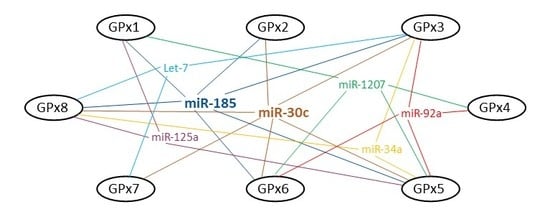
MicroRNAs as Potential Regulators of Glutathione Peroxidases Expression and Their Role in Obesity and Related Pathologies
Abstract
1. Introduction
2. Bioinformatic Analysis: MicroRNAs and GPxs
2.1. GPx1
2.2. GPx2
2.3. GPx3
2.4. GPx4
2.5. GPx5
2.6. GPx6
2.7. GPx7
2.8. GPx8
3. Summary
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
Abbreviations
| 3′UTR | 3′untranslated region |
| 5′UTR | 5′untranslated region |
| A/U | adenin/uracil |
| AMP | adenosine monophosphate |
| ApoE | apolipoprotein E |
| BMI | body mass index |
| cAMP | cyclic adenosine monophosphate |
| C/EBP | CCAAT-enhancer-binding protein |
| cGPx | cytosolic glutathione peroxidase |
| CLASH | crosslinking, ligation, and sequencing of hybrids |
| ER | endoplasmic reticulum |
| FGF19 | fibroblast growth factor 19 |
| GI | gastrointestinal |
| GSH | glutathione |
| GPx | glutathione peroxidase |
| HUVEC | human umbilical vein endothelial cells |
| LDL | low density lipoprotein |
| MAPK7 | mitogen-activated protein kinase 7 |
| mGPx | mitochondrial glutathione peroxidase |
| miRNA | microRNA |
| MnSOD | manganese superoxide dismutase |
| NFκB | nuclear factor-κB |
| NPHGPx | nonselenocysteine containing phospholipid hydroperoxide glutathione peroxidase |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PHGPx | phospholipid hydroperoxide glutathione peroxidase |
| PKA1 | protein kinase A1 |
| PKA/C/EBPβ | protein kinase A/CAAT box/enhancer-binding protein beta |
| PPAR | peroxisome proliferator-activated receptor |
| ROS | reactive oxygen species |
| Se | selenocystein insertion sequence |
| SECIS | serine |
| snGPx | sperm nuclear glutathione peroxidase |
| SNP | single nucleotide polymorphism |
| SOD | superoxide dismutase |
| T2DM | type 2 diabetes mellitus |
| TNFα | tumor necrosis factor alpha |
| TrxR2 | thioredoxin reductase 2 |
| Wnt | wngless/integration-1 |
| WT | wild-type |
References
- Chartoumpekis, D.V.; Zaravinos, A.; Ziros, P.G.; Iskrenova, R.P.; Psyrogiannis, A.I.; Kyriazopoulou, V.E.; Habeos, I.G. Differential Expression of MicroRNAs in Adipose Tissue after Long-Term High-Fat Diet-Induced Obesity in Mice. PLoS ONE 2012, 7, e34872. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Mozaffarian, D.; Pischon, T. Addressing the Perfect Storm: Biomarkers in Obesity and Pathophysiology of Cardiometabolic Risk. Clin. Chem. 2018, 64, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sanchez-Perez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Bar-Or, D.; Bar-Or, R.; Rael, L.T.; Brody, E.N. Oxidative stress in severe acute illness. Redox Biol. 2015, 4, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, A.; Dimova, E.Y.; Petry, A.; Martinez-Ruiz, A.; Hernansanz-Agustin, P.; Rolo, A.P.; Palmeira, C.M.; Kietzmann, T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015, 6, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Herbette, S.; Roeckel-Drevet, P.; Drevet, J.R. Seleno-independent glutathione peroxidases—More than simple antioxidant scavengers. FEBS J. 2007, 274, 2163–2180. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Kipp, A. Glutathione peroxidases in different stages of carcinogenesis. Biochim. Biophys. Acta 2009, 1790, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Ufer, C.; Wang, C.C. The roles of glutathione peroxidases during embryo development. Front. Mol. Neurosci. 2011, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Parra, A.; Trejo, C.; Diaz, C.; Olguin, A.; Perez, A. Association Between Single Nucleotide Polymorphism Pro198Leu of Glutathione Peroxidase and Normal Weight, Overweight and Obesity in Mexican Population. FASEB J. 2013, 27. [Google Scholar]
- McClung, J.P.; Roneker, C.A.; Mu, W.; Lisk, D.J.; Langlais, P.; Liu, F.; Lei, X.G. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc. Natl. Acad. Sci. USA 2004, 101, 8852–8857. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, A.Y.; Choi, J.W.; Kim, M.; Yasue, S.; Son, H.J.; Masuzaki, H.; Park, K.S.; Kim, J.B. Dysregulation of adipose glutathione peroxidase 3 in obesity contributes to local and systemic oxidative stress. Mol. Endocrinol. 2008, 22, 2176–2189. [Google Scholar] [CrossRef] [PubMed]
- Ruperez, A.I.; Olza, J.; Gil-Campos, M.; Leis, R.; Mesa, M.D.; Tojo, R.; Canete, R.; Gil, A.; Aguilera, C.M. Association of Genetic Polymorphisms for Glutathione Peroxidase Genes with Obesity in Spanish Children. J. Nutrigenet. Nutrigenom. 2014, 7, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Hudder, A.; Novak, R.F. miRNAs: Effectors of environmental influences on gene expression and disease. Toxicol. Sci. 2008, 103, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Deiuliis, J.A. MicroRNAs as regulators of metabolic disease: Pathophysiologic significance and emerging role as biomarkers and therapeutics. Int. J. Obes. 2016, 40, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Pescador, N.; Perez-Barba, M.; Ibarra, J.M.; Corbaton, A.; Martinez-Larrad, M.T.; Serrano-Rios, M. Serum Circulating microRNA Profiling for Identification of Potential Type 2 Diabetes and Obesity Biomarkers. PLoS ONE 2013, 8, e77251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.H.; Ku, C.H.; Siow, R.C.M. Regulation of the Nrf2 antioxidant pathway by microRNAs: New players in micromanaging redox homeostasis. Free Radic. Biol. Med. 2013, 64, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Cabrera, R.; Fernandez-Fernandez, S.; Bobo-Jimenez, V.; Escobar, J.; Sastre, J.; Almeida, A.; Bolanos, J.P. γ-Glutamylcysteine detoxifies reactive oxygen species by acting as glutathione peroxidase-1 cofactor. Nat. Commun. 2012, 3, 718. [Google Scholar] [CrossRef] [PubMed]
- Vats, P.; Sagar, N.; Singh, T.P.; Banerjee, M. Association of Superoxide dismutases (SOD1 and SOD2) and Glutathione peroxidase 1 (GPx1) gene polymorphisms with type 2 diabetes mellitus. Free Radic. Res. 2015, 49, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, H.; Fan, Y.; Kong, B.; Hu, H.; Hu, K.; Guo, J.; Mei, Y.; Liu, W. Effects of Downregulation of MicroRNA-181a on H2O2-Induced H9c2 Cell Apoptosis via the Mitochondrial Apoptotic Pathway. Oxid. Med. Cell. Longev. 2014, 2014, 960362. [Google Scholar] [CrossRef] [PubMed]
- La Sala, L.; Cattaneo, M.; De Nigris, V.; Pujadas, G.; Testa, R.; Bonfigli, A.R.; Genovese, S.; Ceriello, A. Oscillating glucose induces microRNA-185 and impairs an efficient antioxidant response in human endothelial cells. Cardiovasc. Diabetol. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Yentrapalli, R.; Azimzadeh, O.; Kraemer, A.; Malinowsky, K.; Sarioglu, H.; Becker, K.F.; Atkinson, M.J.; Moertl, S.; Tapio, S. Quantitative and integrated proteome and microRNA analysis of endothelial replicative senescence. J. Proteom. 2015, 126, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, G.; le Sage, C.; Tekirdag, K.A.; Agami, R.; Gozuacik, D. miR-376b controls starvation and mTOR inhibition-related autophagy by targeting ATG4C and BECN1. Autophagy 2012, 8, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Milagro, F.I.; Miranda, J.; Portillo, M.P.; Fernandez-Quintela, A.; Campion, J.; Martinez, J.A. High-Throughput Sequencing of microRNAs in Peripheral Blood Mononuclear Cells: Identification of Potential Weight Loss Biomarkers. PLoS ONE 2013, 8, e54319. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, H.; Wang, R.; Wang, P.; Tao, Z.; Gao, L.; Yan, F.; Liu, X.; Yu, S.; Ji, X.; et al. MicroRNA-424 Protects Against Focal Cerebral Ischemia and Reperfusion Injury in Mice by Suppressing Oxidative Stress. Stroke 2015, 46, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Marchand, A.; Atassi, F.; Mougenot, N.; Clergue, M.; Codoni, V.; Berthuin, J.; Proust, C.; Tregouet, D.A.; Hulot, J.S.; Lompre, A.M. miR-322 regulates insulin signaling pathway and protects against metabolic syndrome-induced cardiac dysfunction in mice. Biochim. Biophys. Acta 2016, 1862, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, M.; Li, H.; Lan, X.; Liu, L.; Li, J.; Li, Y.; Li, J.; Yi, J.; Du, X.; et al. Upregulation of miR-497 induces hepatic insulin resistance in E3 rats with HFD-MetS by targeting insulin receptor. Mol. Cell. Endocrinol. 2015, 416, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, H.; Zhang, Y.; Zhuang, H. Protection of rats spinal cord ischemia-reperfusion injury by inhibition of MiR-497 on inflammation and apoptosis: Possible role in pediatrics. Biomed. Pharmacother. 2016, 81, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Yao, C.; Li, Z.; Teng, Y.; Li, W.; Wang, J.; Ye, C.; Chang, G.; Huang, X.; Li, X.; et al. Differentially expressed microRNAs at different stages of atherosclerosis in ApoE-deficient mice. Chin. Med. J. 2013, 126, 515–520. [Google Scholar] [PubMed]
- Mimura, S.; Iwama, H.; Kato, K.; Nomura, K.; Kobayashi, M.; Yoneyama, H.; Miyoshii, H.; Tani, J.; Morishita, A.; Himoto, T.; et al. Profile of microRNAs associated with aging in rat liver. Int. J. Mol. Med. 2014, 34, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Diez, C.; Fierro-Fernandez, M.; Sanchez-Gomez, F.; Rodriguez-Pascual, F.; Alique, M.; Ruiz-Ortega, M.; Beraza, N.; Martinez-Chantar, M.L.; Fernandez-Hernando, C.; Lamas, S. Targeting of γ-Glutamyl-Cysteine Ligase by miR-433 Reduces Glutathione Biosynthesis and Promotes TGF-β-Dependent Fibrogenesis. Antioxid. Redox Signal. 2015, 23, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Banning, A.; Deubel, S.; Kluth, D.; Zhou, Z.W.; Brigelius-Flohe, R. The GI-GPx gene is a target for Nrf2. Mol. Cell. Biol. 2005, 25, 4914–4923. [Google Scholar] [CrossRef] [PubMed]
- Florian, S.; Wingler, K.; Schmehl, K.; Jacobasch, G.; Kreuzer, O.J.; Meyerhof, W.; Brigelius-Flohe, R. Cellular and subcellular localization of gastrointestinal glutathione peroxidase in normal and malignant human intestinal tissue. Free Radic. Res. 2001, 35, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Banning, A.; Kipp, A.; Schmitmeier, S.; Lowinger, M.; Florian, S.; Krehl, S.; Thalmann, S.; Thierbach, R.; Steinberg, P.; Brigelius-Flohe, R. Glutathione Peroxidase 2 Inhibits Cyclooxygenase-2-Mediated Migration and Invasion of HT-29 Adenocarcinoma Cells but Supports Their Growth as Tumors in Nude Mice. Cancer Res. 2008, 68, 9746–9753. [Google Scholar] [CrossRef] [PubMed]
- Baek, I.J.; Yon, J.M.; Lee, S.R.; Kim, M.R.; Hong, J.T.; Lee, B.J.; Yun, Y.W.; Nam, S.Y. Differential expression of gastrointestinal glutathione peroxidase (GI-GPx) gene during mouse organogenesis. Anat. Histol. Embryol. 2011, 40, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.J. Insights into the hierarchy of selenium incorporation. Nat. Genet. 2005, 37, 1162–1163. [Google Scholar] [CrossRef] [PubMed]
- Maciel-Dominguez, A.; Swan, D.; Ford, D.; Hesketh, J. Selenium alters miRNA profile in an intestinal cell line: Evidence that miR-185 regulates expression of GPX2 and SEPSH2. Mol. Nutr. Food Res. 2013, 57, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Dai, X.; Zhan, J.; Zhang, Y.; Zhang, H.; Zhang, H.; Zeng, S.; Xi, W. Profiling peripheral microRNAs in obesity and type 2 diabetes mellitus. APMIS 2015, 123, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fang, F.; Zhang, J.Y.; Josson, S.; St. Clair, W.H.; St. Clair, D.K. miR-17 Suppresses Tumorigenicity of Prostate Cancer by Inhibiting Mitochondrial Antioxidant Enzymes. PLoS ONE 2010, 5, e14356. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Dellambra, E.; Ciarapica, R.; Capogrossi, M.C. Oxidative stress, microRNAs and cytosolic calcium homeostasis. Cell Calcium 2016, 60, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Delic, D.; Eisele, C.; Schmid, R.; Luippold, G.; Mayoux, E.; Grempler, R. Characterization of Micro-RNA Changes during the Progression of Type 2 Diabetes in Zucker Diabetic Fatty Rats. Int. J. Mol. Sci. 2016, 17, 665. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Fu, X.; Si, M.; Wang, Y.; Ma, R.; Ren, X.; Lv, H. MicroRNA-185 Targets SOCS3 to Inhibit β-Cell Dysfunction in Diabetes. PLoS ONE 2015, 10, e0116067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, H.D.; Cao, Y.; Dela Cruz, C.S.; Jin, Y. miR-185 mediates lung epithelial cell death after oxidative stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L700–L710. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, W.; Pellicane, C.; Sahyoun, C.; Joseph, B.K.; Gallo-Ebert, C.; Donigan, M.; Pandya, D.; Giordano, C.; Bata, A.; et al. Identification of miR-185 as a regulator of de novo cholesterol biosynthesis and low density lipoprotein uptake. J. Lipid Res. 2014, 55, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhan, X.; Li, X.; Yu, J.; Liu, X. MicroRNA-185 regulates expression of lipid metabolism genes and improves insulin sensitivity in mice with non-alcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 17914–17923. [Google Scholar] [CrossRef] [PubMed]
- Rotllan, N.; Price, N.; Pati, P.; Goedeke, L.; Fernandez-Hernando, C. microRNAs in lipoprotein metabolism and cardiometabolic disorders. Atherosclerosis 2016, 246, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.J.; Cardona-Alvarado, M.I.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Sabater, M.; Fuentes-Batllevell, N.; Ramirez-Chavez, E.; Ricart, W.; Molina-Torres, J.; et al. Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common microRNAs. J. Nutr. Biochem. 2015, 26, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Sangokoya, C.; Telen, M.J.; Chi, J.T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood 2010, 116, 4338–4348. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, D.; Piro, S.; Condorelli, A.G.; Mascali, L.G.; Urbano, F.; Parrinello, N.; Monello, A.; Statello, L.; Ragusa, M.; Rabuazzo, A.M.; et al. miR-296-3p, miR-298-5p and their downstream networks are causally involved in the higher resistance of mammalian pancreatic α cells to cytokine-induced apoptosis as compared to β cells. BMC Genom. 2013, 14, 62. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Ai, D.; Wu, R.; Zhang, T.; Jing, L.; Lu, J.; Zhong, L. Identification of the differential expression of serum microRNA in type 2 diabetes. Biosci. Biotechnol. Biochem. 2016, 80, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Thulasingam, S.; Massilamany, C.; Gangaplara, A.; Dai, H.J.; Yarbaeva, S.; Subramaniam, S.; Riethoven, J.J.; Eudy, J.; Lou, M.; Reddy, J. miR-27b*, an oxidative stress-responsive microRNA modulates nuclear factor-κB pathway in RAW 264.7 cells. Mol. Cell. Biochem. 2011, 352, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Can, U.; Buyukinan, M.; Yerlikaya, F.H. The investigation of circulating microRNAs associated with lipid metabolism in childhood obesity. Pediatr. Obes. 2016, 11, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Yin, D. Glutathione peroxidase-3 produced by the kidney binds to a population of basement membranes in the gastrointestinal tract and in other tissues. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G32–G38. [Google Scholar] [CrossRef] [PubMed]
- Baez-Duarte, B.G.; Zamora-Ginez, I.; Mendoza-Carrera, F.; Ruiz-Vivanco, G.; Torres-Rasgado, E.; Gonzalez-Mejia, M.E.; Garcia-Zapien, A.; Flores-Martinez, S.E.; Perez-Fuentes, R. Serum levels of glutathione peroxidase 3 in overweight and obese subjects from central Mexico. Arch. Med. Res. 2012, 43, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; Enlund, E.; Funcke, J.B.; Tews, D.; Holzmann, K.; Debatin, K.M.; Wabitsch, M.; Fischer-Posovszky, P. miR-146a-mediated suppression of the inflammatory response in human adipocytes. Sci. Rep. 2016, 6, 38339. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, B.; Lumayag, S.; Cowan, C.; Xu, S.B. microRNAs in Early Diabetic Retinopathy in Streptozotocin-Induced Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4402–4409. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tian, F.; Chen, Z.; Li, R.; Ge, Q.; Lu, Z. Amplification-based method for microRNA detection. Biosens. Bioelectron. 2015, 71, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, C.; Kitano, M.; Rimbach, G.; Niklowitz, P.; Menke, T.; Hosoe, K.; Doring, F. Effects of Ubiquinol-10 on MicroRNA-146a Expression In Vitro and In Vivo. Mediat. Inflamm. 2009, 2009, 415437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, L.; Dai, Y.; Ji, C.; Yang, L.; Shi, C.; Xu, G.; Pang, L.; Huang, F.; Zhang, C.; Guo, X. MiR-146b is a regulator of human visceral preadipocyte proliferation and differentiation and its expression is altered in human obesity. Mol. Cell. Endocrinol. 2014, 393, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Van Dooren, E.; Mathieu, C.; Holvoet, P. Decrease of miR-146b-5p in Monocytes during Obesity Is Associated with Loss of the Anti-Inflammatory but Not Insulin Signaling Action of Adiponectin. PLoS ONE 2012, 7, e32794. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, L.; Chen, X.; Gu, N.; Chen, L.; Zhu, L.; Yang, L.; Pang, L.; Guo, X.; Ji, C.; et al. IL-6 and TNF-α Induced Obesity-Related Inflammatory Response Through Transcriptional Regulation of miR-146b. J. Interferon Cytokine Res. 2014, 34, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, S.; Feng, Z.; Zhao, L.; Jia, W.; Liu, P.; Zhu, Y.; Jian, Z.; Xiao, Y. MicroRNA-146b inhibition augments hypoxia-induced cardiomyocyte apoptosis. Mol. Med. Rep. 2015, 12, 6903–6910. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, S.; Liu, J.; Dou, D.; Liu, L.; Chen, Z.; Ye, L.; Liu, H.; He, Q.; Raj, J.U.; et al. Hypoxia induces downregulation of soluble guanylyl cyclase β(1) by miR-34c-5p. J. Cell Sci. 2012, 125, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Baldeon, R.L.; Weigelt, K.; de Wit, H.; Ozcan, B.; van Oudenaren, A.; Sempertegui, F.; Sijbrands, E.; Grosse, L.; van Zonneveld, A.J.; Drexhage, H.A.; et al. Type 2 Diabetes Monocyte MicroRNA and mRNA Expression: Dyslipidemia Associates with Increased Differentiation-Related Genes but Not Inflammatory Activation. PLoS ONE 2015, 10, e0129421. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, C.; Iaffaldano, L.; Ferrigno, M.; Labruna, G.; Maruotti, G.M.; Quaglia, F.; Capobianco, V.; Di Noto, R.; Del Vecchio, L.; Martinelli, P.; et al. Characterization and predicted role of the microRNA expression profile in amnion from obese pregnant women. Int. J. Obes. 2014, 38, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Zaragosi, L.E.; Wdziekonski, B.; Le Brigand, K.; Villageois, P.; Mari, B.; Waldmann, R.; Dani, C.; Barbry, P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011, 12, R64. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.J.; Wang, Z.Y.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A.; et al. Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatr. Res. 2015, 77, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wu, Y.; Wang, J.; Chen, J.; Huang, Y.; Rao, J.; Feng, C. MicroRNA-24 promotes 3T3-L1 adipocyte differentiation by directly targeting the MAPK7 signaling. Biochem. Biophys. Res. Commun. 2016, 474, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.; Wu, H.; Xiao, H.; Chen, X.; Willenbring, H.; Steer, C.J.; Song, G. Inhibition of MicroRNA-24 Expression in Liver Prevents Hepatic Lipid Accumulation and Hyperlipidemia. Hepatology 2014, 60, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Heim, S.; Kiess, M.; Maiorino, M.; Roveri, A.; Wissing, J.; Flohe, L. Dual function of the selenoprotein PHGPx during sperm maturation. Science 1999, 285, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Katunga, L.A.; Gudimella, P.; Efird, J.T.; Abernathy, S.; Mattox, T.A.; Beatty, C.; Darden, T.M.; Thayne, K.A.; Alwair, H.; Kypson, A.P.; et al. Obesity in a model of gpx4 haploinsufficiency uncovers a causal role for lipid-derived aldehydes in human metabolic disease and cardiomyopathy. Mol. Metab. 2015, 4, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Maes, O.C.; An, J.; Sarojini, H.; Wang, E. Murine microRNAs implicated in liver functions and aging process. Mech. Ageing Dev. 2008, 129, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, Y.; Chen, Y.; Yin, C.; Chen, J.; Liu, S. miR-214 protects erythroid cells against oxidative stress by targeting ATF4 and EZH2. Free Radic. Biol. Med. 2016, 92, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, H.; Chen, F.; Li, D.; Zhao, Y. MiR-214 Promotes the Alcohol-Induced Oxidative Stress via Down-Regulation of Glutathione Reductase and Cytochrome P450 Oxidoreductase in Liver Cells. Alcohol. Clin. Exp. Res. 2014, 38, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Badosa, G.; Bonmati, A.; Ortega, F.J.; Mercader, J.M.; Guindo-Martinez, M.; Torrents, D.; Prats-Puig, A.; Martinez-Calcerrada, J.M.; Platero-Gutierrez, E.; De Zegher, F.; et al. Altered Circulating miRNA Expression Profile in Pregestational and Gestational Obesity. J. Clin. Endocrinol. Metab. 2015, 100, E1446–E1456. [Google Scholar] [CrossRef] [PubMed]
- Dharap, A.; Pokrzywa, C.; Murali, S.; Pandi, G.; Vemuganti, R. MicroRNA miR-324-3p Induces Promoter-Mediated Expression of RelA Gene. PLoS ONE 2013, 8, e79467. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Stephan, J.P.; Cruz, D.D.; Merchant, M.; Haley, B.; Bourgon, R.; Classon, M.; Settleman, J. Functional screening implicates miR-371-3p and peroxiredoxin 6 in reversible tolerance to cancer drugs. Nat. Commun. 2016, 7, 12351. [Google Scholar] [CrossRef] [PubMed]
- Bork, S.; Horn, P.; Castoldi, M.; Hellwig, I.; Ho, A.D.; Wagner, W. Adipogenic Differentiation of Human Mesenchymal Stromal Cells Is Down-Regulated by microRNA-369-5p and Up-Regulated by microRNA-371. J. Cell. Physiol. 2011, 226, 2226–2234. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Yang, L.; Pang, L.; Chen, L.; Guo, X.; Ji, C.; Shi, C.; Ni, Y. Expression of obesity-related miR-1908 in human adipocytes is regulated by adipokines, free fatty acids and hormones. Mol. Med. Rep. 2014, 10, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, C.; Chen, L.; Pang, L.; Xu, G.; Gu, N.; Zhu, L.; Guo, X.; Ni, Y.; Ji, C. The biological effects of hsa-miR-1908 in human adipocytes. Mol. Biol. Rep. 2015, 42, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Rejraji, H.; Vernet, P.; Drevet, J.R. GPX5 is present in the mouse caput and cauda epididymidis lumen at three different locations. Mol. Reprod. Dev. 2002, 63, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Robson, A.; Houghton, B.C.; Jepson, C.A.; Ford, W.C.L.; Frayne, J. Epididymal specific, selenium-independent GPX5 protects cells from oxidative stress-induced lipid peroxidation and DNA mutation. Hum. Reprod. 2013, 28, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Xie, W.; Li, F.; Lv, Q.; He, J.; Wu, J.; Gu, D.; Xu, N.; Zhang, Y. MiR-143 enhances adipogenic differentiation of 3T3-L1 cells through targeting the coding region of mouse pleiotrophin. FEBS Lett. 2011, 585, 3303–3309. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yu, J.; Zhou, C.; Ren, G.; Cong, P.; Mo, D.; Chen, Y.; Liu, X. MiR-143 is not essential for adipose development as revealed by in vivo antisense targeting. Biotechnol. Lett. 2013, 35, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Kilic, I.D.; Dodurga, Y.; Uludag, B.; Alihanoglu, Y.I.; Yildiz, B.S.; Enli, Y.; Secme, M.; Bostanci, H.E. microRNA-143 and-223 in obesity. Gene 2015, 560, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Latouche, C.; Natoli, A.; Reddy-Luthmoodoo, M.; Heywood, S.E.; Armitage, J.A.; Kingwell, B.A. MicroRNA-194 Modulates Glucose Metabolism and Its Skeletal Muscle Expression Is Reduced in Diabetes. PLoS ONE 2016, 11, e0155108. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Yao, X.; Zhang, D.; Yang, X.; Tian, S.; Wang, N. Abated microRNA-195 expression protected mesangial cells from apoptosis in early diabetic renal injury in mice. J. Nephrol. 2012, 25, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Krutzfeldt, J.; Rosch, N.; Hausser, J.; Manoharan, M.; Zavolan, M.; Stoffel, M. MicroRNA-194 is a target of transcription factor 1 (Tcf1, HNF1α) in adult liver and controls expression of frizzled-6. Hepatology 2012, 55, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, F.; Didelot, X.; Bruce, K.D.; Cagampang, F.R.; Vatish, M.; Hanson, M.; Lehnert, H.; Ceriello, A.; Byrne, C.D. Maternal high fat diet during pregnancy and lactation alters hepatic expression of insulin like growth factor-2 and key microRNAs in the adult offspring. BMC Genom. 2009, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Goltyaev, M.V.; Varlamova, E.G.; Novoselov, V.I.; Fesenko, E.E. Determination of mgpx6 and mselv gene mRNA expression during mouse postnatal development. Dokl. Biochem. Biophys. 2014, 457, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Shema, R.; Kulicke, R.; Cowley, G.S.; Stein, R.; Root, D.E.; Heiman, M. Synthetic lethal screening in the mammalian central nervous system identifies Gpx6 as a modulator of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2015, 112, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Gracia, A.; Miranda, J.; Fernandez-Quintela, A.; Eseberri, I.; Garcia-Lacarte, M.; Milagro, F.I.; Martinez, J.A.; Aguirre, L.; Portillo, M.P. Involvement of miR-539-5p in the inhibition of de novo lipogenesis induced by resveratrol in white adipose tissue. Food Funct. 2016, 7, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wan, S.; Yang, T.; Niu, D.; Zhang, A.; Yang, C.; Cai, J.; Wu, J.; Song, J.; Zhang, C.; et al. Increased serum microRNAs are closely associated with the presence of microvascular complications in type 2 diabetes mellitus. Sci. Rep. 2016, 6, 20032. [Google Scholar] [CrossRef] [PubMed]
- Mentzel, C.M.J.; Anthon, C.; Jacobsen, M.J.; Karlskov-Mortensen, P.; Bruun, C.S.; Jorgensen, C.B.; Gorodkin, J.; Cirera, S.; Fredholm, M. Gender and Obesity Specific MicroRNA Expression in Adipose Tissue from Lean and Obese Pigs. PLoS ONE 2015, 10, e0131650. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, D.; Roy, U.; Garg, S.; Ghosh, S.; Pathak, S.; Kolthur-Seetharam, U. Sirt1 and mir-9 expression is regulated during glucose-stimulated insulin secretion in pancreatic β-islets. FEBS J. 2011, 278, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.D.; Saaranen, M.J.; Karala, A.R.; Lappi, A.K.; Wang, L.; Raykhel, I.B.; Alanen, H.I.; Salo, K.E.; Wang, C.C.; Ruddock, L.W. Two endoplasmic reticulum PDI peroxidases increase the efficiency of the use of peroxide during disulfide bond formation. J. Mol. Biol. 2011, 406, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, P.C.; Hsu, J.L.; Su, F.; Lee, W.H. NPGPx (GPx7): A novel oxidative stress sensor/transmitter with multiple roles in redox homeostasis. Am. J. Transl. Res. 2016, 8, 1626–1640. [Google Scholar] [PubMed]
- Utomo, A.; Jiang, X.; Furuta, S.; Yun, J.; Levin, D.S.; Wang, Y.; Desai, K.V.; Green, J.E.; Chen, P.; Lee, W.H. Identification of a novel putative non-selenocysteine containing phospholipid hydroperoxide glutathione peroxidase (NPGPx) essential for alleviating oxidative stress generated from polyunsaturated fatty acids in breast cancer cells. J. Biol. Chem. 2004, 279, 43522–43529. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.C.; Hsieh, Y.H.; Su, M.I.; Jiang, X.; Hsu, P.H.; Lo, W.T.; Weng, J.Y.; Jeng, Y.M.; Wang, J.M.; Chen, P.L.; et al. Loss of the oxidative stress sensor NPGPx compromises GRP78 chaperone activity and induces systemic disease. Mol. Cell 2012, 48, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Yu, Y.H.; Shew, J.Y.; Lee, W.J.; Hwang, J.J.; Chen, Y.H.; Chen, Y.R.; Wei, P.C.; Chuang, L.M.; Lee, W.H. Deficiency of NPGPx, an oxidative stress sensor, leads to obesity in mice and human. EMBO Mol. Med. 2013, 5, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.K.; Kim, Y.S.; Kim, J.Y.; Bae, Y.C.; Jung, J.S. miR-137 Controls Proliferation and Differentiation of Human Adipose Tissue Stromal Cells. Cell. Physiol. Biochem. 2014, 33, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, J.; Wei, T.; Li, J. Down-Regulation of MicroRNA-137 Improves High Glucose-Induced Oxidative Stress Injury in Human Umbilical Vein Endothelial Cells by Up-Regulation of AMPKα1. Cell. Physiol. Biochem. 2016, 39, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, R.; Wu, J.; Li, Z. MicroRNA-137 Negatively Regulates H2O2-Induced Cardiomyocyte Apoptosis Through CDC42. Med. Sci. Monit. 2015, 21, 3498–3504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dooley, J.; Garcia-Perez, J.E.; Sreenivasan, J.; Schlenner, S.M.; Vangoitsenhoven, R.; Papadopoulou, A.S.; Tian, L.; Schonefeldt, S.; Serneels, L.; Deroose, C.; et al. The microRNA-29 Family Dictates the Balance Between Homeostatic and Pathological Glucose Handling in Diabetes and Obesity. Diabetes 2016, 65, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Slusarz, A.; Pulakat, L. The two faces of miR-29. J. Cardiovasc. Med. 2015, 16, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Widlansky, M.E.; Jensen, D.M.; Wang, J.; Liu, Y.; Geurts, A.M.; Kriegel, A.J.; Liu, P.; Ying, R.; Zhang, G.; Casati, M.; et al. miR-29 contributes to normal endothelial function and can restore it in cardiometabolic disorders. EMBO Mol. Med. 2018, e8046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Su, D.; Zhu, D.; Li, Q.; Chi, M. MicroRNA-29b Promotes the Adipogenic Differentiation of Human Adipose Tissue-Derived Stromal Cells. Obesity 2016, 24, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Yao, Y.; Fan, X.; Lei, S.; Li, J.; Liu, H.; Zhou, Y. miR-29c-3p promotes senescence of human mesenchymal stem cells by targeting CNOT6 through p53-p21 and p16-pRB pathways. Biochim. Biophys. Acta 2016, 1863, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Verma, G.; Vig, S.; Srivastava, S.; Srivastava, A.K.; Datta, M. miR-29a levels are elevated in the db/db mice liver and its overexpression leads to attenuation of insulin action on PEPCK gene expression in HepG2 cells. Mol. Cell. Endocrinol. 2011, 332, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.J.A.; Olson, E.N. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc. Natl. Acad. Sci. USA 2011, 108, 21075–21080. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Huang, F.; Gu, X.; Zhang, M.; Wen, J.; Wang, X.; You, L.; Cui, X.; Ji, C.; Guo, X. Adipogenic miRNA and Meta-signature miRNAs involved in human adipocyte differentiation and obesity. Oncotarget 2016, 7, 40830–40845. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.H.; Tian, Q.; Steuerwald, N.M.; Schrum, L.W.; Bonkovsky, H.L. The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes. Biochim. Biophys. Acta. 2012, 1819, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Pasut, A.; Soleimani, V.D.; Bentzinger, C.F.; Antoun, G.; Thorn, S.; Seale, P.; Fernando, P.; van Ijcken, W.; Grosveld, F.; et al. MicroRNA-133 Controls Brown Adipose Determination in Skeletal Muscle Satellite Cells by Targeting Prdm16. Cell Metab. 2013, 17, 210–224. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chen, K.; Wang, F.; Zhao, L.; Wan, X.; Wang, L.; Mo, Z. miR-204-5p promotes the adipogenic differentiation of human adipose-derived mesenchymal stem cells by modulating DVL3 expression and suppressing Wnt/β-catenin signaling. Int. J. Mol. Med. 2015, 35, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Chen, J.; Jing, G.; Shalev, A. Thioredoxin-interacting protein regulates insulin transcription through microRNA-204. Nat. Med. 2013, 19, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L.; Shi, C.; Xu, G.; Xu, L.; Zhu, L.; Guo, X.; Ni, Y.; Cui, Y.; Ji, C. MiR-335, an Adipogenesis-Related MicroRNA, is Involved in Adipose Tissue Inflammation. Cell Biochem. Biophys. 2014, 68, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, N.; Nakagawa, Y.; Tokushige, N.; Aoki, N.; Matsuzaka, T.; Ishii, K.; Yahagi, N.; Kobayashi, K.; Yatoh, S.; Takahashi, A.; et al. The up-regulation of microRNA-335 is associated with lipid metabolism in liver and white adipose tissue of genetically obese mice. Biochem. Biophys. Res. Commun. 2009, 385, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Oger, F.; Gheeraert, C.; Mogilenko, D.; Benomar, Y.; Molendi-Coste, O.; Bouchaert, E.; Caron, S.; Dombrowicz, D.; Pattou, F.; Duez, H.; et al. Cell-Specific Dysregulation of MicroRNA Expression in Obese White Adipose Tissue. J. Clin. Endocrinol. Metab. 2014, 99, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- Gerin, I.; Clerbaux, L.-A.; Haumont, O.; Lanthier, N.; Das, A.K.; Burant, C.F.; Leclercq, I.A.; MacDougald, O.A.; Bommer, G.T. Expression of miR-33 from an SREBP2 Intron Inhibits Cholesterol Export and Fatty Acid Oxidation. J. Biol. Chem. 2010, 285, 33652–33661. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Suarez, Y.; Davalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernandez-Hernando, C. MiR-33 Contributes to the Regulation of Cholesterol Homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Rayner, K.J.; Suarez, Y.; Fernandez-Hernando, C. The Role of MicroRNAs in Cholesterol Efflux and Hepatic Lipid Metabolism. Annu. Rev. Nutr. 2011, 31, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Novák, J.; Olejníčková, V.; Tkáčová, N.; Santulli, G. Mechanistic Role of MicroRNAs in Coupling Lipid Metabolism and Atherosclerosis. In microRNA: Basic Science: From Molecular Biology to Clinical Practice; Santulli, G., Ed.; Springer: Cham, Switzerland, 2015; pp. 79–100. [Google Scholar]
- Karunakaran, D.; Richards, L.; Geoffrion, M.; Barrette, D.; Gotfrit, R.J.; Harper, M.E.; Rayner, K.J. Therapeutic Inhibition of miR-33 Promotes Fatty Acid Oxidation but Does Not Ameliorate Metabolic Dysfunction in Diet-Induced Obesity. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Bian, C.; Zhou, H.; Huang, S.; Wang, S.; Liao, L.; Zhao, R. MicroRNA hsa-miR-138 Inhibits Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells through Adenovirus EID-1. Stem Cells Dev. 2011, 20, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Gao, Z.G.; Alarcon, R.M.; Ye, J.P.; Yun, Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009, 276, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Sasano, T.; Sugiyama, K.; Kurokawa, J.; Tamura, N.; Soejima, Y.; Sawabe, M.; Isobe, M.; Furukawa, T. High-fat diet increases vulnerability to atrial arrhythmia by conduction disturbance via miR-27b. J. Mol. Cell. Cardiol. 2016, 90, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Karbiener, M.; Fischer, C.; Nowitsch, S.; Opriessnig, P.; Papak, C.; Ailhaud, G.; Dani, C.; Amri, E.Z.; Scheideler, M. microRNA miR-27b impairs human adipocyte differentiation and targets PPARγ. Biochem. Biophys. Res. Commun. 2009, 390, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Lu, W.; Xu, W.; Anderson, L.; Bacanamwo, M.; Thompson, W.; Chen, Y.E.; Liu, D. MicroRNA-27 (miR-27) Targets Prohibitin and Impairs Adipocyte Differentiation and Mitochondria! Function in Human Adipose-derived Stem Cells. J. Biol. Chem. 2013, 288, 34394–34402. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.C.; Yu, J.; Bi, J.H.; Qi, H.M.; Di, W.J.; Wu, L.; Wang, L.; Zha, J.M.; Lv, S.; Zhang, F.; et al. Glucocorticoids Transcriptionally Regulate miR-27b Expression Promoting Body Fat Accumulation Via Suppressing the Browning of White Adipose Tissue. Diabetes 2015, 64, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, X.; Ning, L.; Jiang, W.; Xing, C.; Tang, Q.; Huang, H. miR-27 Impairs the Adipogenic Lineage Commitment via Targeting Lysyl Oxidase. Obesity 2015, 23, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, X.; Ding, X.; Wang, H.; Chen, X.; Zhao, H.; Jia, Y.; Liu, S.; Liu, Y. miR-27 inhibits adipocyte differentiation via suppressing CREB expression. Acta Biochim. Biophys. Sin. 2014, 46, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Ramming, T.; Hansen, H.G.; Nagata, K.; Ellgaard, L.; Appenzeller-Herzog, C. GPx8 peroxidase prevents leakage of H2O2 from the endoplasmic reticulum. Free Radic. Biol. Med. 2014, 70, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Yoboue, E.D.; Rimessi, A.; Anelli, T.; Pinton, P.; Sitia, R. Regulation of calcium fluxes by GPX8, a type-II transmembrane peroxidase enriched at the mitochondria-associated endoplasmic reticulum membrane. Antioxid. Redox Signal. 2017, 27, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Bosello-Travain, V.; Forman, H.J.; Roveri, A.; Toppo, S.; Ursini, F.; Venerando, R.; Warnecke, C.; Zaccarin, M.; Maiorino, M. Glutathione peroxidase 8 is transcriptionally regulated by HIFα and modulates growth factor signaling in HeLa cells. Free Radic. Biol. Med. 2015, 81, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, K.; Gouttenoire, J.; Hernandez, C.; Dao Thi, V.L.; Tran, H.T.; Lange, C.M.; Dill, M.T.; Heim, M.H.; Donze, O.; Penin, F.; et al. Quantitative proteomics identifies the membrane-associated peroxidase GPx8 as a cellular substrate of the hepatitis C virus NS3-4A protease. Hepatology 2014, 59, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Xu, G.; Ji, C.; Shi, C.; Shen, Y.; Chen, L.; Zhu, L.; Yang, L.; Zhao, Y.; Guo, X. The role of microRNA-26b in human adipocyte differentiation and proliferation. Gene 2014, 533, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Ji, C.; Song, G.; Shi, C.; Shen, Y.; Chen, L.; Yang, L.; Zhao, Y.; Guo, X. Obesity-associated microRNA-26b regulates the proliferation of human preadipocytes via arrest of the G1/S transition. Mol. Med. Rep. 2015, 12, 3648–3654. [Google Scholar] [CrossRef] [PubMed]
- Prats-Puig, A.; Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Bonet, N.; Ricart, W.; Lopez-Bermejo, A.; Fernandez-Real, J.M. Changes in Circulating MicroRNAs Are Associated With Childhood Obesity. J. Clin. Endocrinol. Metab. 2013, 98, E1655–E1660. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Hwang, S.H.; Lee, S.Y.; Shin, K.K.; Cho, H.H.; Bae, Y.C.; Jung, J.S. miR-486-5p Induces Replicative Senescence of Human Adipose Tissue-Derived Mesenchymal Stem Cells and Its Expression Is Controlled by High Glucose. Stem Cells Dev. 2012, 21, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Aziz, F. The emerging role of miR-223 as novel potential diagnostic and therapeutic target for inflammatory disorders. Cell. Immunol. 2016, 303, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.Z.; Qiao, P.; Wang, L. Circulating microRNA-223 as a potential biomarker for obesity. Obes. Res. Clin. Pract. 2015, 9, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Smutny, T.; Tebbens, J.D.; Pavek, P. Bioinformatic analysis of miRNAs targeting the key nuclear receptors regulating CYP3A4 gene expression: The challenge of the CYP3A4 “missing heritability’’ enigma. J. Appl. Biomed. 2015, 13, 181–188. [Google Scholar] [CrossRef]
- Cheng, C.; Bhardwaj, N.; Gerstein, M. The relationship between the evolution of microRNA targets and the length of their UTRs. BMC Genom. 2009, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Brandao, B.B.; Guerra, B.A.; Mori, M.A. Shortcuts to a functional adipose tissue: The role of small non-coding RNAs. Redox Biol. 2017, 12, 82–102. [Google Scholar] [CrossRef] [PubMed]
- Giroud, M.; Karbiener, M.; Pisani, D.F.; Ghandour, R.A.; Beranger, G.E.; Niemi, T.; Taittonen, M.; Nuutila, P.; Virtanen, K.A.; Langin, D.; et al. Let-7i-5p represses brite adipocyte function in mice and humans. Sci. Rep. 2016, 6, 28613. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Yan, L.M.; Li, Y.M.; Zhang, W.Y.; Wang, H.; Tang, A.Z.; Ou, H.S. Inhibitory effect of microRNA-24 on fatty acid-binding protein expression on 3T3-L1 adipocyte differentiation. Genet. Mol. Res. 2013, 12, 5267–5277. [Google Scholar] [CrossRef] [PubMed]
- Orian, L.; Mauri, P.; Roveri, A.; Toppo, S.; Benazzi, L.; Bosello-Travain, V.; De Palma, A.; Maiorino, M.; Miotto, G.; Zaccarin, M.; et al. Selenocysteine oxidation in glutathione peroxidase catalysis: An MS-supported quantum mechanics study. Free Radic. Biol. Med. 2015, 87, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Johansson, L.; Gafvelin, G.; Arner, E.S. Selenocysteine in proteins-properties and biotechnological use. Biochim. Biophys. Acta 2005, 1726, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pasquinelli, A.E. MicroRNAs and their targets: Recognition, regulation and an emerging reciprocal relationship. Nat. Rev. Genet. 2012, 13, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Podshivalova, K.; Salomon, D.R. MicroRNA regulation of T-lymphocyte immunity: Modulation of molecular networks responsible for T-cell activation, differentiation, and development. Crit. Rev. Immunol. 2013, 33, 435–476. [Google Scholar] [CrossRef] [PubMed]
- Ben-Hamo, R.; Efroni, S. MicroRNA regulation of molecular pathways as a generic mechanism and as a core disease phenotype. Oncotarget 2015, 6, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Saydam, O.; Shen, Y.; Wurdinger, T.; Senol, O.; Boke, E.; James, M.F.; Tannous, B.A.; Stemmer-Rachamimov, A.O.; Yi, M.; Stephens, R.M.; et al. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/β-catenin signaling pathway. Mol. Cell. Biol. 2009, 29, 5923–5940. [Google Scholar] [CrossRef] [PubMed]
- Karbiener, M.; Neuhold, C.; Opriessnig, P.; Prokesch, A.; Bogner-Strauss, J.G.; Scheideler, M. MicroRNA-30c promotes human adipocyte differentiation and co-represses PAI-1 and ALK2. RNA Biol. 2011, 8, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.L.; Cheng, I.C.; Hou, Y.C.; Wang, W.; Yeh, S.L. MicroRNA-125a-3p expression in abdominal adipose tissues is associated with insulin signalling gene expressions in morbid obesity: Observations in Taiwanese. Asia Pac. J. Clin. Nutr. 2014, 23, 331–337. [Google Scholar] [PubMed]
- Chen, K.; He, H.; Xie, Y.; Zhao, L.; Zhao, S.; Wan, X.; Yang, W.; Mo, Z. miR-125a-3p and miR-483-5p promote adipogenesis via suppressing the RhoA/ROCK1/ERK1/2 pathway in multiple symmetric lipomatosis. Sci. Rep. 2015, 5, 11909. [Google Scholar] [CrossRef] [PubMed]
- Lavery, C.A.; Kurowska-Stolarska, M.; Holmes, W.M.; Donnelly, I.; Caslake, M.; Collier, A.; Baker, A.H.; Miller, A.M. miR-34a(-/-) Mice are Susceptible to Diet-Induced Obesity. Obesity 2016, 24, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Choi, S.G.; Huang, Z.; Suino-Powell, K.; Xu, H.E.; Kemper, B.; Kemper, J.K. MicroRNA 34a Inhibits Beige and Brown Fat Formation in Obesity in Part by Suppressing Adipocyte Fibroblast Growth Factor 21 Signaling and SIRT1 Function. Mol. Cell. Biol. 2014, 34, 4130–4142. [Google Scholar] [CrossRef] [PubMed]
- Nesca, V.; Guay, C.; Jacovetti, C.; Menoud, V.; Peyot, M.L.; Laybutt, D.R.; Prentki, M.; Regazzi, R. Identification of particular groups of microRNAs that positively or negatively impact on β cell function in obese models of type 2 diabetes. Diabetologia 2013, 56, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Kemper, J.K. MicroRNA-34a and Impaired FGF19/21 Signaling in Obesity. Vitam. Horm. 2016, 101, 175–196. [Google Scholar] [PubMed]
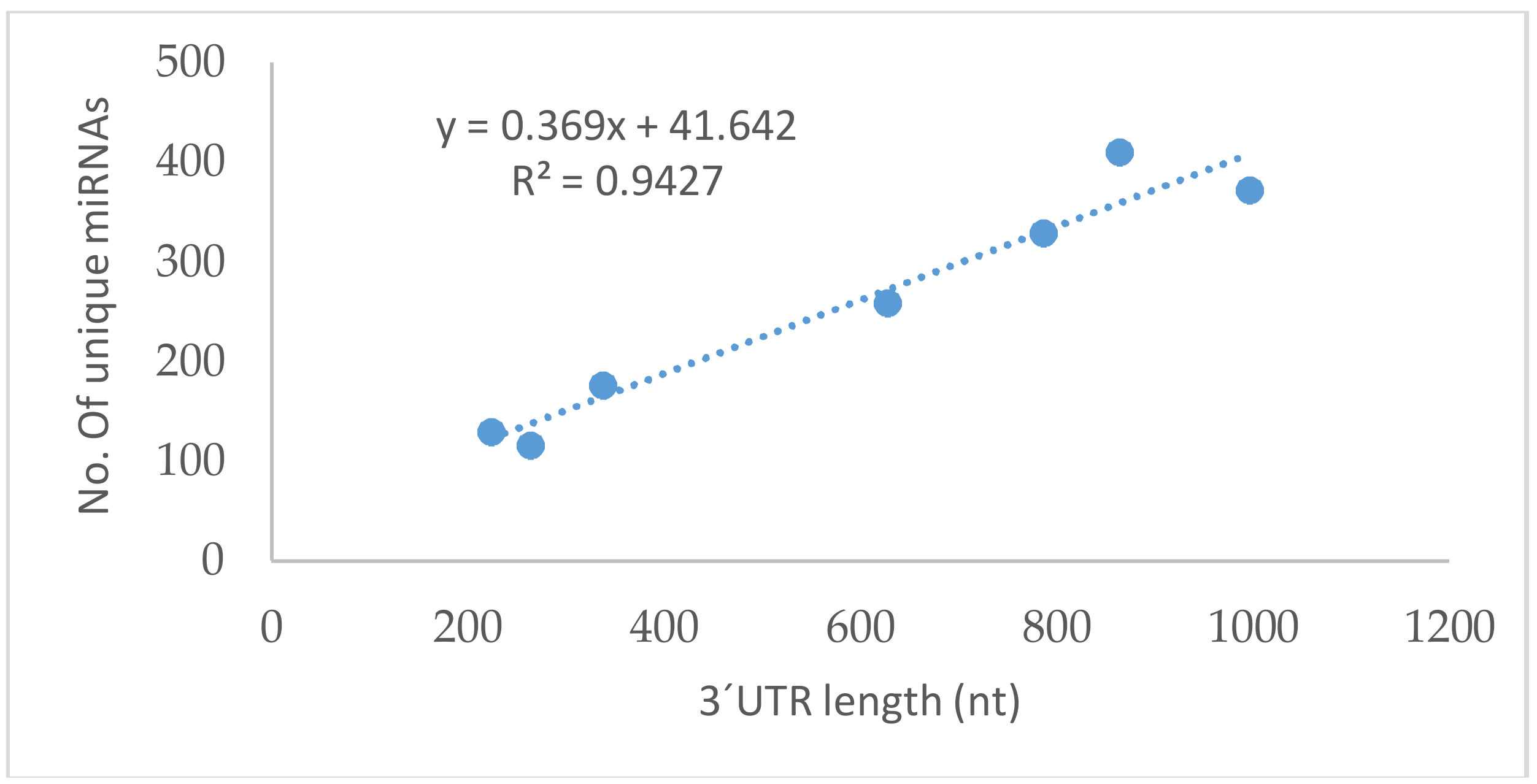

| Gene | Ensembl Gene ID | 3′UTR Length (nt) | TargetScan a | miRDB a | miRanda a | DIANA a |
|---|---|---|---|---|---|---|
| GPx1 | ENSG00000233276.2 | 222 | 99 | 16 | 25 | 37 |
| GPx2 | ENSG00000176153 | 336 | 133 | 10 | 38 | 62 |
| GPx3 | ENSG00000211445.7 | 863 | 440 | 57 | 90 | 54 |
| GPx4 | ENSG00000167468.12 | 263 | 124 | 11 | 10 | 3 |
| GPx5 | ENSG00000224586.2 | 785 | 328 | 35 | 84 | 46 |
| GPx6 | ENSG00000198704 | 996 | 414 | 41 | 101 | 68 |
| GPx7 | ENST00000361314.4 | 626 | 236 | 28 | 100 | 57 |
| GPx8 | ENSG00000164294.9 | 4869 | 15 | 46 | 53 | 111 |
| Gene | Identified miRNA | Observed Effect * | Note | Reference |
|---|---|---|---|---|
| Gpx1 | miR-376a-3p | ↓obese serum | [17] | |
| miR-367b-3p | ↓peripheral blood mononuclear cells | non-responders in weight-loss trial | [25] | |
| Gpx2 | miR-17-5p | ↑obese subjects | [39] | |
| miR-27a-5p | ↑serum of obese children | [53] | ||
| Gpx3 | miR-146b-5p | ↑adipose tissue in mice | high fat diet | [1] |
| ↓in monocytes of obese persons | [61] | |||
| miR-379-5p | ↑adipose tissue in mice | high fat diet | [1] | |
| miR-575 | expressed in the amnion of obese woman only | [66] | ||
| miR-642a-3p | ↑in fat depots of obese subjects | [67] | ||
| miR-4269 | ↓in obese donors | [68] | ||
| miR-24-3p | ↑liver high fat diet mice | [70] | ||
| Gpx4 | miR-324-3p | ↓gestational obesity | associated with pregnancy weight gain | [76] |
| miR-1908-5p | involvement in regulation of obesity development | [80] | ||
| Gpx5 | miR-143-3p | ↓in obese human subject | [86] | |
| miR-194-5p | ↓liver - High fat maternal diet altered | [90] | ||
| Gpx6 | miR-146a/b-5p | ↑adipose tissue in mice | high fat diet | [1] |
| ↓in monocytes of obese persons | [61] | |||
| miR-9 | ↑adipose tissue in obese pigs | [95] | ||
| Gpx7 | miR-29 family | obesity related | [105] | |
| let-7 family | obesity related | [112] | ||
| mir-133b-3p | ↓ adipose tissue in mice | high fat diet | [1] | |
| miR-204-5p | ↑ adipose tissue in mice | high fat diet | [1] | |
| miR-335-5p | ↑adipose tissue in mice | genetically fat mice/high fat diet | [118, 119] | |
| miR-138-5p | ↑serum of obese subjects | potential biomarker of obesity | [17] | |
| miR-27b-3p | ↑atria in mice | high fat diet | [127] | |
| GPx8 | miR-486-5p | ↑plasma of obese children | [139] | |
| miR-223-3p | ↓in serum obese subjects | after life style intervention | [142] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matoušková, P.; Hanousková, B.; Skálová, L. MicroRNAs as Potential Regulators of Glutathione Peroxidases Expression and Their Role in Obesity and Related Pathologies. Int. J. Mol. Sci. 2018, 19, 1199. https://doi.org/10.3390/ijms19041199
Matoušková P, Hanousková B, Skálová L. MicroRNAs as Potential Regulators of Glutathione Peroxidases Expression and Their Role in Obesity and Related Pathologies. International Journal of Molecular Sciences. 2018; 19(4):1199. https://doi.org/10.3390/ijms19041199
Chicago/Turabian StyleMatoušková, Petra, Barbora Hanousková, and Lenka Skálová. 2018. "MicroRNAs as Potential Regulators of Glutathione Peroxidases Expression and Their Role in Obesity and Related Pathologies" International Journal of Molecular Sciences 19, no. 4: 1199. https://doi.org/10.3390/ijms19041199
APA StyleMatoušková, P., Hanousková, B., & Skálová, L. (2018). MicroRNAs as Potential Regulators of Glutathione Peroxidases Expression and Their Role in Obesity and Related Pathologies. International Journal of Molecular Sciences, 19(4), 1199. https://doi.org/10.3390/ijms19041199