Triclosan Lacks (Anti-)Estrogenic Effects in Zebrafish Cells but Modulates Estrogen Response in Zebrafish Embryos
Abstract
1. Introduction
2. Results
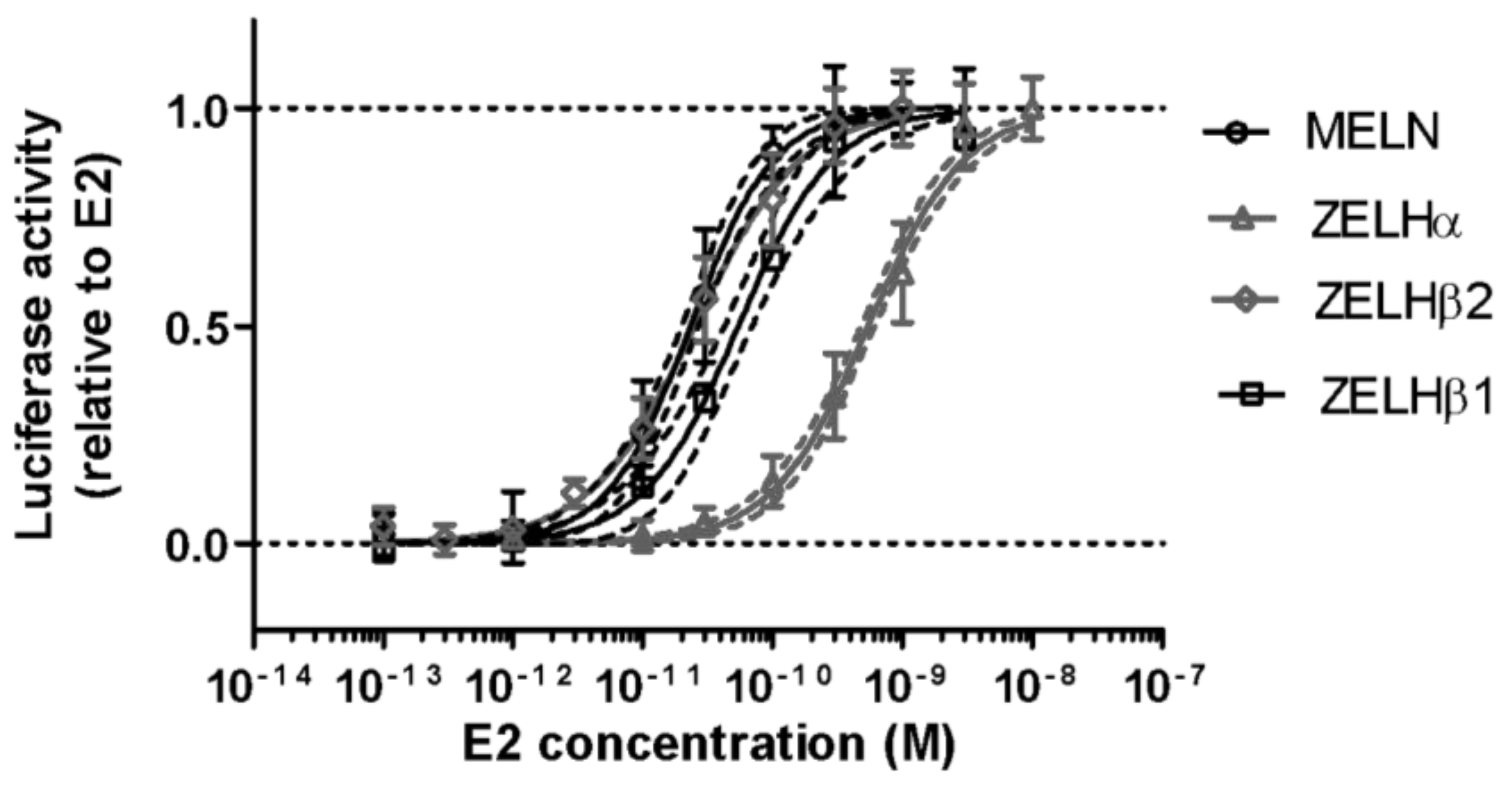
2.1. Triclosan Does Not Alter ZfER Transactivation In Vitro
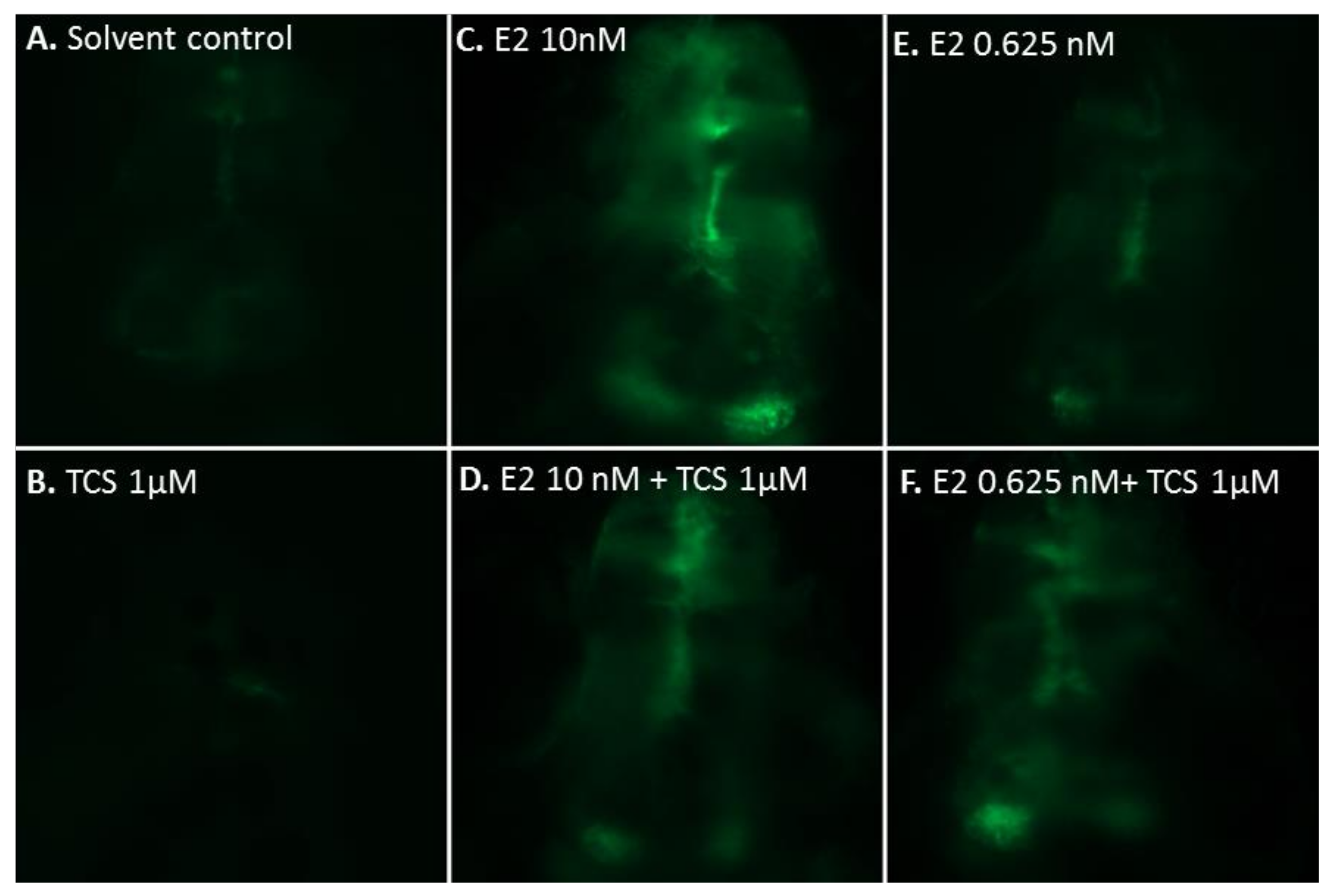
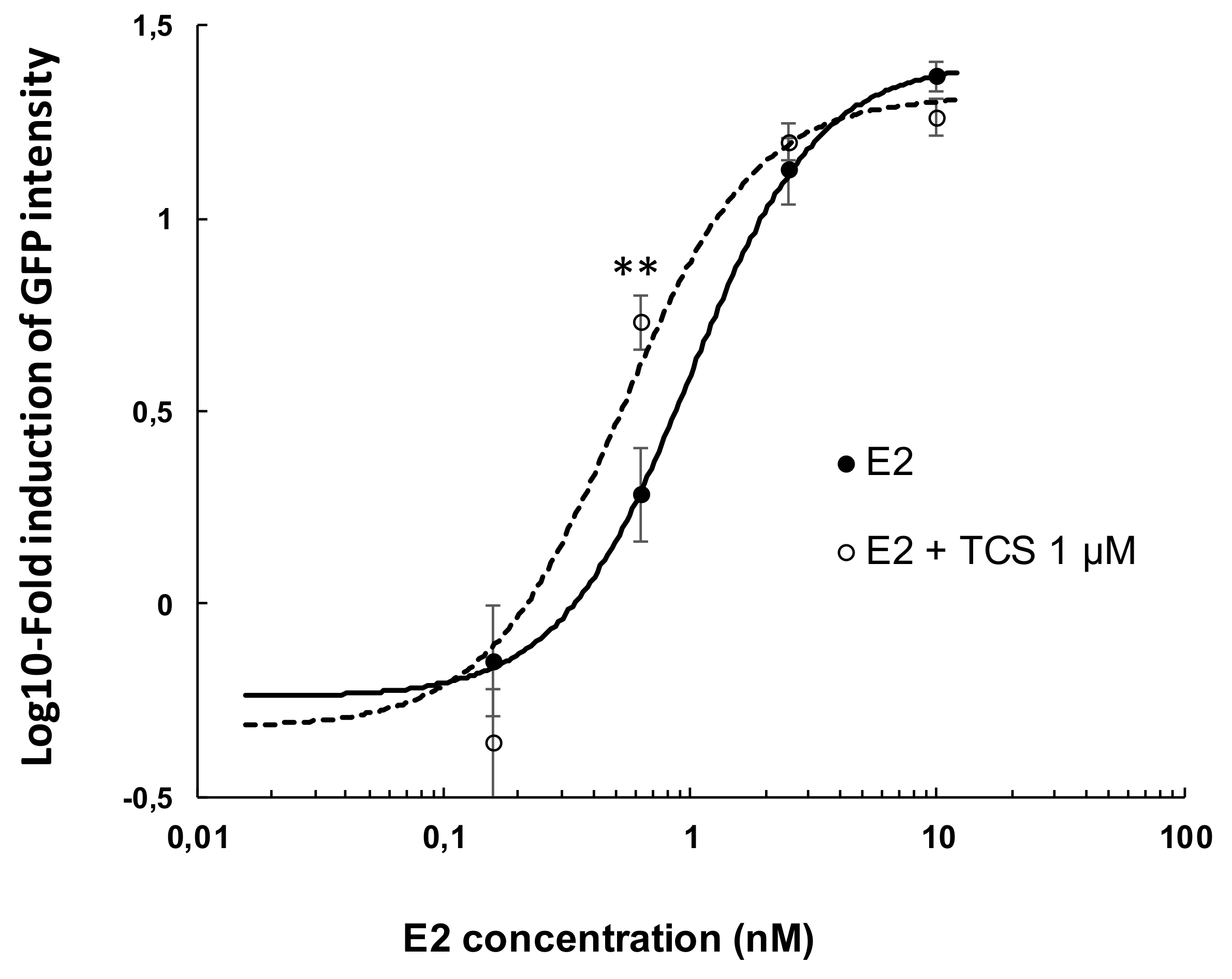
2.2. Effect of Triclosan on Brain Aromatase Expression Using the Cyp19a1b-GFP Transgenic Zebrafish Embryo Assay (EASZY Assay)
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. In Vitro Assays: Cell Culture, Luciferase and Cell Viability Assays
4.3. In Vivo Zebrafish Bioassays
4.4. Data Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- SCCS (Scientific Committee on Consumer Safety). Opinion on Triclosan (Antimicrobial Resistance); SCCS: Brussels, Belgium, 2010. [Google Scholar]
- Dann, A.B.; Hontela, A. Triclosan: Environmental exposure, toxicity and mechanisms of action. J. Appl. Toxicol. 2011, 31, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Loos, R.; Carvalho, R.; Antonio, D.C.; Cornero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.M.; Bartell, S.E.; Schoenfuss, H.L. Effects of Triclosan and Triclocarban, Two Ubiquitous Environmental Contaminants, on Anatomy, Physiology, and Behavior of the Fathead Minnow (Pimephales promelas). Arch. Environ. Contam. Toxicol. 2012, 63, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Foran, C.M.; Bennett, E.R.; Benson, W.H. Developmental evaluation of a potential non-steroidal estrogen: Triclosan. Mar. Environ. Res. 2000, 50, 153–156. [Google Scholar] [CrossRef]
- Raut, S.A.; Angus, R.A. Triclosan has endocrine-disrupting effects in male western mosquitofish, Gambusia affinis. Environ. Toxicol. Chem. 2010, 29, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, H.; Matsumura, N.; Hirano, M.; Matsuoka, M.; Shiratsuchi, H.; Ishibashi, Y.; Takao, Y.; Arizono, K. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat. Toxicol. 2004, 67, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Yamagishi, T.; Takahashi, H.; Iguchi, T.; Tatarazako, N. Effects of triclosan on Japanese medaka (Oryzias latipes) during embryo development, early life stage and reproduction. J. Appl. Toxicol. 2018, 38, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Du, G.Z.; Zhang, W.; Hu, J.L.; Wu, D.; Song, L.; Xia, Y.K.; Wang, X.R. The in vitro estrogenic activities of triclosan and triclocarban. J. Appl. Toxicol. 2014, 34, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Henry, N.D.; Fair, P.A. Comparison of in vitro cytotoxicity, estrogenicity and anti-estrogenicity of triclosan, perfluorooctane sulfonate and perfluorooctanoic acid. J. Appl. Toxicol. 2013, 33, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.C.; Zhao, B.; Chen, J.; Cherednichenko, G.; Sanmarti, E.; Denison, M.S.; Lasley, B.; Pessah, I.N.; Kultz, D.; Chang, D.P.Y.; et al. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 2008, 116, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Gee, R.H.; Charles, A.; Taylor, N.; Darbre, P.D. Oestrogenic and androgenic activity of triclosan in breast cancer cells. J. Appl. Toxicol. 2008, 28, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Tarnow, P.; Tralau, T.; Hunecke, D.; Luch, A. Effects of triclocarban on the transcription of estrogen, androgen and aryl hydrocarbon receptor responsive genes in human breast cancer cells. Toxicol. Vitro 2013, 27, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Lange, A.; Hirakawa, I.; Tohyama, S.; Ogino, Y.; Mizutani, T.; Kagami, Y.; Kusano, T.; Ihara, M.; Tanaka, H.; et al. Differing Species Responsiveness of Estrogenic Contaminants in Fish Is Conferred by the Ligand Binding Domain of the Estrogen Receptor. Environ. Sci. Technol. 2014, 48, 5254–5263. [Google Scholar] [CrossRef] [PubMed]
- Le Fol, V.; Ait-Aissa, S.; Sonavane, M.; Porcher, J.M.; Balaguer, P.; Cravedi, J.P.; Zalko, D.; Brion, F. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol. Environ. Saf. 2017, 142, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, M.; Creusot, N.; Maillot-Marechal, E.; Pery, A.; Brion, F.; Ait-Aissa, S. Zebrafish-based reporter gene assays reveal different estrogenic activities in river waters compared to a conventional human-derived assay. Sci. Total Environ. 2016, 550, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Cosnefroy, A.; Brion, F.; Maillot-Marechal, E.; Porcher, J.M.; Pakdel, F.; Balaguer, P.; Ait-Aissa, S. Selective Activation of Zebrafish Estrogen Receptor Subtypes by Chemicals by Using Stable Reporter Gene Assay Developed in a Zebrafish Liver Cell Line. Toxicol. Sci. 2012, 125, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, P.; Francois, F.; Comunale, F.; Fenet, H.; Boussioux, A.M.; Pons, M.; Nicolas, J.C.; Casellas, C. Reporter cell lines to study the estrogenic effects of xenoestrogens. Sci. Total Environ. 1999, 233, 47–56. [Google Scholar] [CrossRef]
- Brion, F.; Le Page, Y.; Piccini, B.; Cardoso, O.; Tong, S.K.; Chung, B.C.; Kah, O. Screening Estrogenic Activities of Chemicals or Mixtures In vivo Using Transgenic (cyp19a1b-GFP) Zebrafish Embryos. PLoS ONE 2012, 7, e36069. [Google Scholar] [CrossRef] [PubMed]
- Louis, G.W.; Hallinger, D.R.; Stoker, T.E. The effect of triclosan on the uterotrophic response to extended doses of ethinyl estradiol in the weanling rat. Reprod. Toxicol. 2013, 36, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Gonzales, E.L.T.; Yang, S.M.; Bang, M.J.; Choi, C.S.; Shin, C.Y. Effects of Triclosan on Neural Stem Cell Viability and Survival. Biomol. Ther. 2016, 24, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Sitarz, A.M.; Wojtowicz, A.K. Triclosan induces fas receptor-dependent apoptosis in mouse neocrotical neurons in vitro. Neuroscience 2015, 284, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Xu, B.; Han, X.M.; Mao, Z.L.; Chen, M.J.; Du, G.Z.; Talbot, P.; Wang, X.R.; Xia, Y.K. The effects of triclosan on pluripotency factors and development of mouse embryonic stem cells and zebrafish. Arch. Toxicol. 2015, 89, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Q.; Wang, Y.Q.; Fillgrove, K.L.; Anderson, V.E. Triclosan inhibits enoyl-reductase of type I fatty acid synthase in vitro and is cytotoxic to MCF-7 and SKBr-3 breast cancer cells. Cancer Chemother. Pharmacol. 2002, 49, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Le Page, Y.; Menuet, A.; Kah, O.; Pakdel, F. Characterization of a cis-acting element involved in cell-specific expression of the zebrafish brain aromatase gene. Mol. Reprod. Dev. 2008, 75, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Menuet, A.; Pellegrini, E.; Brion, F.; Gueguen, M.M.; Anglade, I.; Pakdel, F.; Kah, O. Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene. J. Comp. Neurol. 2005, 485, 304–320. [Google Scholar] [CrossRef] [PubMed]
- Cano-Nicolau, J.; Garoche, C.; Hinfray, N.; Pellegrini, E.; Boujrad, N.; Pakdel, F.; Kah, O.; Brion, F. Several synthetic progestins disrupt the glial cell specific-brain aromatase expression in developing zebra fish. Toxicol. Appl. Pharmacol. 2016, 305, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Neale, P.A.; Altenburger, R.; Ait-Aissa, S.; Brion, F.; Busch, W.; Umbuzeiro, G.d.A.; Denison, M.S.; Du Pasquier, D.; Hilscherova, K.; Hollert, H.; et al. Development of a bioanalytical test battery for water quality monitoring: Fingerprinting identified micropollutants and their Contribution to effects in surface water. Water Res. 2017, 123, 734–750. [Google Scholar] [CrossRef] [PubMed]
- Pinto, P.I.S.; Guerreiro, E.M.; Power, D.M. Triclosan interferes with the thyroid axis in the zebrafish (Danio rerio). Toxicol. Res. 2013, 2, 60–69. [Google Scholar] [CrossRef]
- Schnitzler, J.G.; Frederich, B.; Dussenne, M.; Klaren, P.H.M.; Silvestre, F.; Das, K. Triclosan exposure results in alterations of thyroid hormone status and retarded early development and metamorphosis in Cyprinodon variegatus. Aquat. Toxicol. 2016, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, I. Thyroid hormones and the central nervous system of mammals (Review). Mol. Med. Rep. 2008, 1, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Noda, M. Possible role of glial cells in the relationship between thyroid dysfunction and mental disorders. Front. Cell. Neurosci. 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.E.; Gibson, E.K.; Zorrilla, L.M. Triclosan Exposure Modulates Estrogen-Dependent Responses in the Female Wistar Rat. Toxicol. Sci. 2010, 117, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guo, X.; Chen, W.; Sun, Y.; Fan, C. Effects of triclosan on hormones and reproductive axis in female Yellow River carp (Cyprinus carpio): Potential mechanisms underlying estrogen effect. Toxicol. Appl. Pharmacol. 2017, 336, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, F.; Chen, W.; Xu, R.; Wang, W. Effects of triclosan (TCS) on hormonal balance and genes of hypothalamus-pituitary-gonad axis of juvenile male Yellow River carp (Cyprinus carpio). Chemosphere 2018, 193, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival —Application to proliferation and cyto-toxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tong, S.K.; Mouriec, K.; Kuo, M.W.; Pellegrini, E.; Gueguen, M.M.; Brion, F.; Kah, O.; Chung, B.C. A cyp19a1b-GFP (Aromatase B) Transgenic Zebrafish Line That Expresses GFP in Radial Glial Cells. Genesis 2009, 47, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Vindimian, E. REGTOX: Macro ExcelTM Pour Dose-Réponse. Available online: http://www.normalesup.org/~vindimian/fr_index.html (accessed on 29 January 2018).


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, H.; Brion, F.; Porcher, J.-M.; Budzinski, H.; Aït-Aïssa, S. Triclosan Lacks (Anti-)Estrogenic Effects in Zebrafish Cells but Modulates Estrogen Response in Zebrafish Embryos. Int. J. Mol. Sci. 2018, 19, 1175. https://doi.org/10.3390/ijms19041175
Serra H, Brion F, Porcher J-M, Budzinski H, Aït-Aïssa S. Triclosan Lacks (Anti-)Estrogenic Effects in Zebrafish Cells but Modulates Estrogen Response in Zebrafish Embryos. International Journal of Molecular Sciences. 2018; 19(4):1175. https://doi.org/10.3390/ijms19041175
Chicago/Turabian StyleSerra, Hélène, François Brion, Jean-Marc Porcher, Hélène Budzinski, and Selim Aït-Aïssa. 2018. "Triclosan Lacks (Anti-)Estrogenic Effects in Zebrafish Cells but Modulates Estrogen Response in Zebrafish Embryos" International Journal of Molecular Sciences 19, no. 4: 1175. https://doi.org/10.3390/ijms19041175
APA StyleSerra, H., Brion, F., Porcher, J.-M., Budzinski, H., & Aït-Aïssa, S. (2018). Triclosan Lacks (Anti-)Estrogenic Effects in Zebrafish Cells but Modulates Estrogen Response in Zebrafish Embryos. International Journal of Molecular Sciences, 19(4), 1175. https://doi.org/10.3390/ijms19041175




