstumshormoninsensitivität und schwerer primärer Mangel an insulinähnlichem Wachstumsfaktor-1: Behandlung im Kindesalter. Monatsschrift Kinderheilkunde 2014, 162, 309–314. [Google Scholar] [CrossRef]
- Schreiner, F.; Schoenberger, S.; Koester, B.; Domené, H.M.; Woelfle, J. Novel acid-labile subunit (IGFALS) mutation p.T145K (c.434C > A) in a patient with ALS deficiency, normal stature and immunological dysfunction. Horm. Res. Paediatr. 2013, 80, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Bang, P.; Polak, M.; Woelfle, J.; Houchard, A.; EU IGFD Registry Study Group. Effectiveness and Safety of rhIGF-1 Therapy in Children: The European Increlex® Growth Forum Database Experience. Horm. Res. Paediatr. 2015, 83, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B.; Wölfle, J.; Schnabel, D.; Bettendorf, M. Treatment of dwarfism with recombinant human insulin-like growth factor-1. Dtsch. Arztebl. Int. 2009, 106, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Ardon, O.; Procter, M.; Tvrdik, T.; Longo, N.; Mao, R. Sequencing analysis of insulin receptor defects and detection of two novel mutations in INSR gene. Mol. Genet. Metab. Rep. 2014, 1, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Wang, Y.; Smith, S.A.; Langley, S.D.; DiMeglio, L.A.; Giannella-Neto, D. Genotype–phenotype correlation in inherited severe insulin resistance. Hum. Mol. Genet. 2002, 11, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdelaziz, R.; Ben Chehida, A.; Azzouz, H.; Boudabbous, H.; Lascols, O.; Ben Turkia, H.; Tebib, N. A novel homozygous missense mutation in the insulin receptor gene results in an atypical presentation of Rabson-Mendenhall syndrome. Eur. J. Med. Genet. 2016, 59, 16–19. [Google Scholar] [CrossRef] [PubMed]
- De Kerdanet, M.; Caron-Debarle, M.; Nivot, S.; Gaillot, T.; Lascols, O.; Fremont, B.; Bonnaure-Mallet, M.; Gie, S.; Massart, C.; Capeau, J. Ten-year improvement of insulin resistance and growth with recombinant human insulin-like growth factor 1 in a patient with insulin receptor mutations resulting in leprechaunism. Diabetes Metab. 2015, 41, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, A.; Stuart, G.; Harding, L. Donohue syndrome: A review of literature, case series, and anesthetic considerations. Pediatr. Anesth. 2018, 28, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Simpkin, A.; Cochran, E.; Cameron, F.; Dattani, M.; de Bock, M.; Dunger, D.B.; Forsander, G.; Guran, T.; Harris, J.; Isaac, I.; et al. Insulin receptor and the kidney: Nephrocalcinosis in patients with recessive INSR mutations. Nephron Physiol. 2014, 128, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Sharma, N.; Gill, P.S.; Igarashi, P.; Kahn, C.R.; Wade, J.B.; Ecelbarger, C.M. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc. Natl. Acad. Sci. USA 2008, 105, 6469–6474. [Google Scholar] [CrossRef] [PubMed]
- Accili, D.; Drago, J.; Lee, E.J.; Johnson, M.D.; Cool, M.H.; Salvatore, P.; Asico, L.D.; José, P.A.; Taylor, S.I.; Westphal, H. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat. Genet. 1996, 12, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Ogilvy-Stuart, A.L.; Soos, M.A.; Hands, S.J.; Anthony, M.Y.; Dunger, D.B.; O’Rahilly, S. Hypoglycemia and resistance to ketoacidosis in a subject without functional insulin receptors. J. Clin. Endocrinol. Metab. 2001, 86, 3319–3326. [Google Scholar] [CrossRef] [PubMed]
- Wertheimer, E.; Lu, S.; Backeljauw, P.F.; Davenport, M.L.; Taylor, S.I. Homozygous deletion of the human insulin receptor gene results in leprechaunism. Nat. Genet. 1993, 5, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, T.; Murakami, T.; Tanaka, H.; Miyawaki, S.; Yamashiro, T.; Takano-Yamamoto, T. Dental and craniofacial characteristics in a patient with leprechaunism treated with insulin-like growth factor-I. Angle Orthod. 2008, 78, 745–751. [Google Scholar] [CrossRef]
- Bathi, R.J.; Parveen, S.; Mutalik, S.; Rao, R. Rabson-Mendenhall syndrome: two case reports and a brief review of the literature. Odontology 2010, 98, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Musso, C.; Cochran, E.; Moran, S.A.; Skarulis, M.C.; Oral, E.A.; Taylor, S.; Gorden, P. Clinical course of genetic diseases of the insulin receptor (type A and Rabson-Mendenhall syndromes): A 30-year prospective. Medicine 2004, 83, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Abe, Y.; Watanabe, T. Renal tubular dysfunction in patients with molecular defects of the insulin receptor gene. Eur. J. Pediatr. 2014, 173, 263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abe, Y.; Sato, T.; Takagi, M.; Watanabe, T.; Nagayama, Y.; Hasegawa, T.; Abe, T. A case of Rabson-Mendenhall syndrome with a novel mutation in the tyrosine kinase domain of the insulin receptor gene complicated by medullary sponge kidney. J. Pediatr. Endocrinol. Metab. 2012, 25, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Baqir, Z.S.; Al-Lawati, T.T.; Al Hussaini, S.O.; Al-Sinani, A.; Al-Said, K.; Al-Rashdi, I. A novel leprechaunism mutation, Cys807Arg, in an Arab infant: a rare cause of hypoglycaemia. Paediatr. Int. Child Health 2012, 32, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Hovnik, T.; Brantanic, N.; Podkrajšek, K.T.; Kovač, J.; Paro, D.; Podnar, T.; Bratina, N.; Battelino, T. Severe progressive obstructive cardiomyopathy and renal tubular dysfunction in Donohue syndrome with decreased insulin receptor autophosphorylation due to a novel INSR mutation. Eur. J. Pediatr. 2013, 172, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Desbois-Mouthon, C.; Magré, J.; Duprey, J.; Caron, M.; Blivet-Van Eggelpoel, M.J.; Daubas, C.; Gourmelen, M.; Chevallier, B.; Rizkalla, S.; Robert, J.J.; et al. Major circadian variations of glucose homeostasis in a patient with Rabson-Mendenhall syndrome and primary insulin resistance due to a mutation(Cys284 → Tyr) in the insulin receptor alpha-subunit. Pediatr. Res. 1997, 42, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Singh, R.; Elsas, L.J. Decreased half-life of insulin-like growth factor I in Rabson-Mendenhall syndrome. J. Inherit. Metab. Dis. 2001, 24, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Carrasco de la Fuente, M.; Barrio Castellanos, R.; Alonso Blanco, M.; de la Calle Blasco, H. Long survival in Rabson-Mendenhall syndrome. Diabetes Res. Clin. Pract. 2010, 89, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.O.; Zagury, R.L.; Nascimento, T.S. Multidrug therapy in a patient with Rabson-Mendenhall syndrome. Diabetologia 2010, 53, 2454–2455. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.H.; Taylor, B.J.; Wheeler, B.J. Renal manifestations of severe Rabson-Mendenhall syndrome: A case report. J. Diabetes Metab. Disord. 2013, 12, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moore, M.M.; Bailey, A.M.; Flannery, A.H.; Baum, R.A. Treatment of diabetic ketoacidosis with intravenous U-500 insulin in a patient with Rabson-Mendenhall syndrome: A case report. J. Pharm. Pract. 2017, 30, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Skarulis, M.C.; Celi, F.S.; Mueller, E.; Zemskova, M.; Malek, R.; Hugendubler, L.; Cochran, C.; Solomon, J.; Chen, C.; Gorden, P. Thyroid hormone induced brown adipose tissue and amelioration of diabetes in a patient with extreme insulin resistance. JCEM 2010, 95, 256–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gorden, P.; Zadeh, E.S.; Cochran, E.; Brown, R.J. Syndromic insulin resistance: Models for the therapeutic basis of the metabolic syndrome and other targets of insulin resistance. Endocr. Pract. 2012, 18, 763–771. [Google Scholar] [CrossRef] [PubMed]
- LeRoith, D.; Yakar, S. Mechanisms of disease: Metabolic effects of growth hormone and insulin-like growth factor 1. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Li, T.; Chen, Z.B.; Luo, B.; Sun, R.P. Prevention of beta cell dysfunction and apoptosis by adenoviral gene transfer of rat insulin-like growth factor 1. Chin. Med. J. 2009, 122, 2159–2164. [Google Scholar] [PubMed]
- Escribano, O.; Guillén, C.; Nevado, C.; Gómez-Hernández, A.; Kahn, C.R.; Benito, M. Beta-Cell hyperplasia induced by hepatic insulin resistance: Role of a liver-pancreas endocrine axis through insulin receptor A isoform. Diabetes 2009, 584, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Kuzuya, H.; Matsuura, N.; Sakamoto, M.; Makino, H.; Sakamoto, Y.; Kadowaki, T.; Suzuki, Y.; Kobayashi, M.; Akazawa, Y.; Nomura, M.; et al. Trial of insulin like growth factor I therapy for patients with extreme insulin resistance syndromes. Diabetes 1993, 42, 696–70572. [Google Scholar] [CrossRef] [PubMed]
- Bondy, C.A.; Underwood, L.E.; Clemmons, D.R.; Guler, H.P.; Bach, M.A.; Skarulis, M. Clinical uses of insulin-like growth factor I. Ann. Intern. Med. 1994, 120, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Backeljauw, P.F.; Alves, C.; Clemmons, D.R.; Guler, H.P.; Bach, M.A.; Skarulis, M. Effect of intravenous insulin-like growth factor I in two patients with leprechaunism. Pediatr. Res. 1994, 36, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Nishimura, R.; Utsunomiya, A.; Kagawa, R.; Funata, H.; Fujimoto, M.; Hanaki, K.; Kanzaki, S. Leprechaunism (Donohue syndrome): A case bearing novel compound heterozygous mutations in the insulin receptor gene. Endocr. J. 2013, 60, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Sudo, S.; Nakamura, A.; Endo, D.; Konno, Y.; Ishizu, K.; Tajima, T. Development of endometrial carcinoma in a patient with leprechaunism (donohue syndrome). Clin. Pediatr. Endocrinol. 2013, 22, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Kitamei, H.; Yokoi, M.; Kase, M.; Ohno, S. Retinal neovascularization during treatment with IGF-1 for insulin resistance syndrome. Graefes Arch. Clin. Exp. Opthalmol. 2005, 243, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Regan, F.M.; Williams, R.M.; McDonald, A.; Umpleby, A.M.; Acerini, C.L.; O’Rahilly, S.; Hovorka, R.; Semple, R.K.; Dunger, D.B. Treatment with recombinant human insulin-like growth factor (rhIGF)-I/rhIGF binding protein-3 complex improves metabolic control in subjects with severe insulin resistance. JCEM 2010, 95, 2113–2122. [Google Scholar] [PubMed]
- Ranke, M.B. Insulin-like growth factor-I treatment of growth disorders, diabetes mellitus and insulin resistance. Trends Endocrinol. Metab. 2005, 16, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Brisigotti, M.; Fabbretti, G.; Pesce, F.; Gatti, R.; Cohen, A.; Parenti, G.; Callea, F. Congenital bilateral juvenile granulosa cell tumor of the ovary in leprechaunism: A case report. Pediatr. Pathol. 1993, 13, 549–558. [Google Scholar] [CrossRef] [PubMed]
| DS | RMS | Type A Insulin Resistance | |
|---|---|---|---|
| Molecular genetics | Homozygous mutation in the insulin receptor (INSR) gene | Compound heterozygous mutation in INSR gene | Mutation in the insulin receptor gene (autosomal-dominant or autosomal-recessive) |
| Face | Proptosis | Resembling DS | Normal phenotype |
| Infraorbital folds | or milder phenotype | ||
| Large, posteriorly rotated ears | |||
| Thick lips | |||
| Gingival hyperplasia | |||
| Broad nasal tip | |||
| Other | Large hands and feet (relative to body) Gingival hypertrophy | Early dentition and dental crowding Nail dysplasia | Usually not obese |
| Abdominal distension | |||
| Reduced lateral thoracic dimension | |||
| Hyperplasia of nipples | Hyperplasia of nipples | ||
| Genital enlargement | Genital enlargement | ||
| Intrauterine growth restriction | Growth retardation (less severe than in DS) | Normal growth | |
| Postnatal failure to thrive | |||
| Organ pathologies | Organomegaly (kidney, liver, spleen) | Organomegaly (kidney, liver, spleen) | |
| Hypertrophic cardiomyopathy (HCOM) | Hypertrophic cardiomyopathy | ||
| Nephrocalcinosis Renal tubular dysfunction | Nephrocalcinosis Renal tubular dysfunction | ||
| Enlarged polycystic ovaries | Enlarged polycystic ovaries | Polycystic ovaries | |
| Rectal prolapse | Second decade: microvascular complications | ||
| Cholestasis | Retinopathy Peripheral neuropathy Renal vascular complications | ||
| Skin Features | Hypertrichosis | Hypertrichosis | Hirsutism |
| Acanthosis nigricans | Acanthosis nigricans | Acanthosis nigricans | |
| Hyperkeratosis | |||
| Thick, hyperelastic skin | |||
| Dry skin | |||
| Decreased subcutaneous fat mass | |||
| Biochemical Findings | Hyperinsulinemia | Same as DS in first year | Hyperinsulinemia or |
| Extremely elevated plasma insulin and C-peptide levels | of live Insulin level decline steadily | extreme resistance to exogenous insulin | |
| Hyperglycemia | Resulting in increased | Hyperandrogenism | |
| Fasting hypoglycemia | glucose levels, fewer hypoglycemic events | Increased serum testosterone | |
| Absence of ketoacidosis | Risk of ketoacidosis | ||
| Decreased IGF-I and IGFBP-3 serum concentrations Hypercalciuria | Decreased IGF-I levels and IGFBP-3 Low triglyceride levels, high HDL levels Hypercalciuria | ||
| Neurological Findings | Severe global developmental delay | Variable developmental | No general impairment |
| Axial hypotonia | delay to normal | ||
| Muscle atrophy | Intelligence | ||
| Life expectancy | Usually death within first two years of life, due to intercurrent infections, severe hypoglycemia, cardiomyopathy | Usually death within second decade of life, due to ketoacidosis or microvascular complications |
| Treatment Option | DS | RMS | Type A IR |
|---|---|---|---|
| Nutrition: | |||
| Frequent/continuous feeding | Prevents fasting hypoglycaemia | Prevents fasting hypoglycaemia | |
| Avoidance of high carbohydrate diet | Useful | Useful | |
| Insulin sensitizer | No effect | Early usage recommended. Improvement of hyperglycaemia | Early usage recommended. Improvement of hyperglycaemia |
| High dose insulin | No effect | Improvement of hyperglycaemia, catabolic state, weight loss and microvascular complications [14] | Improvement of hyperglycaemia, catabolic state and microvascular complications [14] |
| Metreleptin | No assessment available | Improvement of HbA1c [18] | No study with type A IR patients available. |
| Mecasermin (rhIGF-I) | See Table 3 | See Table 3 | See Table 3 |
| Author | Treatment Duration of rhIGF-I | SIR Syndrome (n) | Dosage | Efficacy and Safety |
|---|---|---|---|---|
| Quin et al., 1990 [23] | Short term 1 dose | RMS (1 patient) | 100 μg/kg/day i.v. | Blood glucose, plasma insulin, C-Peptide and growth hormone (GH) declined |
| Schoenle et al., 1991 [24] | Short term 2 doses | Type A IR (3 patients) | 100 μg/kg/day i.v. | Blood glucose, plasma insulin and C-peptide decreased |
| Hussain et al., 1993 [22] | Short term 4 days, BID | Type A IR (1 patient) | 160 μg/kg/day s.c. | Lowering of fasting and postprandial blood glucose, insulin and C-peptide levels |
| Kuzuya et al., 1993 [73] | Up to 16 months | Type A IR (6 patients) DS (2 patients) Congenital lipodystrophy (2 patients) Other (1 patient) | 100 to 400 μg/kg/day | Lowering of fasting and postprandial glucose. Decrease of HbA1c and fructosamine |
| Morrow et al., 1994 [27] | 3–4 weeks | Type A without insulin receptor mutations (2 patients) | 100 μg s.c. | Reduction of blood glucose level, enhancement of insulin sensitivity |
| Backeljauw, 1994 [75] | 66 h and 62 h | DS (2 patients) | Up to 110 μg/kg/h and 40 μg/kg/h i.v. | No apparent glucose lowering effect, decrease in insulin concentration |
| Zenobi, 1994 [25] | 5 days | Type A IR (2 patients) | 150 μg/kg 2x/day s.c. | Decrease of fasting blood glucose. Decrease in fasting insulin and C-peptide by 65% |
| Longo et al., 1994 [63] | 16 months | RMS (1 patient) | Up to 100 μg/kg/day s.c. | No significant effect on glycaemic control and growth |
| Nakashima et al., 1995 [28] | 9 months | Type A IR (1 patient) | 0.08–0.2 mg/kg/day s.c. | Decrease in blood glucose level, free fatty acid concentration, HbA1c; enhanced insulin sensitivity, improvement of acanthosis nigricans |
| Vestergaard et al., 1997 [26] | 2 weeks high dose rhIGF-I | SIR (4 patients) | 60 μg/kg 2x/day s.c. | Reduction of fasting blood glucose and insulin |
| 10 weeks low dose rhIGF-I | SIR (3 patients) | 40 μg/kg/day s.c. | ||
| Takahashi et al., 1997 [32] | 6 months | Leprechaunism—1 patient | 100 μg/kg/day up to 1000 μg/kg/day | Fasting blood glucose, insulin, HbA1c, body weight and development improved |
| Nakae et al., 1998 [29] | 6 years and 10 months | DS (?) or RMS (?) (1 patient at different time points) | Up to 1.6 mg/kg/day | Maintained growth rate, HbA1c near normal range |
| Kitamei et al., 2004 [78] | Intermittent and continuous s.c injection | Adult height was 143 cm (−2.7 SDS for Japanese girls) | ||
| Jo et al., 2013 [77] | Withdrawl of rhIGF-I treatment at 18 years, due to diabetic ketoacidosis and start of high dose insulin | Recurrent episodes of ketoacidosis. HbA1c up to 12–13% | ||
| Regan et al., 2010 [79] | 16 weeks | Type A IR (5 patients) | 0.5–2 mg/kg rhIGF-I/rhIGFBP-3 | HbA1c improvement, reduction of acanthosis nigricans |
| Weber et al., 2014 [30] | 16 months (from 19 months up to 35 months—death of the patient) | DS (1 patient) | BID 80 up to 640 μg/kg/day s.c.; At 30 months: continuous s.c. infusion via insulin pump: 800–1200 μg/kg/day | HbA1c improvement from 9.5 to 7.7%, rebound to 9.8% Improvement of HbA1c from 9.8 to 8.8%, Moderate weight gain |
| De Kerdanet et al., 2015 [48] | 8.7 years; 2 years | DS (1 patient) | IGF-I/IGFBP3 combination, subsequ. IGF-I alone 50 μg/kg/day s.c. | Decrease in mean glycaemia with large variation. Improvement of growth. |
| Carmody et al., 2016 [31] | 5 years | RMS (1 patient) | rhIGF-I up to 1.72 U/kg 2x/day s.c. + metformin | Decrease in insulin, homa index and HbA1c. Growth maintained within target height range. |
| Adverse Effects | Indication Other than SIR [20] | SIR |
|---|---|---|
| Hypoglycaemia | 50–86% | +++ |
| Mild pain/erythema at injection side | +++ | ++ [27,79] |
| Paresthesia and painful toes | ++ [28] | |
| Lipohypertrophy | 1/3 | |
| (Asymptomatic) tachycardia | +++ | + |
| Parotid swelling | + | |
| Facial nerve palsy | + | |
| Retinopathy/worsening of retinopathy | + | + [1,20,28,77,78,79] [77,78] # (patient’s age 12 years) |
| Muscle pain * | + | + [1,20] |
| Fluid retention/edema * | + | + [1,20] |
| Arthritis * | + | + [20] |
| Benign intracranial hypertension /papilledema | 5% | + [1] |
| Lymphoid tissue hypertrophy: tonsillar hypertrophy Adenoidal hypertrophy | 25% (>10% require surgical intervention) | + [29] # (patient’s age 3 years) + [76] |
| Thymic hypertrophy | 35% (X-ray) | |
| Mastitis | ? [77] # (age 16 years) | |
| Endometrial cancer | + [77] # (age 24 years) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plamper, M.; Gohlke, B.; Schreiner, F.; Woelfle, J. Mecasermin in Insulin Receptor-Related Severe Insulin Resistance Syndromes: Case Report and Review of the Literature. Int. J. Mol. Sci. 2018, 19, 1268. https://doi.org/10.3390/ijms19051268
Plamper M, Gohlke B, Schreiner F, Woelfle J. Mecasermin in Insulin Receptor-Related Severe Insulin Resistance Syndromes: Case Report and Review of the Literature. International Journal of Molecular Sciences. 2018; 19(5):1268. https://doi.org/10.3390/ijms19051268
Chicago/Turabian StylePlamper, Michaela, Bettina Gohlke, Felix Schreiner, and Joachim Woelfle. 2018. "Mecasermin in Insulin Receptor-Related Severe Insulin Resistance Syndromes: Case Report and Review of the Literature" International Journal of Molecular Sciences 19, no. 5: 1268. https://doi.org/10.3390/ijms19051268
APA StylePlamper, M., Gohlke, B., Schreiner, F., & Woelfle, J. (2018). Mecasermin in Insulin Receptor-Related Severe Insulin Resistance Syndromes: Case Report and Review of the Literature. International Journal of Molecular Sciences, 19(5), 1268. https://doi.org/10.3390/ijms19051268




