Tumor-Associated Macrophages and Mast Cells Positive to Tryptase Are Correlated with Angiogenesis in Surgically-Treated Gastric Cancer Patients
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Immunohistochemistry
4.3. Morphometrical Assay
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhattacharyya, S.P.; Drucker, I.; Reshef, T.; Kirshenbaum, A.S.; Metcalfe, D.D.; Mekori, Y.A. Activated T lymphocytes induce degranulation and cytokine production by human mast cells following cell-to-cell contact. J. Leukoc. Biol. 1998, 63, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Ammendola, M.; Sacco, R.; Sammarco, G.; Zuccalà, V.; Zizzo, N.; Leporini, C.; Luposella, M.; Patruno, R.; Filippelli, G.; et al. Tumour-associated macrophages correlate with microvascular bed extension in colorectal cancer patients. J. Cell. Mol. Med. 2016, 20, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Patruno, R.; Marech, I.; Zizzo, N.; Ammendola, M.; Nardulli, P.; Gadaleta, C.; Introna, M.; Capriuolo, G.; Rubini, R.A.; Ribatti, D.; et al. c-Kit expression, angiogenesis, and grading in canine mast cell tumour: A unique model to study c-Kit driven human malignancies. Biomed. Res. Int. 2014, 2014, 730246. [Google Scholar] [CrossRef] [PubMed]
- Wasiuk, A.; de Vries, V.C.; Hartmann, K.; Roers, A.; Noelle, R.J. Mast cells as regulators of adaptive immunity to tumours. Clin. Exp. Immunol. 2009, 155, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. Mast cells and angiogenesis. APMIS 2002, 111, 355–371. [Google Scholar] [CrossRef]
- Ribatti, D.; Ranieri, G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Varricchi, G.; Loffredo, S.; Granata, F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharmacol. 2015, 778, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, W.; Mize, G.J.; Takayama, T.K.; True, L.D.; Vessella, R.L. Protease-activated receptor 2 signaling up regulates angiogenic growth factors in renal cell carcinoma. Exp. Mol. Pathol. 2013, 94, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.G.; Riis, S.E.; Frobert, O.; Yang, S.; Kastrup, J.; Zachar, V.; Simonsen, U.; Fink, T. Activation of protease-activated receptor 2 induces VEGF independently of HIF-1. PLoS ONE 2012, 7, e46087. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.H.; Pan, S.L.; Lai, C.Y.; Tsai, A.C.; Teng, C.M. Activated PAR-2 regulates pancreatic cancer progression through ILK/HIF-α-induced TGF-α expression and MEK/VEGF-A-mediated angiogenesis. Am. J. Pathol. 2013, 183, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Piardi, T.; Zuccalà, V.; Patruno, R.; Zullo, A.; Zizzo, N.; Nardo, B.; Marech, I.; et al. Mast Cells positive to tryptase, endothelial cells positive to protease-activated receptor-2, and microvascular density correlate among themselves in hepatocellular carcinoma patients who have undergone surgery. OncoTargets Ther. 2016, 9, 4465–4471. [Google Scholar]
- Ammendola, M.; Sacco, R.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Patruno, R.; Giordano, M.; Ruggieri, E.; Zizzo, N.; et al. Microvascular density and endothelial area correlate with Ki-67 proliferative index in surgically-treated pancreatic ductal adenocarcinoma patients. Oncol Lett. 2015, 10, 967–971. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Luposella, M.; Patruno, R.; Gadaleta, C.D.; de Sarro, G.; Ranieri, G. Mast Cell-Targeted Strategies in Cancer Therapy. Transfus. Med. Hemother. 2016, 43, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Ammendola, M.; Sacco, R.; Capriuolo, G.S.; Patruno, R.; Rubini, R.; Luposella, M.; Zuccalà, V.; Savino, E.; Gadaleta, C.D.; et al. Serum tryptase, mast cells positive to tryptase and microvascular density evaluation in early breast cancer patients: Possible translational significance. BMC Cancer 2014, 14, 534. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Ammendola, M.; Gadaleta, C.; Zizzo, N.; Oakley, C.; Gadaleta, C.D.; Ranieri, G. Possible biological and translational significance of mast cells density in colorectal cancer. World J. Gastroenterol. 2014, 20, 8910–8920. [Google Scholar] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Montemurro, S.; Ruggieri, E.; Patruno, R.; Marech, I.; Cariello, M.; Vacca, A.; et al. Correlation between serum tryptase, mast cells positive to tryptase and microvascular density in colo-rectal cancer patients: Possible biological-clinical significance. PLoS ONE 2014, 9, e99512. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Zuccalà, V.; Romano, R.; Luposella, M.; Patruno, R.; Vallicelli, C.; Verdecchia, G.M.; et al. Mast Cells Positive to Tryptase and c-Kit Receptor Expressing Cells Correlates with Angiogenesis in Gastric Cancer Patients Surgically Treated. Gastroenterol. Res. Pract. 2013, 2013, 703163. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Donato, G.; Zuccalà, V.; Russo, E.; Luposella, M.; Vescio, G.; Rizzuto, A.; Patruno, R.; De Sarro, G.; et al. Mast cell positivity to tryptase correlates with metastatic lymph nodes in gastrointestinal cancer patients treated surgically. Oncology 2013, 85, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Leporini, C.; Ammendola, M.; Porcelli, M.; Gadaleta, C.D.; Russo, E.; de Sarro, G.; Ranieri, G. Classical and non classical proangiogenic factors as a target of antiangiogenic therapy in tumor microenvironment. Cancer Lett. 2015, 380, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Ranieri, G.; Nico, B.; Benagiano, V.; Crivellato, E. Tryptase and chymase are angiogenic in vivo in the chorioallantoic membrane assay. Int. J. Dev. Biol. 2011, 55, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.J.; Meng, H.; Marchese, M.J.; Ren, S.; Schwartz, L.B.; Tonnesen, M.G.; Gruber, B.L. Human mast cells stimulate vascular tube formation. Tryptase is a novel, potent angiogenic factor. J. Clin. Investig. 1997, 99, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Sendo, T.; Oishi, R. Physiology and pathophysiology of proteinase-activated receptors (PARs): Role of tryptase/PAR-2 in vascular endothelial barrier function. J. Pharmacol. Sci. 2005, 97, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.; Portell, C.; Kell, P.J.; Vinson, S.M.; McHowat, J. Protease-activated receptor stimulation activates a Ca2+-independent phospholipase A2 in bladder microvascular endothelial cells. Am. J. Physiol. Renal Physiol. 2005, 288, F714–F721. [Google Scholar] [CrossRef] [PubMed]
- Matej, R.; Mandàkovà, P.; Netikovà, I.; Pouckova, P.; Olejár, T. Proteinase-activated receptor-2 expression in breast cancer and the role of trypsin on growth and metabolism of breast cancer cell line MDA MB-231. Physiol. Res. 2007, 56, 475–484. [Google Scholar] [PubMed]
- Morris, D.R.; Ding, Y.; Ricks, T.K.; Gullapalli, A.; Wolfe, B.L.; Trejo, J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006, 66, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Leporini, C.; Marech, I.; Gadaleta, C.D.; Scognamillo, G.; Sacco, R.; Sammarco, G.; de Sarro, G.; Russo, E.; Ranieri, G. Targeting mast cells tryptase in tumor microenvironment: A potential antiangiogenetic strategy. BioMed Int. Res. 2014, 2014, 154702. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Sammarco, G.; Donato, G.; Zuccalà, V.; Luposella, M.; Patruno, R.; Marech, I.; Montemurro, S.; Zizzo, N.; et al. Mast cells density positive to tryptase correlates with angiogenesis in pancreatic ductal adenocarcinoma patients having undergone surgery. Gastroenterol. Res. Pract. 2014, 2014, 951957. [Google Scholar] [CrossRef] [PubMed]
- Donato, G.; Conforti, F.; Camastra, C.; Ammendola, M.; Donato, A.; Renzulli, A. The role of mast cell tryptases in cardiac myxoma: Histogenesis and development of a challenging tumor. Oncol. Lett. 2014, 8, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Zuccalà, V.; Patruno, R.; Russo, E.; Luposella, M.; Amorosi, A.; Vescio, G.; Sammarco, G.; Montemurro, S.; de Sarro, G.; et al. Tryptase-positive mast cells and angiogenesis in keloids: A new possible post-surgical target for prevention. Updates Surg. 2013, 65, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Ammendola, M.; Patruno, R.; Celano, G.; Zito, F.A.; Montemurro, S.; Rella, A.; di Lecce, V.; Gadaleta, C.D.; de Sarro, G.B.; et al. Tryptase-positive mast cells correlate with angiogenesis in early breast cancer patients. Int. J. Oncol. 2009, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Zizzo, N.; Patruno, R.; Crovace, A.; Ruggieri, E.; Zito, A.F.; et al. Infiltrating mast cells correlate with angiogenesis in bone metastases from gastric cancer patients. Int. J. Mol. Sci. 2015, 16, 3237–3250. [Google Scholar] [CrossRef] [PubMed]
- Malfettone, A.; Silvestris, N.; Saponaro, C.; Ranieri, G.; Russo, A.; Caruso, S.; Popescu, O.; Simone, G.; Paradiso, A.; Mangia, A. High density of tryptase-positive mast cells in human colorectal cancer: A poor prognostic factor related to protease-activated receptor 2 expression. J. Cell. Mol. Med. 2013, 17, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Soreide, K.; Janssen, E.A.; Körner, H.; Baak, J.P. Trypsin in colorectal cancer: Molecular biological mechanisms of proliferation, invasion, and metastasis. J. Pathol. 2006, 209, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Darmoul, D.; Marie, J.C.; Devaud, H.; Gratio, V.; Laburthe, M. Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. Br. J. Cancer 2001, 85, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo-Jarvinen, H.; Kurokawa, T.; Mueller, B.M.; Andrade-Gordon, P.; Friedlander, M.; Ruf, W. Role of protease activated receptor 1 and 2 signaling in hypoxia-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mueller, B.M. Protease-activated receptor-2 regulates vascular endothelial growth factor expression in MDA-MB-231 cells via MAPK pathways. Biochem. Biophys. Res. Commun. 2006, 344, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Caronni, N.; Savino, B.; Bonecchi, R. Myeloid cells in cancer-related inflammation. Immunobiology 2015, 220, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front. Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, D.; Link, V.M.; Romanoski, C.E.; Fonseca, G.J.; Eichenfield, D.Z.; Spann, N.J.; Stender, J.D.; Chun, H.B.; Garner, H.; Geissmann, F.; et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 2014, 159, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Sunderkotter, C.; Steinbrink, K.; Goebeler, M.; Bhardwaj, R.A.; Sorg, C. Macrophages and angiogenesis. J. Leukoc. Biol. 1994, 55, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef] [PubMed]
- Polverini, P.J.; Leibovich, S.J. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab. Investig. 1984, 51, 635–642. [Google Scholar] [PubMed]
- Polverini, P.J. How the extracellular matrix and macrophages contribute to angiogenesis-dependent diseases. Eur. J. Cancer 1996, 32, 2430–2437. [Google Scholar] [CrossRef]
- Mantovani, A. Tumor-associated macrophages in neoplastic progression: A paradigm for the in vivo function of chemokines. Lab. Investig. 1994, 71, 5–16. [Google Scholar] [PubMed]
- Yano, H.; Kinuta, M.; Tateishi, H.; Nakano, Y.; Matsui, S.; Monden, T.; Okamura, J.; Sakai, M.; Okamoto, S. Mast cell infiltration around gastric cancer cells correlates with tumour angiogenesis and metastasis. Gastric Cancer 1999, 2, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sedda, S.; Marafini, I.; Caruso, R.; Pallone, F.; Monteleone, G. Proteinase activated-receptors-associated signaling in the control of gastric cancer. World J. Gastroenterol. 2014, 20, 11977–11984. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Guidolin, D.; Marzullo, A.; Nico, B.; Annese, T.; Benagiano, V.; Crivellato, E. Mast cells and angiogenesis in gastric carcinoma. Int. J. Exp. Pathol. 2010, 91, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.J.; Wang, Y.B.; Li, D.N.; Deng, B.B. Expression of protease-activated receptor-2 in human gastric stromal tumor and its clinic-pathological significance. Hepatogastroenterology 2013, 60, 2125–2128. [Google Scholar] [PubMed]
- Zhang, C.; Gao, G.R.; Lv, C.G.; Zhang, B.L.; Zhang, Z.L.; Zhang, X.F. Protease-activated receptor-2 induces expression of vascular endothelial growth factor and cyclooxygenase-2 via the mitogen-activated protein kinase pathway in gastric cancer cells. Oncol. Rep. 2012, 28, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Patruno, R.; Sacco, R.; Marech, I.; Sammarco, G.; Zuccalà, V.; Luposella, M.; Zizzo, N.; Gadaleta, C.; Porcelli, M.; et al. Mast cells positive to tryptase and tumour-associated macrophages correlate with angiogenesis in locally advanced colorectal cancer patients undergone to surgery. Expert Opin. Ther. Targets 2016, 20, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Fang, J.; Yang, C. Overexpression of both VEGF-A and VEGF-C in gastric cancer correlates with prognosis, and silencing of both is effective to inhibit cancer growth. Int. J. Clin. Exp. Pathol. 2013, 6, 586–597. [Google Scholar] [PubMed]
- Khazaie, K.; Blatner, N.R.; Khan, M.W.; Gounari, F.; Gounaris, E.; Dennis, K.; Bonertz, A.; Tsai, F.N.; Strouch, M.J.; Cheon, E.; et al. The significant role of mast cells in cancer. Cancer Metast. Rev. 2011, 30, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. The role of angiogenesis in tumor growth. Semin. Cancer Biol. 1992, 3, 65–71. [Google Scholar] [PubMed]
- Anaka, T.; Ishikawa, H. Mast cells and inflammation-associated colorectal carcinogenesis. Semin. Immunopathol. 2013, 35, 245–254. [Google Scholar]
- Ribatti, D.; Ranieri, G.; Basile, A.; Azzariti, A.; Paradiso, A.; Vacca, A. Tumor endothelial markers as a target in cancer. Expert Opin. Ther. Targets 2012, 16, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, K.; Cai, K.; Zhai, R.; Tao, K.; Wang, G.; Wang, J. Increased numbers of gastric-infiltrating mast cells and regulatory T cells are associated with tumor stage in gastric adenocarcinoma patients. Oncol. Lett. 2012, 4, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Bandyopadhyay, G.; Dutta, C.; Bhattacharya, A.; Karmakar, R.; Barui, G. Evaluation of endoscopic biopsy in gastric lesions with a special reference to the significance of mast cell density. Indian J. Pathol. MicroBiol. 2009, 52, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Pimentel-Nunes, P.; Goncalves, N.; Boal-Carvalho, I.; Afonso, L.; Lopes, P.; Roncon-Albuquerque, R.; Soares, J.B.; Cardoso, E.; Henrique, R.; Moreira-Dias, L.; et al. Decreased Toll-interacting protein and peroxisome proliferator-activated receptor gamma are associated with increased expression of Toll-like receptors in colon carcinogenesis. J. Clin. Pathol. 2012, 65, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Imajo-Ohmi, S.; Fukuda, H.; Kano, S.; Miyake, K.; Watanabe, N. Mast cell-mediated immune responses through IgE antibody and Toll-like receptor 4 by malarial peroxiredoxin. Eur. J. Immunol. 2008, 38, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Zorn, C.N.; Keck, S.; Hendriks, R.W.; Leitges, M.; Freudenberg, M.A.; Huber, M. Bruton’s tyrosine kinase is dispensable for the Toll-like receptor-mediated activation of mast cells. Cell. Signal. 2009, 21, 79–86. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Q.; Wang, L.; Chen, W.; Li, N.; Cao, X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol. Immunol. 2007, 44, 2850–2859. [Google Scholar] [CrossRef] [PubMed]
- Wolska, A.; Lech-Maranda, E.; Robak, T. Toll-like receptors and their role in carcinogenesis and anti-tumor treatment. Cell. Mol. Biol. Lett. 2009, 14, 248–272. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Vescio, G.; Zuccalà, V.; Luposella, M.; Patruno, R.; Zizzo, N.; Gadaleta, C.; Marech, I.; Ruggieri, R.; et al. Tryptase mast cell density, protease-activated receptor-2 microvascular density, and classical microvascular density evaluation in gastric cancer patients undergoing surgery: Possible translational relevance. Therap. Adv. Gastroenterol. 2017, 10, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Sacco, R.; Zuccalà, V.; Luposella, M.; Patruno, R.; Gadaleta, P.; Zizzo, N.; Gadaleta, C.D.; de Sarro, G.; Sammarco, G.; et al. Mast Cells Density Positive to Tryptase Correlate with Microvascular Density in both Primary Gastric Cancer Tissue and Loco-Regional Lymph Node Metastases from Patients That Have Undergone Radical Surgery. Int. J. Mol. Sci. 2016, 17, 1905. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, M.; Gadaleta, C.D.; Frampton, A.E.; Piardi, T.; Memeo, R.; Zuccalà, V.; Luposella, M.; Patruno, R.; Zizzo, N.; Gadaleta, P.; et al. The Density of Mast Cells c-Kit+ and Tryptase+ correlates with each other and with Angiogenesis in Pancreatic Cancer Patients. Oncotarget 2017, 8, 70463–70471. [Google Scholar] [CrossRef] [PubMed]
- Erba, F.; Fiorucci, L.; Pascarella, S.; Menegatti, E.; Ascenzi, P.; Ascoli, F. Selective inhibition of human mast cell tryptase by gabexate mesylate, an antiproteinase drug. Biochem. Pharmacol. 2001, 61, 271–276. [Google Scholar] [CrossRef]
- Mori, S.; Itoh, Y.; Shinohata, R.; Sendo, T.; Oishi, R.; Nishibori, M. Nafamostat mesilate is an extremely potent inhibitor of human tryptase. J. Pharmacol. Sci. 2003, 92, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Castéran, N.; Letard, S.; Hanssens, K.; Iovanna, J.; Finetti, P.; Bertucci, F.; Bader, T.; Mansfield, C.D.; Moussy, A.; et al. Masitinib combined with standard gemcitabine chemotherapy: In vitro and in vivo studies in human pancreatic tumour cell lines and ectopic mouse model. PLoS ONE 2010, 5, e9430. [Google Scholar] [CrossRef] [PubMed]
- Marech, I.; Patruno, R.; Zizzo, N.; Gadaleta, C.; Introna, M.; Zito, A.F.; Gadaleta, C.D.; Ranieri, G. Masitinib (AB1010), from canine tumour model to human clinical development: Where we are? Crit. Rev. Oncol. Hematol. 2013, 91, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Deplanque, G.; Demarchi, M.; Hebbar, M.; Flynn, P.; Melichar, B.; Atkins, J.; Nowara, E.; Moyé, L.; Piquemal, D.; Ritter, D.; et al. A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann. Oncol. 2015, 26, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Washington, K. 7th Edition of the AJCC Cancer Staging Manual: Stomach. Ann. Surg. Oncol. 2010, 17, 3077–3079. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cai, H.; Shi, Y.; Wang, Y. Prognsotic factors in patients with node-negative gastric cancer: A single center experience from China. J. Gastrointest. Surg. 2012, 16, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Sjo, O.H.; Merok, M.A.; Svindland, A.; Nesbakken, A. Prognostic impact of lymph node harvest and lymph node ratio in patients with colon cancer. Dis. Colon Rectum 2012, 55, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Takeno, A.; Miki, H. Lymph node dissection in curative gastrectomy for advanced gastric cancer. Int. J. Surg. Oncol. 2011, 2011, 748745. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Grammatica, L.; Patruno, R.; Zito, A.F.; Valerio, P.; Iacobellis, S.; Gadaleta, C.; Gasparini, G.; Ribatti, D. A possible role of thymidine phosphorylase expression and 5-fluorouracil increased sensitivity in oropharyngeal cancer patients. J. Cell. Mol. Med. 2007, 11, 362–368. [Google Scholar] [CrossRef] [PubMed]
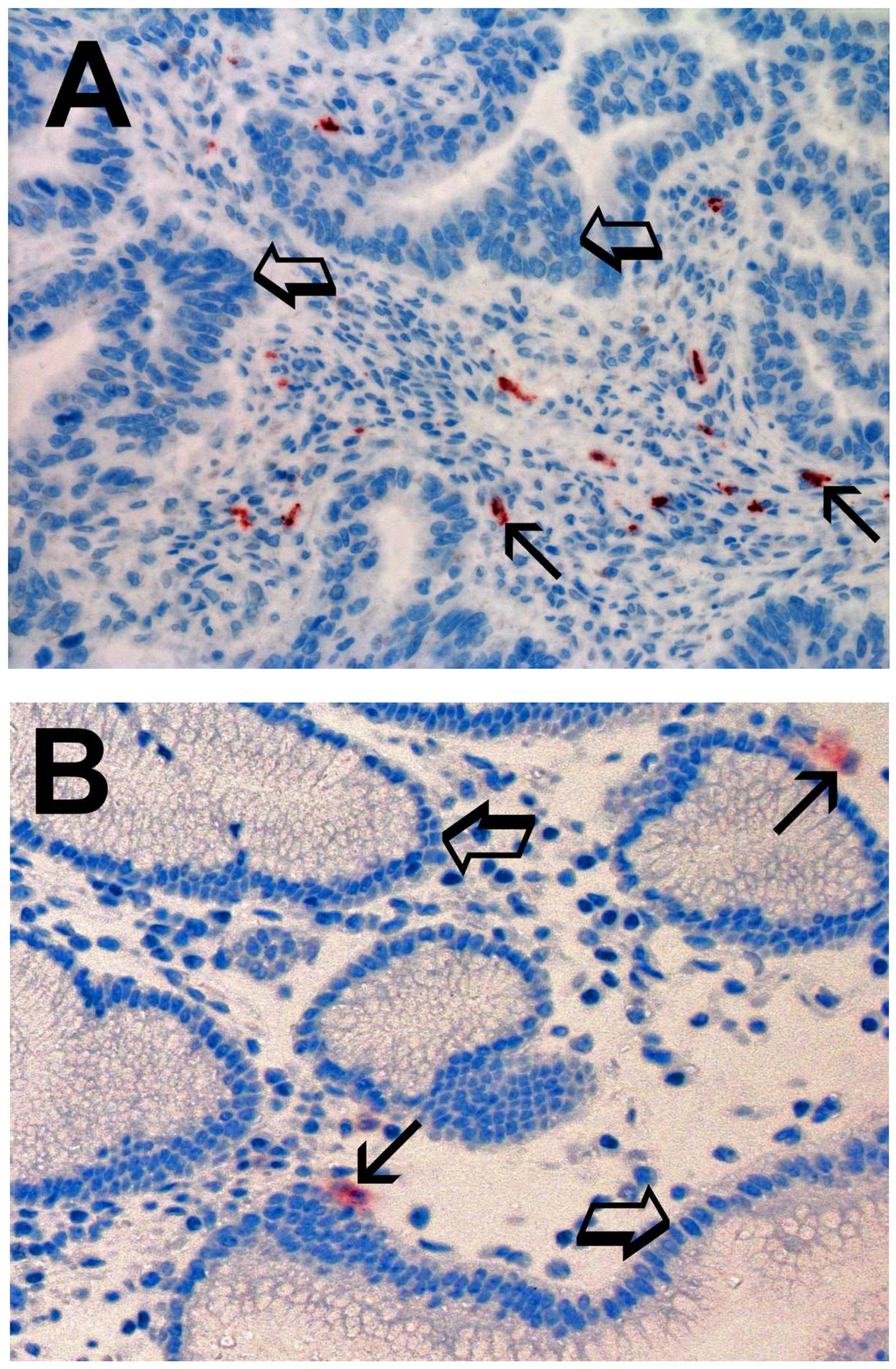

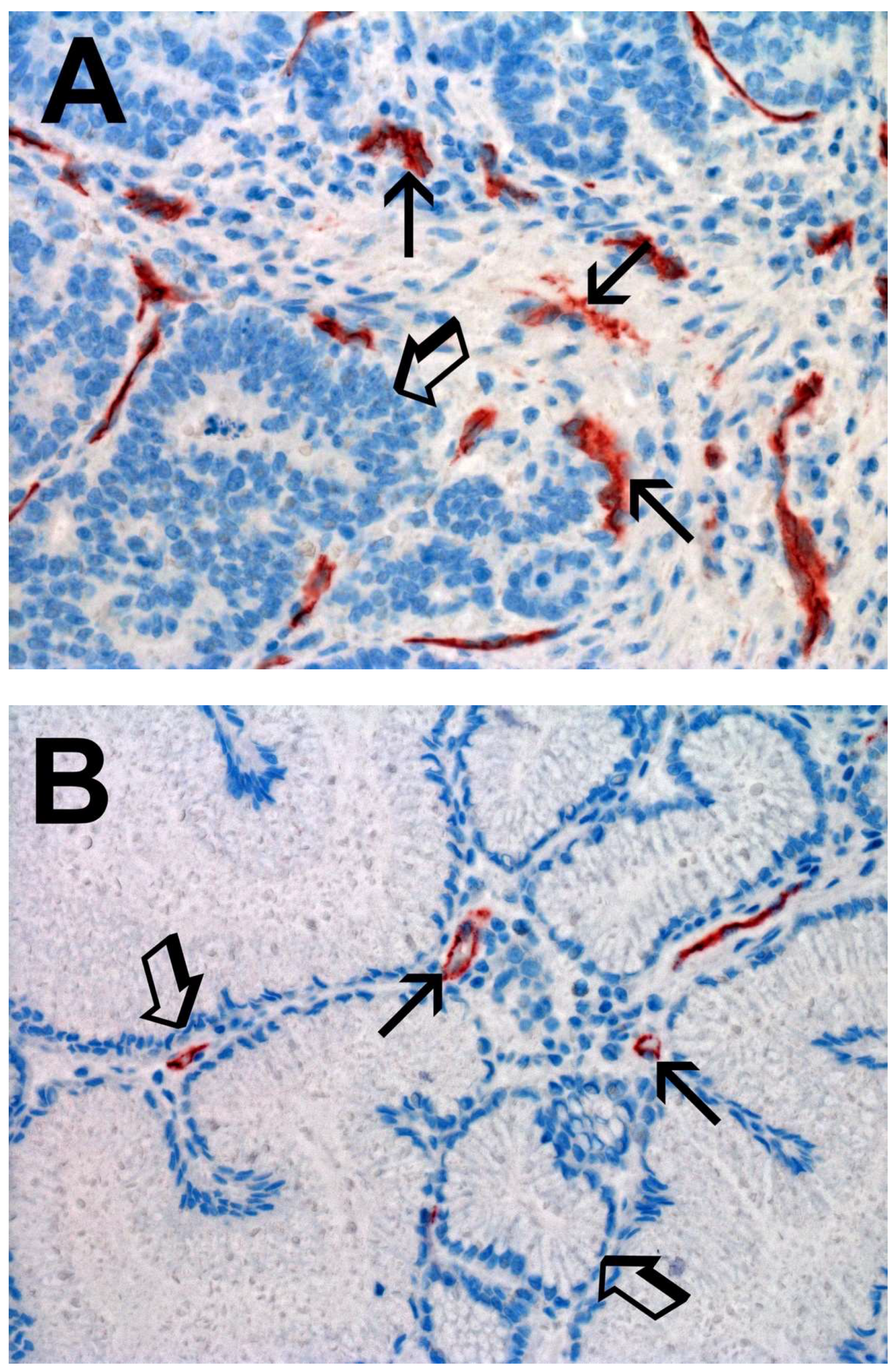
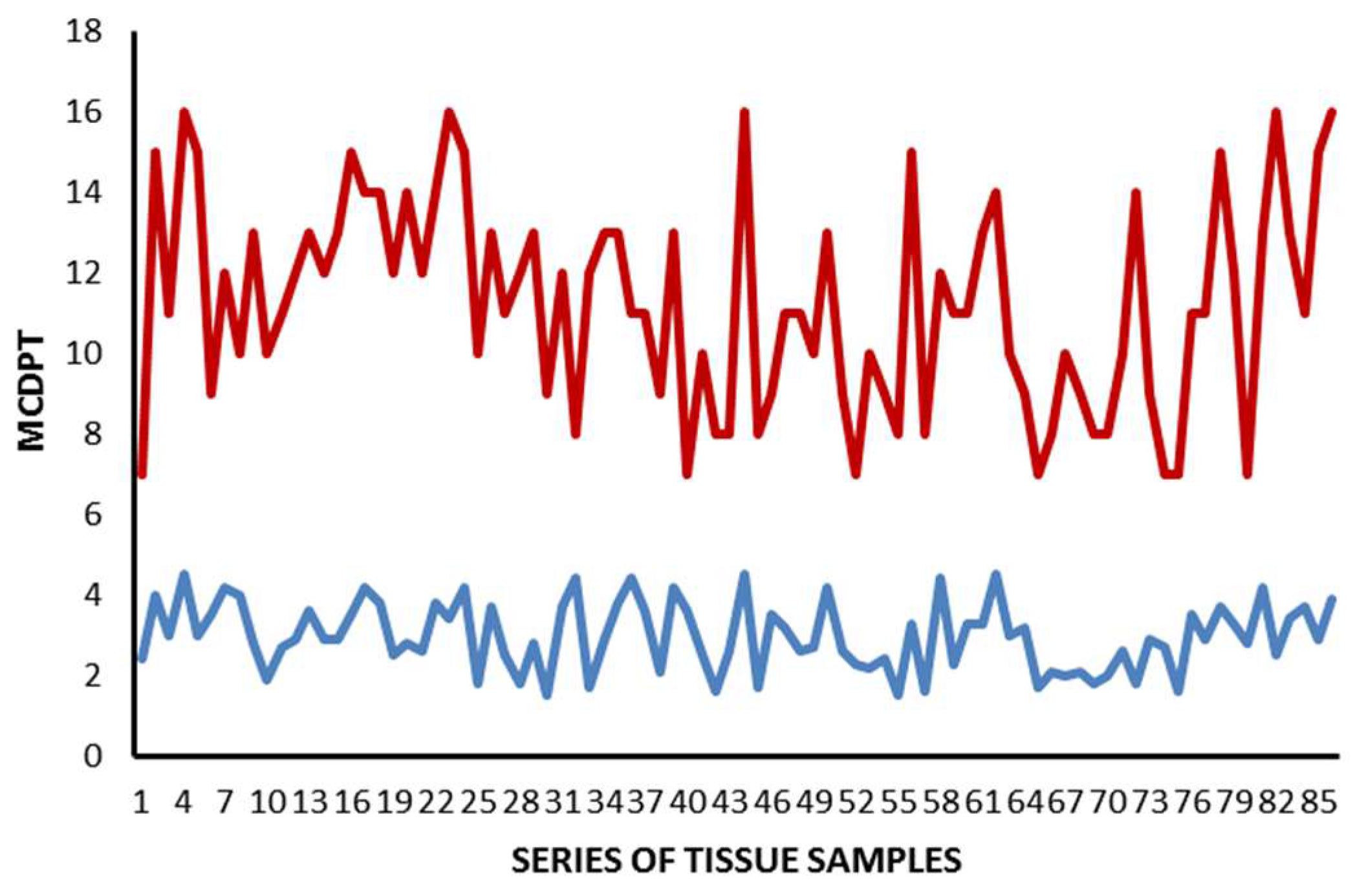
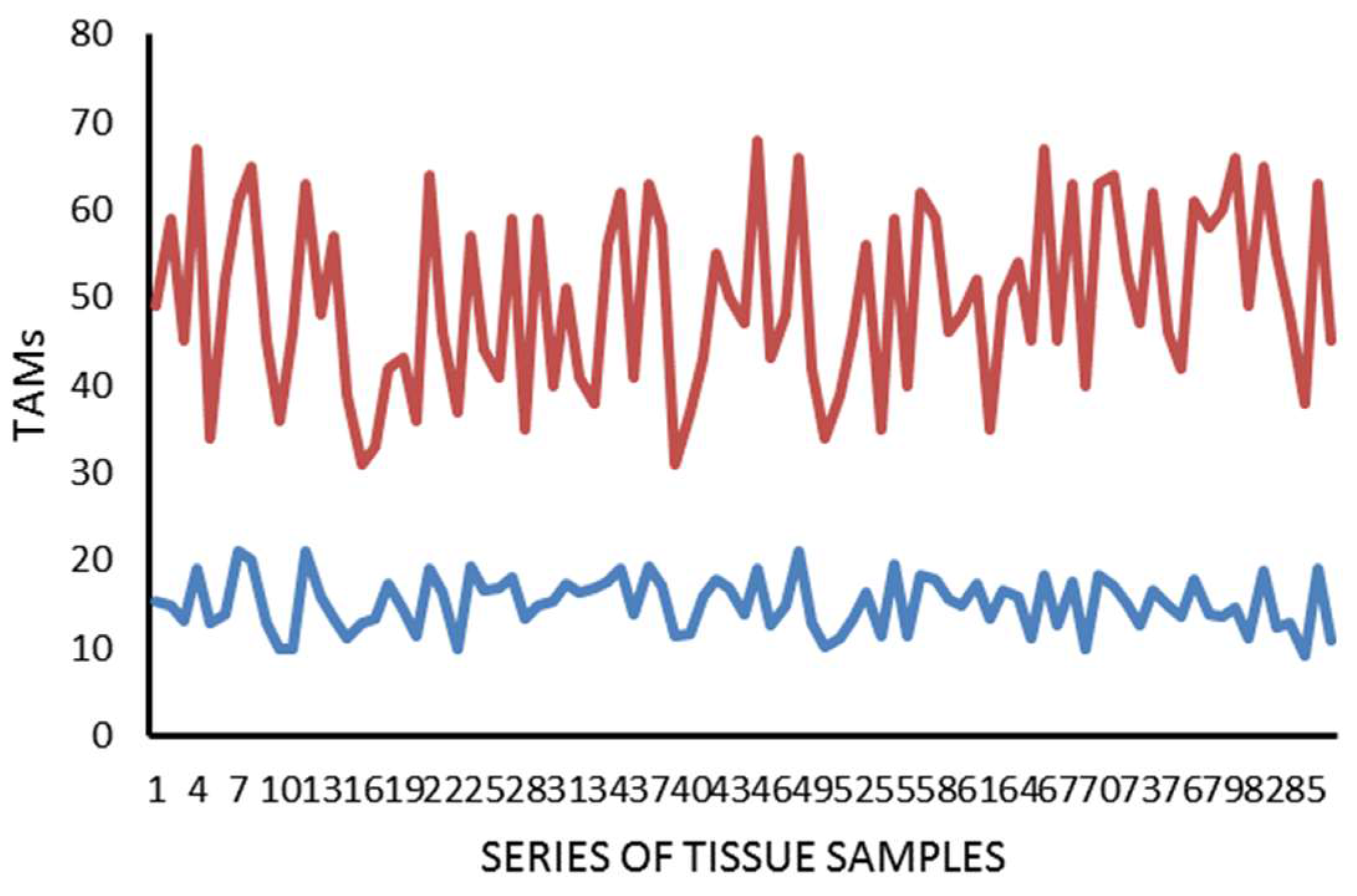
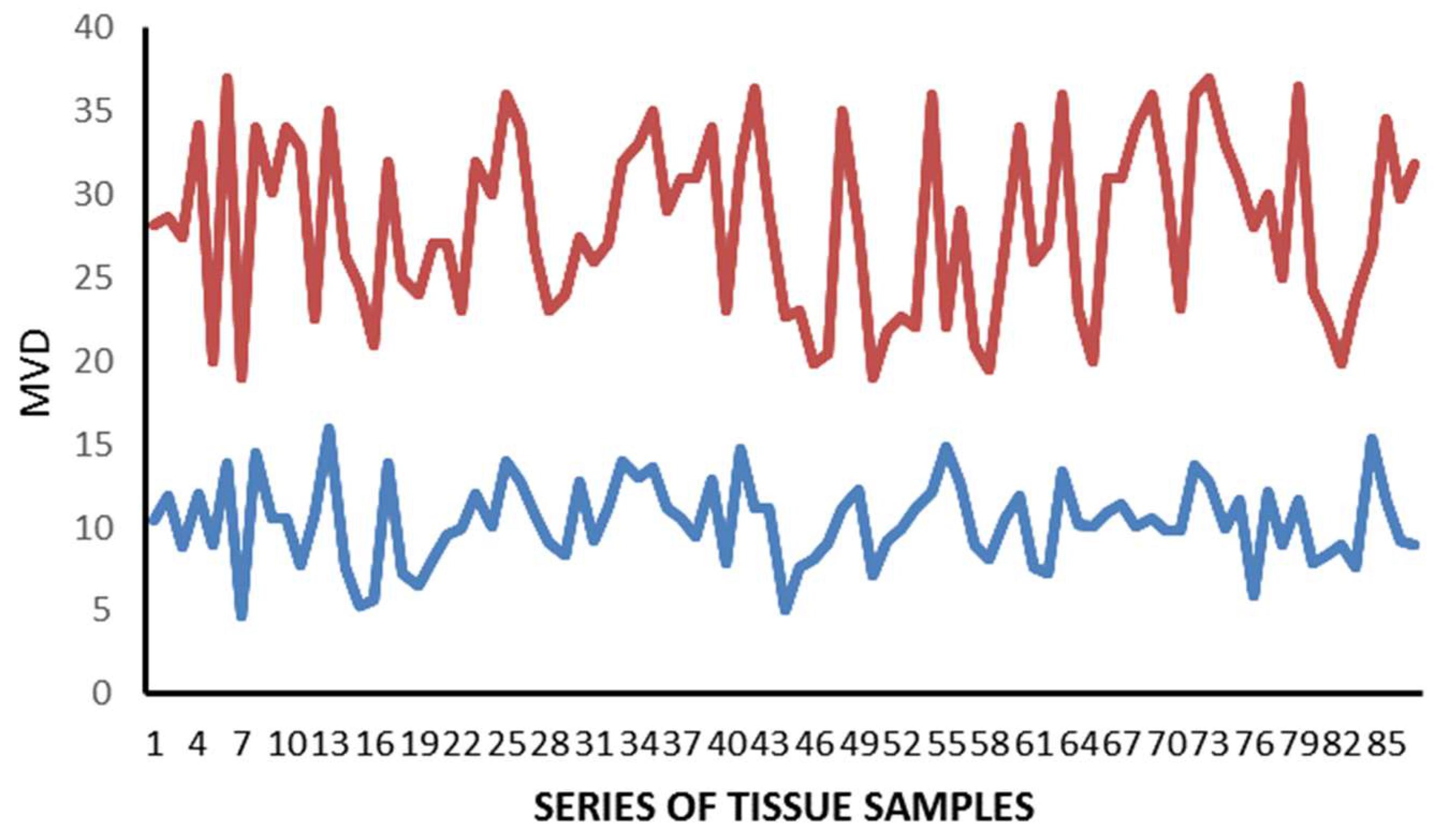
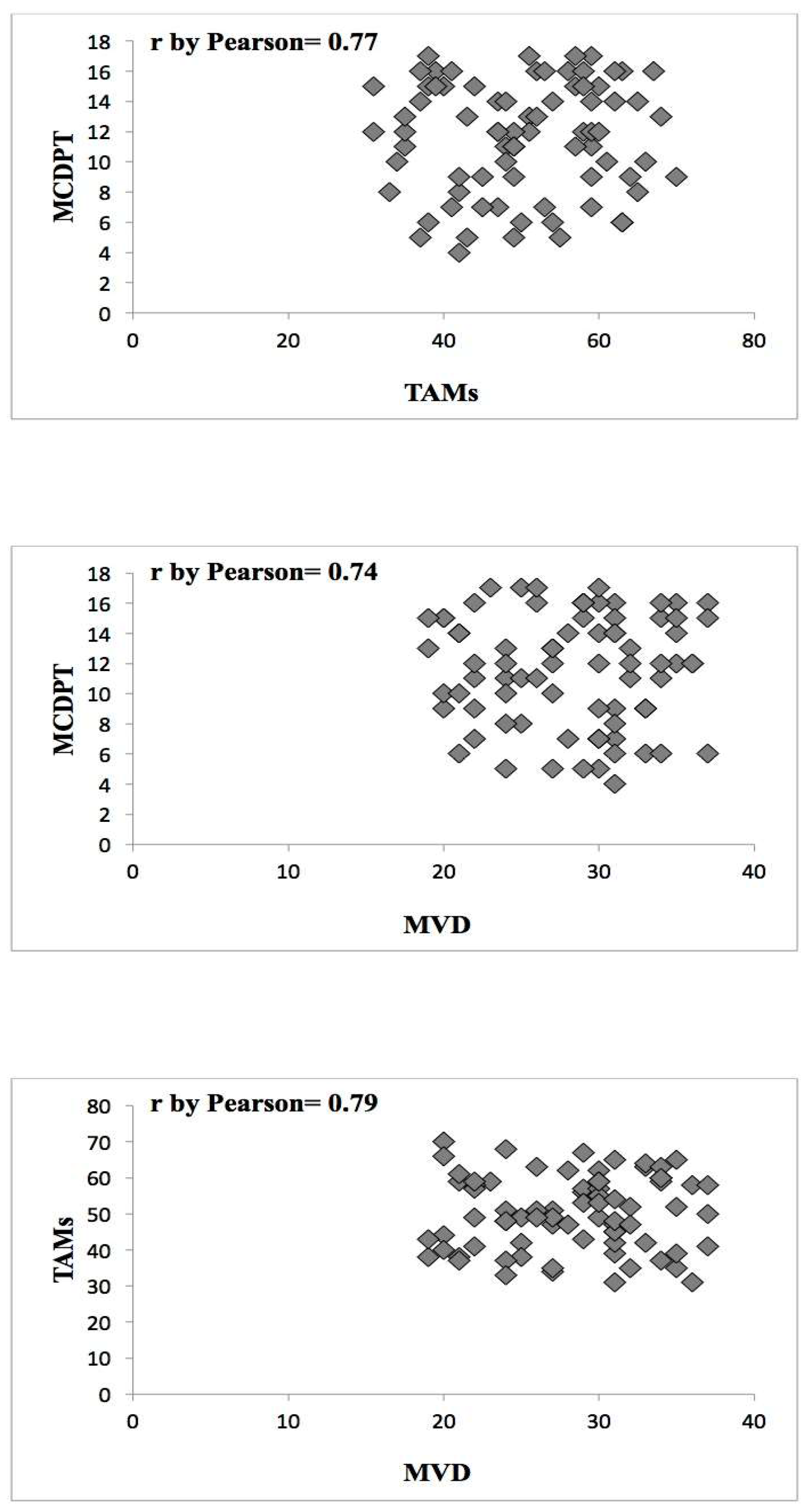
| Tissue | MCDPT ×400 Magnification (0.19 mm2 Area) | TAMs ×400 Magnification (0.19 mm2 Area) | MVD ×400 Magnification (0.19 mm2 Area) |
|---|---|---|---|
| TT | 11.38 ± 4.32 a | 49.17 ± 17.56 a | 28.12 ± 8.98 a |
| ANT | 2.98 ± 1.45 a | 15.34 ± 6.21 a | 10,39 ± 5.62 a |
| t-test | p = 0.001 | p = 0.001 | p = 0.002 |
| Variable | No. of Patients | No. of Tumors with High MCDPT a (%) | No. of Tumors with High MVD b (%) | No. of Tumors with High TAMs c (%) |
|---|---|---|---|---|
| Age years | 86 | |||
| ≤65 | 35 | 19 (54) | 18 (51) | 17 (49) |
| ≥65 | 51 | 25 (49) | 24 (47) | 26 (51) |
| Gender | ||||
| ►Male | 52 | 25 (48) | 24 (46) | 27 (52) |
| ►Female | 34 | 17 (50) | 18 (53) | 16 (47) |
| Tumor site | ||||
| ►Cardia, Lesser and Greater curvature | 31 | 17 (55) | 15 (48) | 16 (52) |
| ►Body and fundus | 26 | 13 (50) | 14 (54) | 13 (50) |
| ►Pyloric area | 29 | 14 (48) | 15 (52) | 14 (48) |
| TNM by AJCC Stage | ||||
| ►T2–3N2M0 | 52 | 24 (46) | 28 (54) | 27 (52) |
| ►T2–3N3M0 | 34 | 18 (53) | 16 (47) | 17 (50) |
| Tumor type by Lauren Classification | ||||
| ►Intestinal type | 55 | 26 (47) | 27 (49) | 29 (53) |
| ►Diffuse type | 31 | 15 (48) | 17 (55) | 16 (52) |
| Histologic grade | ||||
| ►G1–G2 | 42 | 22 (52) | 21 (50) | 23 (55) |
| ►G3 | 44 | 24 (54) | 21 (47) | 20 (45) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammarco, G.; Gadaleta, C.D.; Zuccalà, V.; Albayrak, E.; Patruno, R.; Milella, P.; Sacco, R.; Ammendola, M.; Ranieri, G. Tumor-Associated Macrophages and Mast Cells Positive to Tryptase Are Correlated with Angiogenesis in Surgically-Treated Gastric Cancer Patients. Int. J. Mol. Sci. 2018, 19, 1176. https://doi.org/10.3390/ijms19041176
Sammarco G, Gadaleta CD, Zuccalà V, Albayrak E, Patruno R, Milella P, Sacco R, Ammendola M, Ranieri G. Tumor-Associated Macrophages and Mast Cells Positive to Tryptase Are Correlated with Angiogenesis in Surgically-Treated Gastric Cancer Patients. International Journal of Molecular Sciences. 2018; 19(4):1176. https://doi.org/10.3390/ijms19041176
Chicago/Turabian StyleSammarco, Giuseppe, Cosmo Damiano Gadaleta, Valeria Zuccalà, Emre Albayrak, Rosa Patruno, Pietro Milella, Rosario Sacco, Michele Ammendola, and Girolamo Ranieri. 2018. "Tumor-Associated Macrophages and Mast Cells Positive to Tryptase Are Correlated with Angiogenesis in Surgically-Treated Gastric Cancer Patients" International Journal of Molecular Sciences 19, no. 4: 1176. https://doi.org/10.3390/ijms19041176
APA StyleSammarco, G., Gadaleta, C. D., Zuccalà, V., Albayrak, E., Patruno, R., Milella, P., Sacco, R., Ammendola, M., & Ranieri, G. (2018). Tumor-Associated Macrophages and Mast Cells Positive to Tryptase Are Correlated with Angiogenesis in Surgically-Treated Gastric Cancer Patients. International Journal of Molecular Sciences, 19(4), 1176. https://doi.org/10.3390/ijms19041176






