Neuroimmune Tau Mechanisms: Their Role in the Progression of Neuronal Degeneration
Abstract
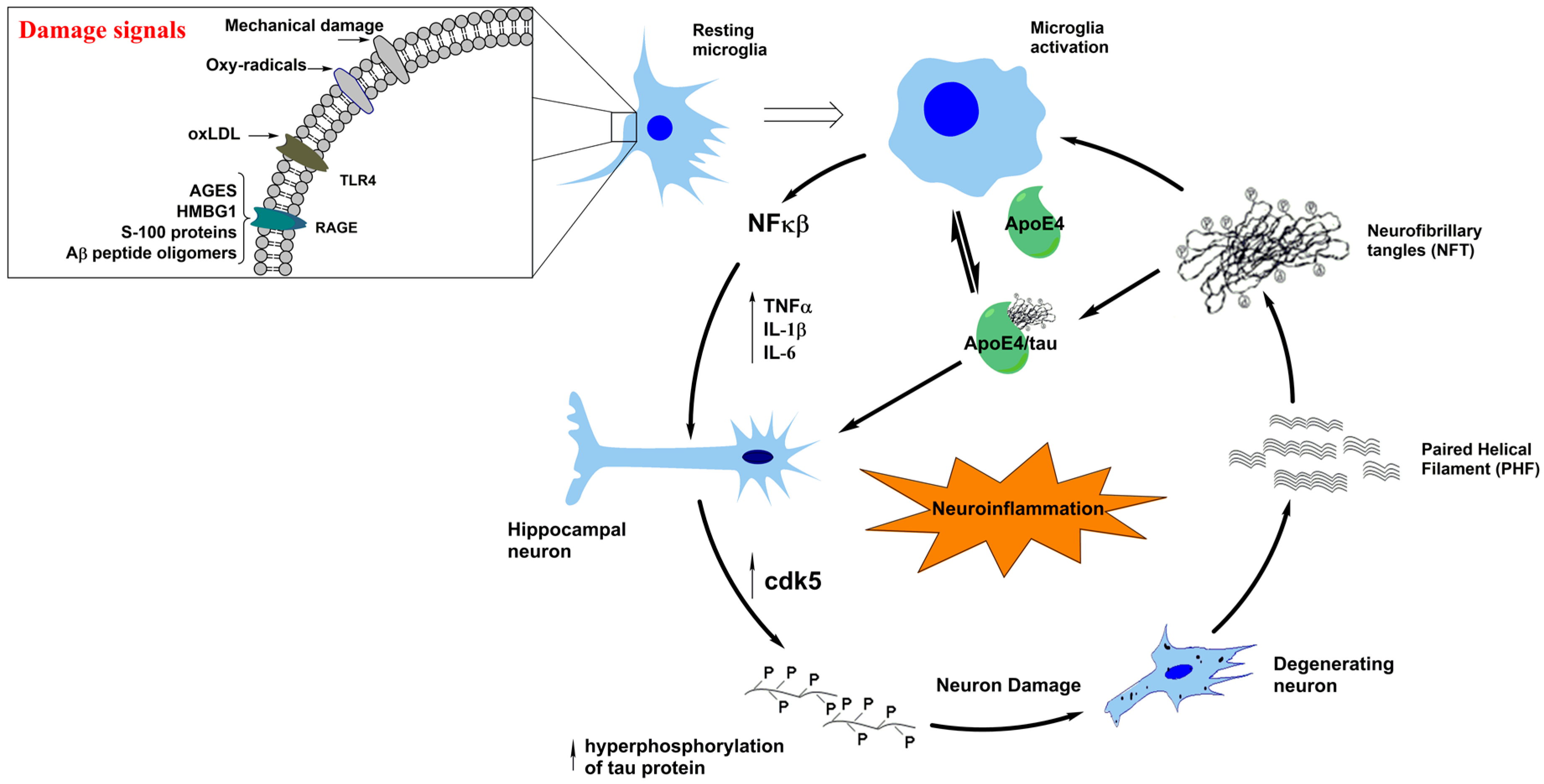
1. Tau Protein the Context of Alzheimer’s Disease
2. Molecular and Structural Aspects of Tauopathies and AD
3. Neuroinflammation in Tauopathies
4. A Typical Tauopathy: Frontotemporal Dementia (FTD)
5. Molecular Interactions and the Links between Tauopathies and Parkinson’s Disease
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| WHO | World Health Organization |
| NFT | Neurofibrillary tangles |
| PHF | Paired helical filaments |
| SP | Senile plaques |
| Aβ | Amyloid-β |
| APP | Amyloid precursor protein |
| MAP | Microtubule-associated proteins |
| NFκB | Nuclear factor κB |
| CNS | Central nervous system |
| EEG | Electroencephalogram |
| NMDA | N-methyl-d-aspartate |
| CDK5 | Cyclin-dependent kinase 5 |
| GSK3-β | Glycogen synthase kinase 3-β |
| JNK | c-Jun-N-terminal kinase |
| Akt | Serine/threonine kinase |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| ERK | Extracellular signal-regulated kinases |
| MEMRI | Manganese-enhanced magnetic resonance imaging |
| PET | Positron-emission tomography |
| PSP | Supranuclear palsy |
| FTD | Frontotemporal dementia |
| bvFTD | Behavioural variant of Frontotemporal dementia |
| nfFTD | Nonfluent variant of Frontotemporal dementia |
| MNFTD | Motoneuron disorder |
| PSP | Progressive supranuclear palsy |
| svFTD | Semantic variant of Frontotemporal dementia |
| CBS | Corticobasal syndromes |
| FTLD | Frontotemporal lobar degeneration |
| TDP | Transactive response of DNA-binding protein |
| FET | FUS, EWS, and TAF15 protein family |
| UPS | Ubiquitin Proteasome System |
| PiD | Picks disease |
| GGT | Globular glial tauopathies |
| AGD | Argyrophilic grain disease |
| C9ORF72 | Chromosome 9 open reading frame 72 |
| TNF-α | Tumor necrosis factor α |
| TGF-β | Transforming growth factor β |
| IL | Interleukin |
| COX2 | Cyclooxygenase-2 |
| TSPO | Translocator protein |
| CSF | Cerebrospinal fluid |
| 5-HT | Serotonin |
| SSRIs | Selective serotonin reuptake inhibitors |
| PD | Parkinson’s disease |
| α-syn | α-Synuclein |
| LB | Lewy Bodies |
| NAC | Non-amyloid component |
| PDD | Parkinson’s disease with dementia |
| DLB | Lewy body dementia |
| MSA | Multiple system atrophy |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| A53T SNCA | Ala53Thr synuclein α |
| PKA | Protein Kinase A |
| FIDA | Fluorescence intensity distribution analysis |
References
- Bettens, K.; Sleegers, K.; Van Broeckhoven, C. Current status on Alzheimer disease molecular genetics: From past, to present, to future. Hum. Mol. Genet. 2010, 19, R4–R11. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B. Introductory remarks. Molecular, biological and clinical aspects of Alzheimer’s disease. Arch. Med. Res. 2012, 43, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, R.B.; Munoz, J.P.; Barbeito, L. The molecular bases of Alzheimer’s disease and other neurodegenerative disorders. Arch. Med. Res. 2001, 32, 367–381. [Google Scholar] [CrossRef]
- Farias, G.; Cornejo, A.; Jimenez, J.; Guzman, L.; Maccioni, R.B. Mechanisms of tau self-aggregation and neurotoxicity. Curr. Alzheimer Res. 2011, 8, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Farias, G.A.; Maccioni, R.B. Tau Oligomers as Potential Targets for Alzheimer’s Diagnosis and Novel Drugs. Front. Neurol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.C.; Schraen-Maschke, S.; Richard, F.; Fievet, N.; Rouaud, O.; Berr, C.; Dartigues, J.F.; Tzourio, C.; Alpérovitch, A.; Buée, L.; et al. Association of plasma amyloid β with risk of dementia: The prospective Three-City Study. Neurology 2009, 73, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, D.W.; Hwo, S.Y.; Kirschner, M.W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J. Mol. Biol. 1977, 116, 207–225. [Google Scholar] [CrossRef]
- Seeds, N.W.; Maccioni, R.B. Proteins from morphologically differentiated neuroblastoma cells promote tubulin polymerization. J. Cell Biol. 1978, 76, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.A.; Rojo, L.; Kuljis, R.O.; Maccioni, R.B. The damage signals hypothesis of Alzheimer’s disease pathogenesis. J. Alzheimer Dis. 2008, 14, 329–333. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Rojo, L.E.; Fernandez, J.A.; Kuljis, R.O. The role of neuroimmunomodulation in Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2009, 1153, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Farias, G.; Maccioni, R.B. Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation 2010, 17, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Guzman-Martinez, L.; Cerda-Troncoso, C.; Farias, G.A.; Maccioni, R.B. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014, 8, 112. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Fernandez, J.A.; Maccioni, A.A.; Jimenez, J.M.; Maccioni, R.B. Neuroinflammation: Implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch. Med. Res. 2008, 39, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.; Jimenez, J.M.; Mancilla, M.; Maccioni, R.B. Tau oligomers and fibrils induce activation of microglial cells. J. Alzheimer Dis. 2013, 37, 849–856. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.L.; Yardin, C.; Terro, F. Tau protein kinases: Involvement in Alzheimer’s disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Wang, C.; Jiang, T.; Tan, L.; Xing, A.; Yu, J.T. The Role of Cdk5 in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 4328–4342. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Sasamoto, K.; Nagai, J.; Yamazaki, Y.; Saito, K.; Goshima, Y.; Inoue, T.; Ohshima, T. Phosphorylation of CRMP2 by Cdk5 Regulates Dendritic Spine Development of Cortical Neuron in the Mouse Hippocampus. Neural Plast. 2016, 2016, 6790743. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Noble, W. Functional implications of glycogen synthase kinase-3-mediated tau phosphorylation. Int. J. Alzheimers Dis. 2011, 2011, 352805. [Google Scholar] [CrossRef] [PubMed]
- Meunier, C.N.; Cancela, J.M.; Fossier, P. Lack of GSK3-β activation and modulation of synaptic plasticity by dopamine in 5-HT1A-receptor KO mice. Neuropharmacology 2017, 113 Pt A, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Maixner, D.W.; Weng, H.R. The role of glycogen synthase kinase 3 β in neuroinflammation and pain. J. Pharm. Pharmacol. 2013, 1, 1. [Google Scholar] [CrossRef][Green Version]
- Lee, H.G.; Ogawa, O.; Zhu, X.; O’Neill, M.J.; Petersen, R.B.; Castellani, R.J.; Ghanbari, H.; Perry, G.; Smith, M.A. Aberrant expression of metabotropic glutamate receptor 2 in the vulnerable neurons of Alzheimer’s disease. Acta Neuropathol. 2004, 107, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Florenzano, F.; Veronica, C.; Ciasca, G.; Ciotti, M.T.; Pittaluga, A.; Olivero, G.; Feligioni, M.; Iannuzzi, F.; Latina, V.; Maria Sciacca, M.F.; et al. Extracellular truncated tau causes early presynaptic dysfunction associated with Alzheimer’s disease and other tauopathies. Oncotarget 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; McInnes, J.; Wierda, K.; Holt, M.; Herrmann, A.G.; Jackson, R.J.; Wang, Y.-C.; Swerts, J.; Beyens, J.; Miskiewicz, K.; et al. Tau association with synaptic vesicles causes presynaptic dysfunction. Nat. Commun. 2017, 8, 15295. [Google Scholar] [CrossRef] [PubMed]
- Swanson, E.; Breckenridge, L.; McMahon, L.; Som, S.; McConnell, I.; Bloom, G.S. Extracellular Tau Oligomers Induce Invasion of Endogenous Tau into the Somatodendritic Compartment and Axonal Transport Dysfunction. J. Alzheimer Dis. 2017, 58, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.N.; Ingram, A.; Cloyd, R.A.; Meier, S.E.; Miller, E.; Lyons, D.; Nation, G.K.; Mechas, E.; Weiss, B.; Lanzillotta, C.; et al. Identification of changes in neuronal function as a consequence of aging and tauopathic neurodegeneration using a novel and sensitive magnetic resonance imaging approach. Neurobiol. Aging 2017, 56, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kepe, V.; Bordelon, Y.; Boxer, A.; Huang, S.C.; Liu, J.; Thiede, F.C.; Mazziotta, J.C.; Mendez, M.F.; Donoghue, N.; Small, G.W.; et al. PET imaging of neuropathology in tauopathies: Progressive supranuclear palsy. J. Alzheimer Dis. 2013, 36, 145–153. [Google Scholar] [CrossRef]
- Andrade, V.; Cortes, N.; Guzmán-Martínez, L.; Maccioni, R.B. An overview of the links between behavioral disorders and Alzheimer’ disease. JSM Alzheimer’s Dis. Relat. Dement. 2017, 4, 1031. [Google Scholar]
- Bellucci, A.; Westwood, A.J.; Ingram, E.; Casamenti, F.; Goedert, M.; Spillantini, M.G. Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein. Am. J. Pathol. 2004, 165, 1643–1652. [Google Scholar] [CrossRef]
- Bellucci, A.; Bugiani, O.; Ghetti, B.; Spillantini, M.G. Presence of reactive microglia and neuroinflammatory mediators in a case of frontotemporal dementia with P301S mutation. Neurodegener. Dis. 2011, 8, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, M.; Folkesson, S.; Blennow, K.; Tarkowski, E. Increased intrathecal inflammatory activity in frontotemporal dementia: Pathophysiological implications. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.M.; Iwata, N.; Saido, T.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Wes, P.D.; Easton, A.; Corradi, J.; Barten, D.M.; Devidze, N.; DeCarr, L.B.; Truong, A.; He, A.; Barrezueta, N.X.; Polson, C.; et al. Tau overexpression impacts a neuroinflammation gene expression network perturbed in Alzheimer’s disease. PLoS ONE 2014, 9, e106050. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, A.; Rossor, M.; Sampson, E.L.; Mackinnon, T.; Banati, R.B. In vivo detection of microglial activation in frontotemporal dementia. Ann. Neurol. 2004, 56, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Venneti, S.; Wiley, C.A.; Kofler, J. Imaging microglial activation during neuroinflammation and Alzheimer’s disease. J. Neuroimmune Pharmacol. 2009, 4, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Mapping neuroinflammation in frontotemporal dementia with molecular PET imaging. J. Neuroinflamm. 2015, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Neary, D.; Snowden, J.S.; Gustafson, L.; Passant, U.; Stuss, D.; Black, S.; Freedman, M.; Kertesz, A.; Robert, P.H.; Albert, M.; et al. Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria. Neurology 1998, 51, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Olney, N.T.; Spina, S.; Miller, B.L. Frontotemporal Dementia. Neurol. Clin. 2017, 35, 339–374. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.E.; Rittman, T.; Regenthal, R.; Robbins, T.W.; Rowe, J.B. Improving response inhibition systems in frontotemporal dementia with citalopram. Brain J. Neurol. 2015, 138 Pt 7, 1961–1975. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Roberts, R.O. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J. Mol. Neurosci. 2011, 45, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Coyle-Gilchrist, I.T.; Dick, K.M.; Patterson, K.; Vazquez Rodriquez, P.; Wehmann, E.; Wilcox, A.; Lansdall, C.J.; Dawson, K.E.; Wiggins, J.; Mead, S.; et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 2016, 86, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Hodges, J.R.; Davies, R.; Xuereb, J.; Kril, J.; Halliday, G. Survival in frontotemporal dementia. Neurology 2003, 61, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Onyike, C.U.; Diehl-Schmid, J. The epidemiology of frontotemporal dementia. Int. Rev. Psychiatry 2013, 25, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Ratnavalli, E.; Brayne, C.; Dawson, K.; Hodges, J.R. The prevalence of frontotemporal dementia. Neurology 2002, 58, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain J. Neurol. 2011, 134 Pt 9, 2456–2477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2015, 17, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Cairns, N.J.; Grossman, M.; McMillan, C.T.; Lee, E.B.; Van Deerlin, V.M.; Lee, V.M.; Trojanowski, J.Q. Frontotemporal lobar degeneration: Defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2015, 129, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, I.R.; Neumann, M.; Bigio, E.H.; Cairns, N.J.; Alafuzoff, I.; Kril, J.; Kovacs, G.G.; Ghetti, B.; Halliday, G.; Holm, I.E.; et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: An update. Acta Neuropathol. 2010, 119, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sieben, A.; Van Langenhove, T.; Engelborghs, S.; Martin, J.J.; Boon, P.; Cras, P.; De Deyn, P.P.; Santens, P.; Van Broeckhoven, C.; Cruts, M. The genetics and neuropathology of frontotemporal lobar degeneration. Acta Neuropathol. 2012, 124, 353–372. [Google Scholar] [CrossRef] [PubMed]
- Ghetti, B.; Oblak, A.L.; Boeve, B.F.; Johnson, K.A.; Dickerson, B.C.; Goedert, M. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: A chameleon for neuropathology and neuroimaging. Neuropathol. Appl. Neurobiol. 2015, 41, 24–46. [Google Scholar] [CrossRef] [PubMed]
- Bevan Jones, W.R.; Cope, T.E.; Passamonti, L.; Fryer, T.D.; Hong, Y.T.; Aigbirhio, F.; Kril, J.J.; Forrest, S.L.; Allinson, K.; Coles, J.P.; et al. [18F]AV-1451 PET in behavioral variant frontotemporal dementia due to MAPT mutation. Ann. Clin. Transl. Neurol. 2016, 3, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Ishiki, A.; Harada, R.; Okamura, N.; Tomita, N.; Rowe, C.C.; Villemagne, V.L.; Yanai, K.; Kudo, Y.; Arai, H.; Furumoto, S.; et al. Tau imaging with [18F]THK-5351 in progressive supranuclear palsy. Eur. J. Neurol. 2017, 24, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Sahara, N.; Kumata, K.; Ji, B.; Ni, R.; Koga, S.; Dickson, D.W.; Trojanowski, J.Q.; Lee, V.M.; Yoshida, M.; et al. Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegenerative tauopathies. Brain J. Neurol. 2017, 140, 764–780. [Google Scholar] [CrossRef] [PubMed]
- Meeter, L.H.; Kaat, L.D.; Rohrer, J.D.; van Swieten, J.C. Imaging and fluid biomarkers in frontotemporal dementia. Nat. Rev. Neurol. 2017, 13, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Struyfs, H.; Van Broeck, B.; Timmers, M.; Fransen, E.; Sleegers, K.; Van Broeckhoven, C.; De Deyn, P.P.; Streffer, J.R.; Mercken, M.; Engelborghs, S. Diagnostic Accuracy of Cerebrospinal Fluid Amyloid-β Isoforms for Early and Differential Dementia Diagnosis. J. Alzheimer Dis. 2015, 45, 813–822. [Google Scholar] [CrossRef]
- Bastin, C.; Feyers, D.; Souchay, C.; Guillaume, B.; Pepin, J.L.; Lemaire, C.; Degueldre, C.; Collette, F.; Salmon, E. Frontal and posterior cingulate metabolic impairment in the behavioral variant of frontotemporal dementia with impaired autonoetic consciousness. Hum. Brain Mapp. 2012, 33, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Black, S.E.; Chow, T.; Cappell, J.; Tang-Wai, D.F.; Lanctot, K.L. Serotonergic function and treatment of behavioral and psychological symptoms of frontotemporal dementia. Am. J. Geriatr. Psychiatry 2012, 20, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Lebert, F.; Stekke, W.; Hasenbroekx, C.; Pasquier, F. Frontotemporal dementia: A randomised, controlled trial with trazodone. Dement. Geriatr. Cogn. Disord. 2004, 17, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Seripa, D.; Imbimbo, B.P.; Lozupone, M.; Santamato, A.; Zecca, C.; Barulli, M.R.; Bellomo, A.; Pilotto, A.; et al. Tau-Centric Targets and Drugs in Clinical Development for the Treatment of Alzheimer’s Disease. BioMed Res. Int. 2016, 2016, 3245935. [Google Scholar] [CrossRef] [PubMed]
- Munoz, Y.; Carrasco, C.M.; Campos, J.D.; Aguirre, P.; Nunez, M.T. Parkinson’s Disease: The Mitochondria-Iron Link. Parkinson’s Dis. 2016, 2016, 7049108. [Google Scholar] [CrossRef]
- Forno, L.S. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J. Neurochem. 2007, 103, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Maarouf, C.L.; Beach, T.G.; Adler, C.H.; Shill, H.A.; Sabbagh, M.N.; Wu, T.; Walker, D.G.; Kokjohn, T.A.; Roher, A.E.; Consortium, A.P.D. Cerebrospinal fluid biomarkers of neuropathologically diagnosed Parkinson’s disease subjects. Neurol. Res. 2012, 34, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Beal, M.F. Parkinson’s disease. Hum. Mol. Genet. 2007, R183–R194. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Dickson, D.W.; Lowe, J.; Emre, M.; O’Brien, J.T.; Feldman, H.; Cummings, J.; Duda, J.E.; Lippa, C.; Perry, E.K.; et al. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 2005, 65, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E.; Miller, V.M. Proteinopathy-induced neuronal senescence: A hypothesis for brain failure in Alzheimer’s and other neurodegenerative diseases. Alzheimer’s Res. Ther. 2009, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Intrinsically disordered proteins and their environment: Effects of strong denaturants, temperature, pH, counter ions, membranes, binding partners, osmolytes, and macromolecular crowding. Protein J. 2009, 28, 305–325. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. The neuropathologic substrate of Parkinson disease dementia. Acta Neuropathol. 2010, 119, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Botond, G.; Budka, H. Protein coding of neurodegenerative dementias: The neuropathological basis of biomarker diagnostics. Acta Neuropathol. 2010, 119, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y.; Yamazaki, M.; Mori, O.; Muramatsu, H.; Asano, G.; Katayama, Y. Α-synuclein-positive structures in cases with sporadic Alzheimer’s disease: Morphology and its relationship to tau aggregation. Brain Res. 2001, 888, 287–296. [Google Scholar] [CrossRef]
- Iseki, E.; Togo, T.; Suzuki, K.; Katsuse, O.; Marui, W.; de Silva, R.; Lees, A.; Yamamoto, T.; Kosaka, K. Dementia with Lewy bodies from the perspective of tauopathy. Acta Neuropathol. 2003, 105, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.A.; Li, J.L.; Li, Y.; Wilson, R.S.; Kordower, J.H.; Bennett, D.A. Substantia nigra tangles are related to gait impairment in older persons. Ann. Neurol. 2006, 59, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Joachim, C.L.; Morris, J.H.; Kosik, K.S.; Selkoe, D.J. Tau antisera recognize neurofibrillary tangles in a range of neurodegenerative disorders. Ann. Neurol. 1987, 22, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Jones, J.; Haggerty, T.; Duka, V.; Joyce, J.N.; Sidhu, A. Elevated tauopathy and α-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp. Neurol. 2010, 225, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, T.; Credle, J.; Rodriguez, O.; Wills, J.; Oaks, A.W.; Masliah, E.; Sidhu, A. Hyperphosphorylated Tau in an α-synuclein-overexpressing transgenic model of Parkinson’s disease. Eur. J. Neurosci. 2011, 33, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Duka, T.; Sidhu, A. The neurotoxin, MPP+, induces hyperphosphorylation of Tau, in the presence of α-Synuclein, in SH-SY5Y neuroblastoma cells. Neurotox. Res. 2006, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Duka, T.; Rusnak, M.; Drolet, R.E.; Duka, V.; Wersinger, C.; Goudreau, J.L.; Sidhu, A. Α-synuclein induces hyperphosphorylation of Tau in the MPTP model of parkinsonism. FASEB J. 2006, 20, 2302–2312. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Interaction between α-synuclein and tau in Parkinson’s disease comment on Wills et al.: Elevated tauopathy and α-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp. Neurol. 2010; 225: 210–218. Exp. Neurol. 2011, 227, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kraybill, M.L.; Larson, E.B.; Tsuang, D.W.; Teri, L.; McCormick, W.C.; Bowen, J.D.; Kukull, W.A.; Leverenz, J.B.; Cherrier, M.M. Cognitive differences in dementia patients with autopsy-verified, A.D.; Lewy body pathology, or both. Neurology 2005, 64, 2069–2073. [Google Scholar] [CrossRef] [PubMed]
- Langlais, P.J.; Thal, L.; Hansen, L.; Galasko, D.; Alford, M.; Masliah, E. Neurotransmitters in basal ganglia and cortex of Alzheimer’s disease with and without Lewy bodies. Neurology 1993, 43, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- Olichney, J.M.; Galasko, D.; Salmon, D.P.; Hofstetter, C.R.; Hansen, L.A.; Katzman, R.; Thal, L.J. Cognitive decline is faster in Lewy body variant than in Alzheimer’s disease. Neurology 1998, 51, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Giasson, B.I.; Forman, M.S.; Higuchi, M.; Golbe, L.I.; Graves, C.L.; Kotzbauer, P.T.; Trojanowski, J.Q.; Lee, V.M. Initiation and synergistic fibrillization of tau and α-synuclein. Science 2003, 300, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M.; Giasson, B.I.; Trojanowski, J.Q. More than just two peas in a pod: Common amyloidogenic properties of tau and α-synuclein in neurodegenerative diseases. Trends Neurosci. 2004, 27, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.H.; Hager, H.; Nielsen, M.S.; Hojrup, P.; Gliemann, J.; Jakes, R. α-synuclein binds to Tau and stimulates the protein kinase A-catalyzed tau phosphorylation of serine residues 262 and 356. J. Biol. Chem. 1999, 274, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Muntane, G.; Dalfo, E.; Martinez, A.; Ferrer, I. Phosphorylation of tau and α-synuclein in synaptic-enriched fractions of the frontal cortex in Alzheimer’s disease, and in Parkinson’s disease and related α-synucleinopathies. Neuroscience 2008, 152, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Frasier, M.; Walzer, M.; McCarthy, L.; Magnuson, D.; Lee, J.M.; Haas, C.; Kahle, P.; Wolozin, B. Tau phosphorylation increases in symptomatic mice overexpressing A30P α-synuclein. Exp. Neurol. 2005, 192, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.Y.; Crary, J.F.; Rao, C.; Sacktor, T.C.; Mirra, S.S. Atypical protein kinase C in neurodegenerative disease II: PKCiota/lambda in tauopathies and α-synucleinopathies. J. Neuropathol. Exp. Neurol. 2006, 65, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Riedel, M.; Goldbaum, O.; Richter-Landsberg, C. α-Synuclein promotes the recruitment of tau to protein inclusions in oligodendroglial cells: Effects of oxidative and proteolytic stress. J. Mol. Neurosci. 2009, 39, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Duka, T.; Duka, V.; Joyce, J.N.; Sidhu, A. Α-Synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB J. 2009, 23, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Ciaccioli, G.; Martins, A.; Rodrigues, C.; Vieira, H.; Calado, P. A powerful yeast model to investigate the synergistic interaction of α-synuclein and tau in neurodegeneration. PLoS ONE 2013, 8, e55848. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, F.; Suzuki, M.; Shimada, N.; Kagiya, G.; Ohta, E.; Tamura, K.; Maruyama, H.; Ichikawa, T. Stimulatory effect of α-synuclein on the tau-phosphorylation by GSK-3β. FEBS J. 2011, 278, 4895–4904. [Google Scholar] [CrossRef] [PubMed]
- Wills, J.; Credle, J.; Haggerty, T.; Lee, J.H.; Oaks, A.W.; Sidhu, A. Tauopathic changes in the striatum of A53T α-synuclein mutant mouse model of Parkinson’s disease. PLoS ONE 2011, 6, e17953. [Google Scholar] [CrossRef] [PubMed]
- Kaul, T.; Credle, J.; Haggerty, T.; Oaks, A.W.; Masliah, E.; Sidhu, A. Region-specific tauopathy and synucleinopathy in brain of the α-synuclein overexpressing mouse model of Parkinson’s disease. BMC Neurosci. 2011, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Oaks, A.W.; Frankfurt, M.; Finkelstein, D.I.; Sidhu, A. Age-dependent effects of A53T α-synuclein on behavior and dopaminergic function. PLoS ONE 2013, 8, e60378. [Google Scholar] [CrossRef] [PubMed]
- Nubling, G.; Bader, B.; Levin, J.; Hildebrandt, J.; Kretzschmar, H.; Giese, A. Synergistic influence of phosphorylation and metal ions on tau oligomer formation and coaggregation with α-synuclein at the single molecule level. Mol. Neurodegener. 2012, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Jakes, R.; Spillantini, M.G.; Hasegawa, M.; Smith, M.J.; Crowther, R.A. Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans. Nature 1996, 383, 550–553. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, N.; Andrade, V.; Guzmán-Martínez, L.; Estrella, M.; Maccioni, R.B. Neuroimmune Tau Mechanisms: Their Role in the Progression of Neuronal Degeneration. Int. J. Mol. Sci. 2018, 19, 956. https://doi.org/10.3390/ijms19040956
Cortés N, Andrade V, Guzmán-Martínez L, Estrella M, Maccioni RB. Neuroimmune Tau Mechanisms: Their Role in the Progression of Neuronal Degeneration. International Journal of Molecular Sciences. 2018; 19(4):956. https://doi.org/10.3390/ijms19040956
Chicago/Turabian StyleCortés, Nicole, Víctor Andrade, Leonardo Guzmán-Martínez, Matías Estrella, and Ricardo B. Maccioni. 2018. "Neuroimmune Tau Mechanisms: Their Role in the Progression of Neuronal Degeneration" International Journal of Molecular Sciences 19, no. 4: 956. https://doi.org/10.3390/ijms19040956
APA StyleCortés, N., Andrade, V., Guzmán-Martínez, L., Estrella, M., & Maccioni, R. B. (2018). Neuroimmune Tau Mechanisms: Their Role in the Progression of Neuronal Degeneration. International Journal of Molecular Sciences, 19(4), 956. https://doi.org/10.3390/ijms19040956



