Telomerase Inhibition by a New Synthetic Derivative of the Aporphine Alkaloid Boldine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity of Boldine Derivatives
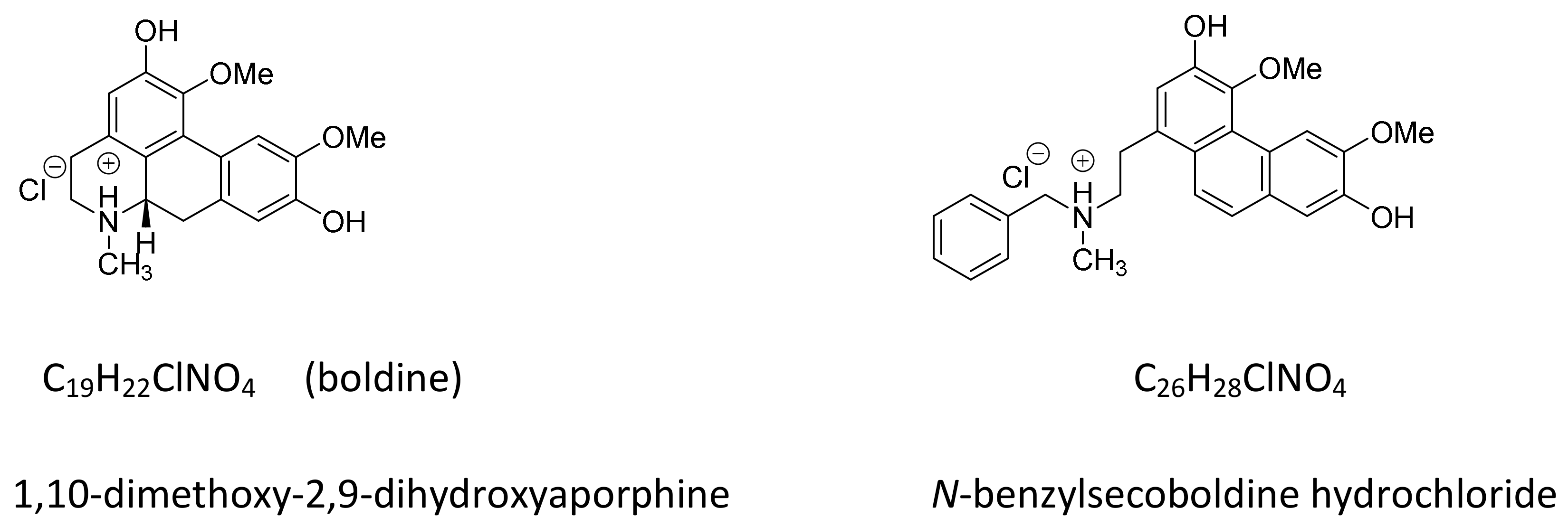
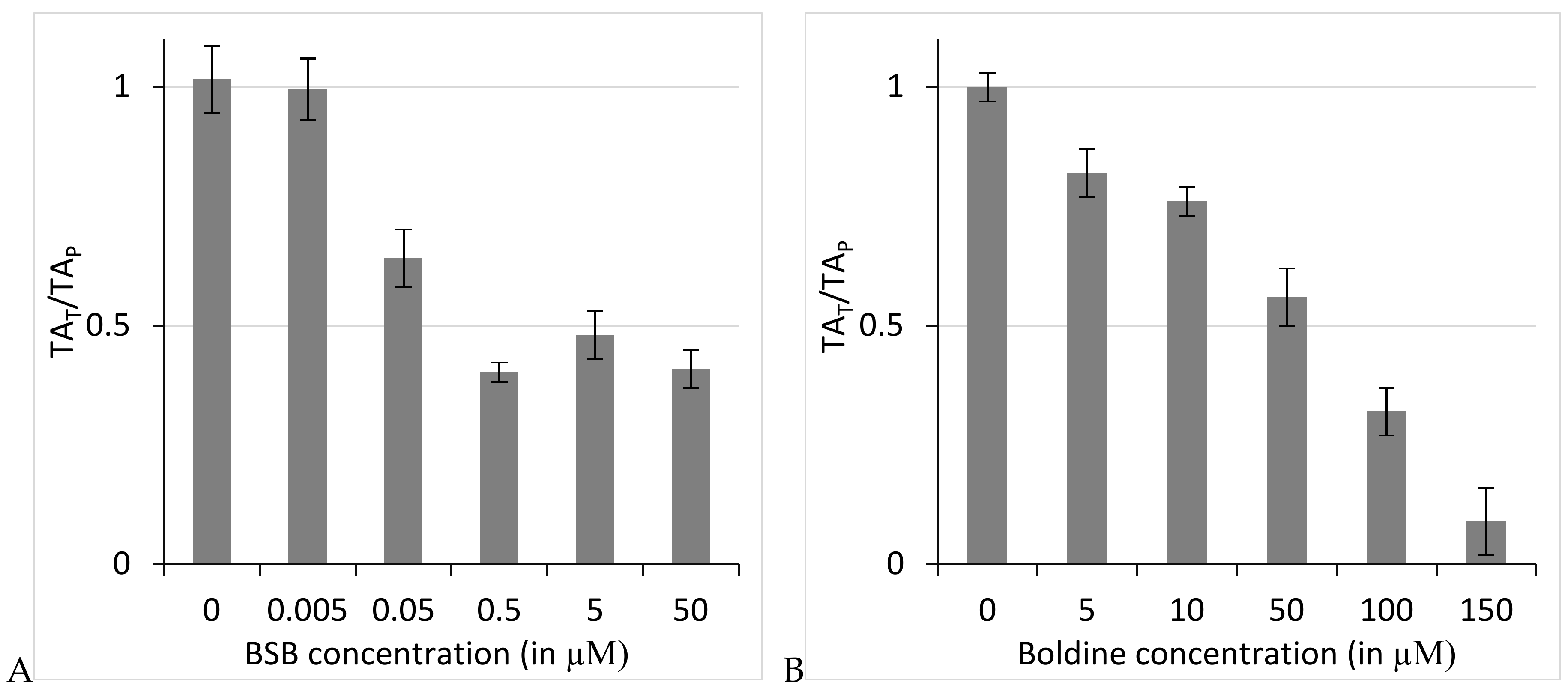
2.2. N-Benzylsecoboldine (BSB) Inhibited Telomerase at Nanomolar Concentrations in a Direct Interaction
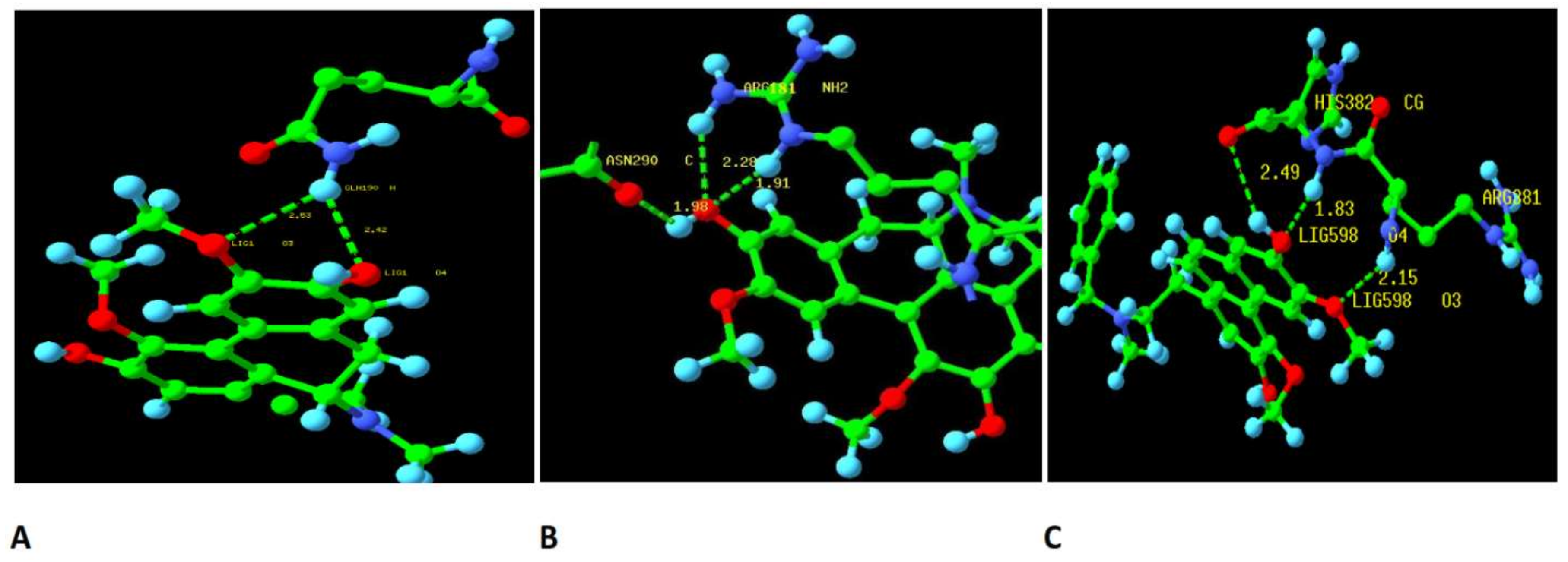
2.3. Binding Site of BSB on TERT
2.3.1. Docking Studies of Boldine and Derivatives to Telomerase
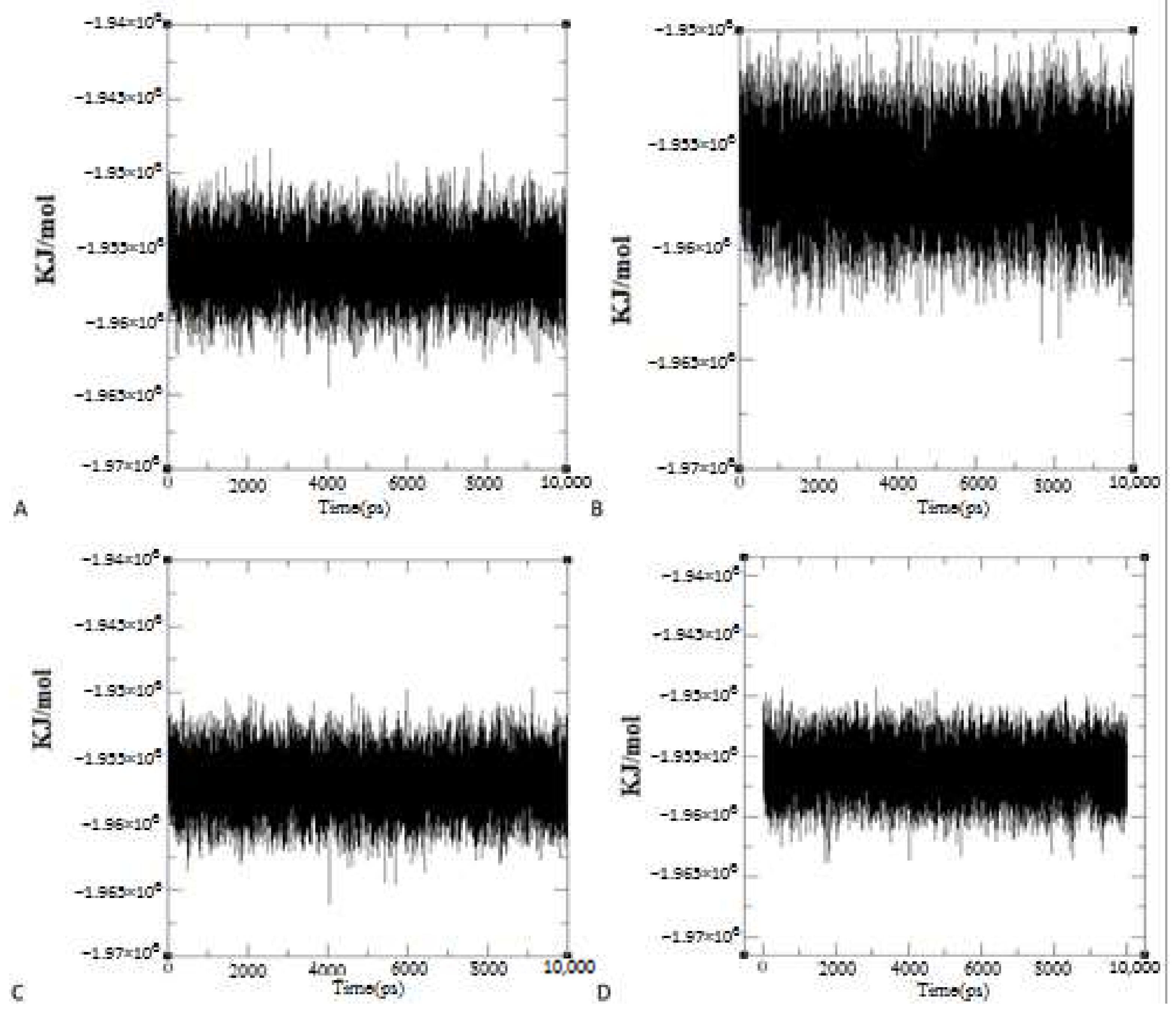
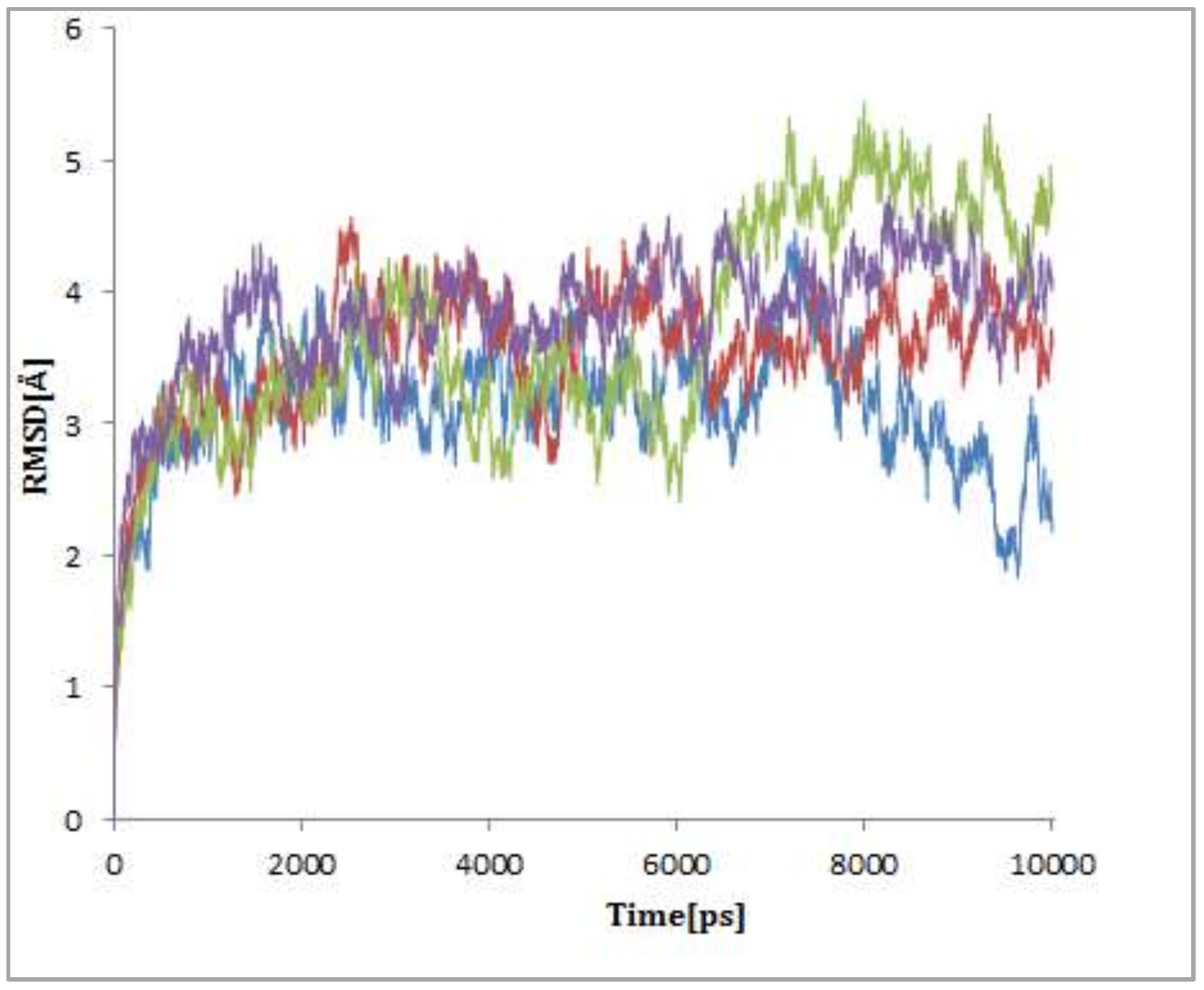
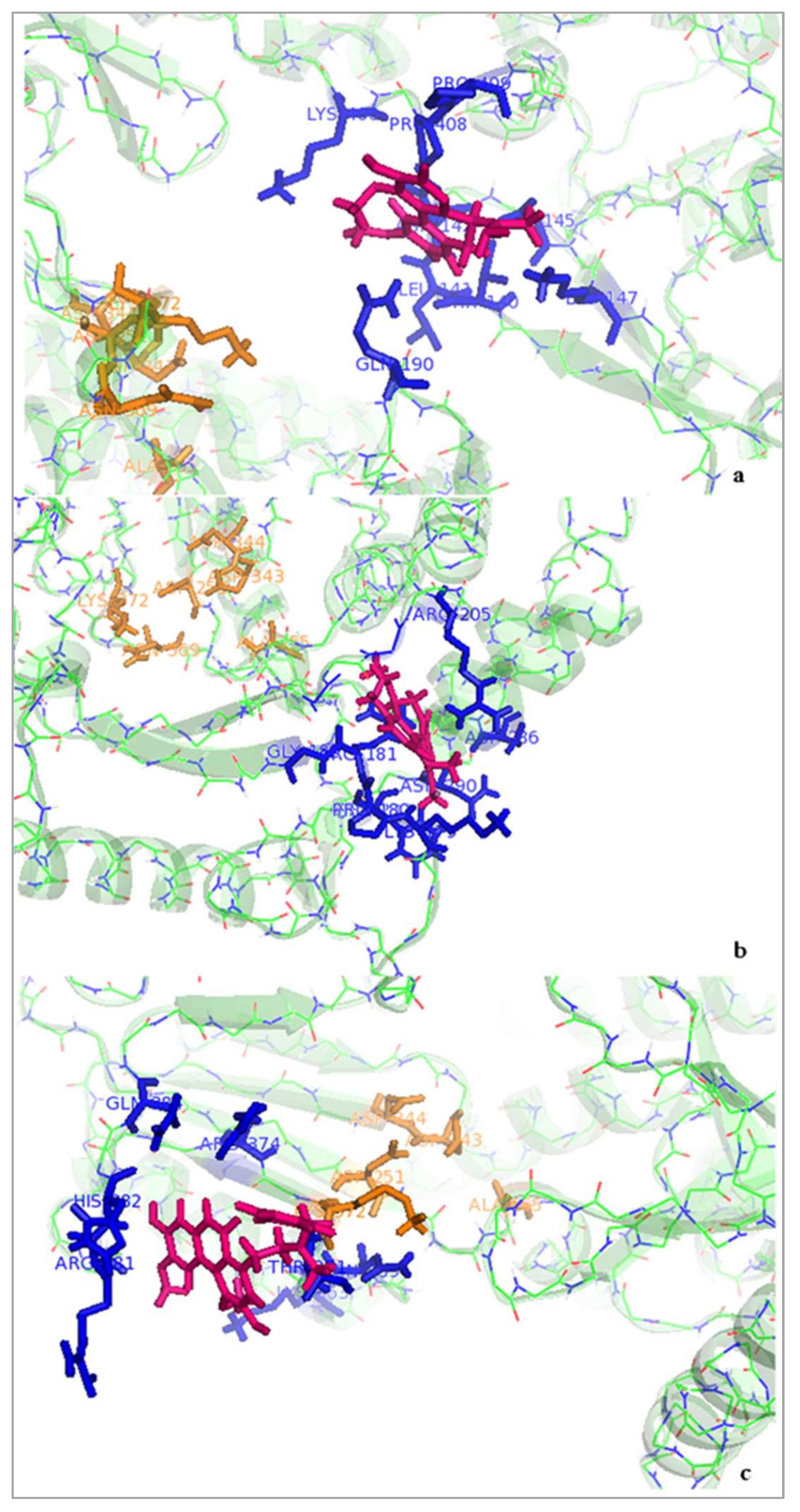
2.3.2. Dynamics Simulation Results of Ligand-Protein Complexes
System Energy
Overall Structural Stability
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture and Cytotoxicity Assay
4.3. q-TRAP-Ligand Assay
4.4. Computational Studies
4.4.1. Protein and Ligand Preparation
4.4.2. Molecular Docking
4.4.3. Molecular Dynamics Simulation
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O’Brien, P.; Carrasco-Pozo, C.; Speisky, H. Boldine and its Antioxidant or Health-Promoting Properties. Chem.-Biol. Interact. 2006, 159, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zheng, Y.; Chen, N.; Luan, L.; Zhou, C.; Gan, L.; Wu, Y. Simultaneous Determination of Four Alkaloids in Lindera aggregata by Ultra-High-Pressure Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. A 2008, 1212, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, Y.; Branfman, A.R.; Baldessarini, R.J.; Neumeyer, J.L. Advances in Development of Dopaminergic Aporphinoids. J. Med. Chem. 2007, 50, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Walstab, J.; Wohlfarth, C.; Hovius, R.; Schmitteckert, S.; Röth, R.; Lasitschka, F.; Wink, M.; Bönisch, H.; Niesler, B. Natural compounds boldine and menthol are antagonists of human 5-HT3 receptors: Implications for treating gastrointestinal disorders. Neurogastroenterol. Motil. 2014, 26, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Ling, W.C.; Murugan, D.; Mustafa, M.R. Boldine Ameliorates Vascular Oxidative Stress and Endothelial Dysfunction: Therapeutic Implication for Hypertension and Diabetes. J. Cardiovasc. Pharmacol. 2015, 65, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Salinas, R.; Vielma, A.Z.; Arismendi, M.N.; Boric, M.P.; Sáez, J.C.; Velarde, V. Boldine prevents renal alterations in diabetic rats. J. Diabetes Res. 2013, 2013, 593672. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Tian, X.Y.; Mustafa, M.R.; Murugan, D.; Liu, J.; Zhang, Y.; Lau, C.W.; Huang, Y. Boldine improves endothelial function in diabetic db/db mice through inhibition of angiotensin II-mediated BMP4-oxidative stress cascade. Br. J. Pharmacol. 2013, 170, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, D.; Bertola, G.; Dietrich, F.; Figueiró, F.; Zanotto-Filho, A.; Fonseca, J.C.M.; Morrone, F.B.; Barrios, C.H.; Battastini, A.M.O.; Salbego, C.G. Salbego Boldine induces cell cycle arrest and apoptosis in T24 human bladder cancer cell line via regulation of ERK, AKT, and GSK-3β. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 36.e1–36.e9. [Google Scholar]
- Tomšík, P.; Mičuda, S.; Muthná, D.; Čermáková, E.; Havelek, R.; Rudolf, E.; Hroch, M.; Kadová, Z.; Řezáčová, M.; Ćmielová, J.; et al. Boldine Inhibits Mouse Mammary Carcinoma in vivo and Human MCF-7 Breast Cancer Cells in vitro. Planta Med. 2016, 82, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Shi, L.; Zhuang, H.; Zhang, H.; Wang, J.; Wang, L.; Sun, P.; Yu, L.; Liu, L. Cerebrovascular Protective Effect of Boldine Against Neural Apoptosis via Inhibition of Mitochondrial Bax Translocation and Cytochrome C Release. Med. Sci. Monit. 2017, 23, 4109–4116. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.K.; Wink, M. Dose-dependent cytotoxic effects of boldine in HepG-2 cells-telomerase inhibition and apoptosis induction. Molecules 2015, 20, 3730–3743. [Google Scholar] [CrossRef] [PubMed]
- Kazemi Noureini, S.; Tanavar, F. Boldine, a natural aporphine alkaloid, inhibits telomerase at non-toxic concentrations. Chem. Biol. Interact. 2015, 231, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Steczkiewicz, K.; Zimmermann, M.T.; Kurcinski, M.; Lewis, B.A.; Dobbs, D.; Kloczkowski, A.; Jernigan, R.L.; Kolinski, A.; Ginalski, K. Human telomerase model shows the role of the TEN domain in advancing the double helix for the next polymerization step. Proc. Natl. Acad. Sci. USA 2011, 108, 9443–9448. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.J.; Schuller, A.P.; Skordalakes, E. Structure of the Tribolium castaneum telomerase catalytic subunit TERT. Nature 2008, 455, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Funk, D.W.; Wang, S.S.; Weinrich, S.L.; Avilion, A.A.; Chiu, P.C.; Adams, R.R.; Chang, E.; Allsopp, R.C.; Yu, J.; et al. The RNA component of human telomerase. Science 1995, 269, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.M.; Morin, G.B.; Chapman, K.B.; Weinrich, S.L.; Andrews, W.H.; Lingner, J.; Harley, C.B.; Cech, T.R. Telomerase catalytic subunit homologs from fission yeast and human. Science 1997, 277, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Greider, C.W. An emerging consensus for telomerase RNA structure. Proc. Natl. Acad. Sci. USA 2004, 101, 14683–14684. [Google Scholar] [CrossRef] [PubMed]
- Ly, H.; Blackburn, E.H.; Parslow, T.G. Comprehensive structure–function analysis of the core domain of human telomerase RNA. Mol. Cell. Biol. 2003, 23, 6849–6856. [Google Scholar] [CrossRef] [PubMed]
- Autexier, C.; Lue, N.F. The structure and function of telomerase reverse transcriptase. Annu. Rev. Biochem. 2006, 75, 493–517. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, H.D.; West, S.C.; Beattie, T.L. InTERTpreting telomerase structure and function. Nucleic. Acids Res. 2010, 38, 5609–5622. [Google Scholar] [CrossRef] [PubMed]
- Sekaran, V.G.; Soares, J.; Jarstfer, M.B. Structures of telomerase subunits provide functional insights. Biochim. Biophys. Acta 2010, 1804, 1190–1201. [Google Scholar] [CrossRef] [PubMed]
- Steitz, T.A. DNA and RNA polymerases: Structural diversity and common mechanisms. Harvey Lect. 1997, 93, 75–93. [Google Scholar] [PubMed]
- Hossain, S.; Singh, S.; Lue, N.F. Functional analysis of the C-terminal extension of telomerase reverse transcriptase. A putative “thumb” domain. J. Biol. Chem. 2002, 277, 36174–36180. [Google Scholar] [CrossRef] [PubMed]
- Asencio, M.; Cassels, B.K.; Manríquez, V.; Boys, D. (S)-1,10-Dimethoxy-2,9-dihydroxyaporphinium chloride (boldine hydrochloride), C19H22NO4Cl. Acta Crystallogr. 1996, C52, 1581–1583. [Google Scholar] [CrossRef]
- Teng, C.M.; Yu, S.M.; Pan, C.P.; Lee, S.S. Mechanism of vasorelaxation caused by IM-benzylsecoboldine in rat thoracic aorta. J. Biomed. Sci. 1994, 1, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sobarzo-Sánchez, E.; Cassels, B.K.; Saitz-Barría, C.; Jullian, C. Oxazine- and oxazole-fused derivatives of the alkaloid boldine and their complete structural and spectral assignment by HMQC and HMBC experiments. Magn. Reson. Chem. 2001, 39, 361–366. [Google Scholar] [CrossRef]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hou, M.; Xu, D.; Björkholm, M.; Gruber, A. Real-time quantitative telomeric repeat amplification protocol assay for the detection of telomerase activity. Clin. Chem. 2001, 47, 519–524. [Google Scholar] [PubMed]
- Kazemi Noureini, S.; Wink, M. Antiproliferative Effects of Crocin in HepG2 Cells by Telomerase Inhibition and hTERT Down-Regulation. Asian Pac. J. Cancer Prev. 2012, 13, 2305–2309. [Google Scholar] [CrossRef]
- Mitchell, M.; Gillis, A.; Futahashi, M.; Fujiwara, H.; Skordalakes, E. Structural basis for telomerase catalytic subunit TERT binding to RNA template and telomeric DNA. Nat. Struct. Mol. Biol. 2010, 17, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Lemkul, J.A.; Allen, W.J.; Bevan, D.R. Practical Considerations for Building GROMOS-Compatible Small-Molecule Topologies. J. Chem. Inf. Model. 2010, 50, 2221–2235. [Google Scholar] [CrossRef] [PubMed]
- Oostenbrink, C.; Villa, A.; Mark, A.E.; Gunsteren, W.F.V. A Biomolecular Force Field Based on the Free Enthalpy of Hydration and Solvation: The GROMOS Force-Field Parameter Sets 53A5 and 53A6. J. Comput. Chem. 2004, 25, 1656–1676. [Google Scholar] [CrossRef] [PubMed]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integrationof the Cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An Nlog (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Tuckerman, M.; Berne, B.J.; Martyna, G.J. Reversible multiple time scale molecular dynamics. J. Chem. Phys. 1992, 97, 1990–2001. [Google Scholar] [CrossRef]
- Bou-Rabee, N. Time Integrators for Molecular Dynamics. Entropy 2014, 16, 138–162. [Google Scholar] [CrossRef]
- Wong-ekkabut, J.; Karttunen, M. Assessment of Common Simulation Protocols for Simulations of Nanopores, Membrane Proteins, and Channels. J. Chem. Theory Comput. 2012, 8, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, H.A.; McCarthy, A.N.; Grigera, J.R. A Molecular Dynamics Approach to Ligand-Receptor Interactionin the Aspirin-Human Serum Albumin Complex. Biophys. J. 2012, 2012, 642745. [Google Scholar]
- Prathab, B.; Aminabhavi, T.M. Molecular Modeling Study on Surface, Thermal, Mechanical and Gas Diffusion Properties of Chitosan. Polym. Phys. 2007, 45, 1260–1270. [Google Scholar] [CrossRef]
- Schuttelkopf, A.W.; van Aalten, D.M.F. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Maunz, A.; Gütlein, M.; Rautenberg, M.; Vorgrimmler, D.; Gebele, D.; Helma, C. Lazar: A modular predictive toxicology framework. Front. Pharmacol. 2013, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL User’s Manual; DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
| Focused Docking | Blind Docking | |||
|---|---|---|---|---|
| Boldine | BSB | Boldine | BSB | |
| Ki (μM) | 9.15 | 0.22108 | 9.15 | 0.130 |
| Binding Energy (kJ/mol) | −6.87 | −9.08 | −6.64 | −9.39 |
| Number of Hydrogen Bonds | 3 | 1 | 1 | 2 |
| Amino Acids | Ala255 Asn369 Lys372 | Ile252 | Arg181 | Arg181 Pro180 |
| RMSD (Å) | 84.54 | 81.53 | 72.13 | 73.82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazemi Noureini, S.; Kheirabadi, M.; Masoumi, F.; Khosrogerdi, F.; Zarei, Y.; Suárez-Rozas, C.; Salas-Norambuena, J.; Kennedy Cassels, B. Telomerase Inhibition by a New Synthetic Derivative of the Aporphine Alkaloid Boldine. Int. J. Mol. Sci. 2018, 19, 1239. https://doi.org/10.3390/ijms19041239
Kazemi Noureini S, Kheirabadi M, Masoumi F, Khosrogerdi F, Zarei Y, Suárez-Rozas C, Salas-Norambuena J, Kennedy Cassels B. Telomerase Inhibition by a New Synthetic Derivative of the Aporphine Alkaloid Boldine. International Journal of Molecular Sciences. 2018; 19(4):1239. https://doi.org/10.3390/ijms19041239
Chicago/Turabian StyleKazemi Noureini, Sakineh, Mitra Kheirabadi, Fatima Masoumi, Farve Khosrogerdi, Younes Zarei, Cristian Suárez-Rozas, Julio Salas-Norambuena, and Bruce Kennedy Cassels. 2018. "Telomerase Inhibition by a New Synthetic Derivative of the Aporphine Alkaloid Boldine" International Journal of Molecular Sciences 19, no. 4: 1239. https://doi.org/10.3390/ijms19041239
APA StyleKazemi Noureini, S., Kheirabadi, M., Masoumi, F., Khosrogerdi, F., Zarei, Y., Suárez-Rozas, C., Salas-Norambuena, J., & Kennedy Cassels, B. (2018). Telomerase Inhibition by a New Synthetic Derivative of the Aporphine Alkaloid Boldine. International Journal of Molecular Sciences, 19(4), 1239. https://doi.org/10.3390/ijms19041239





