Glucose-Dependent Insulinotropic Polypeptide Mitigates 6-OHDA-Induced Behavioral Impairments in Parkinsonian Rats
Abstract
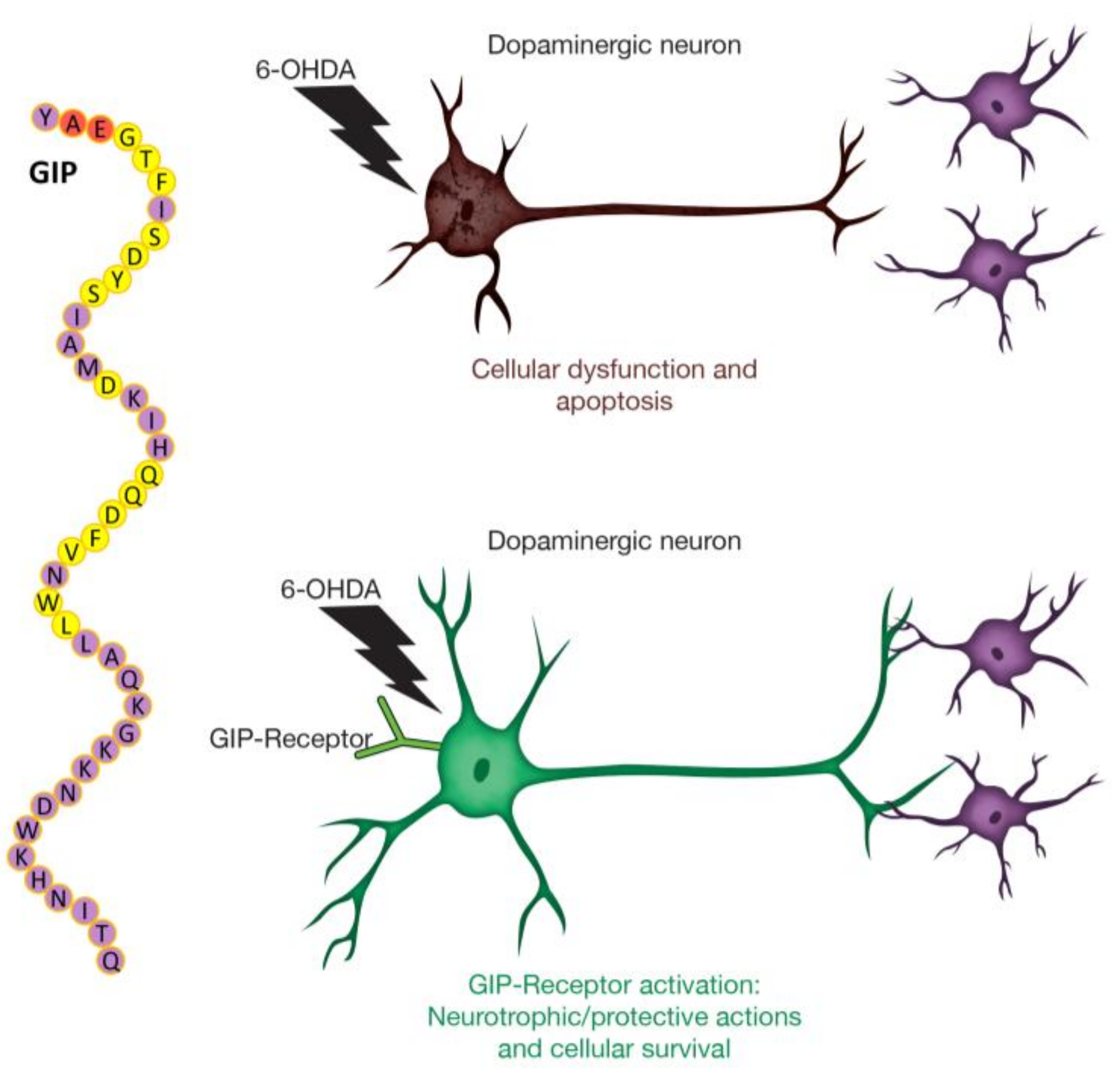
1. Introduction
2. Results
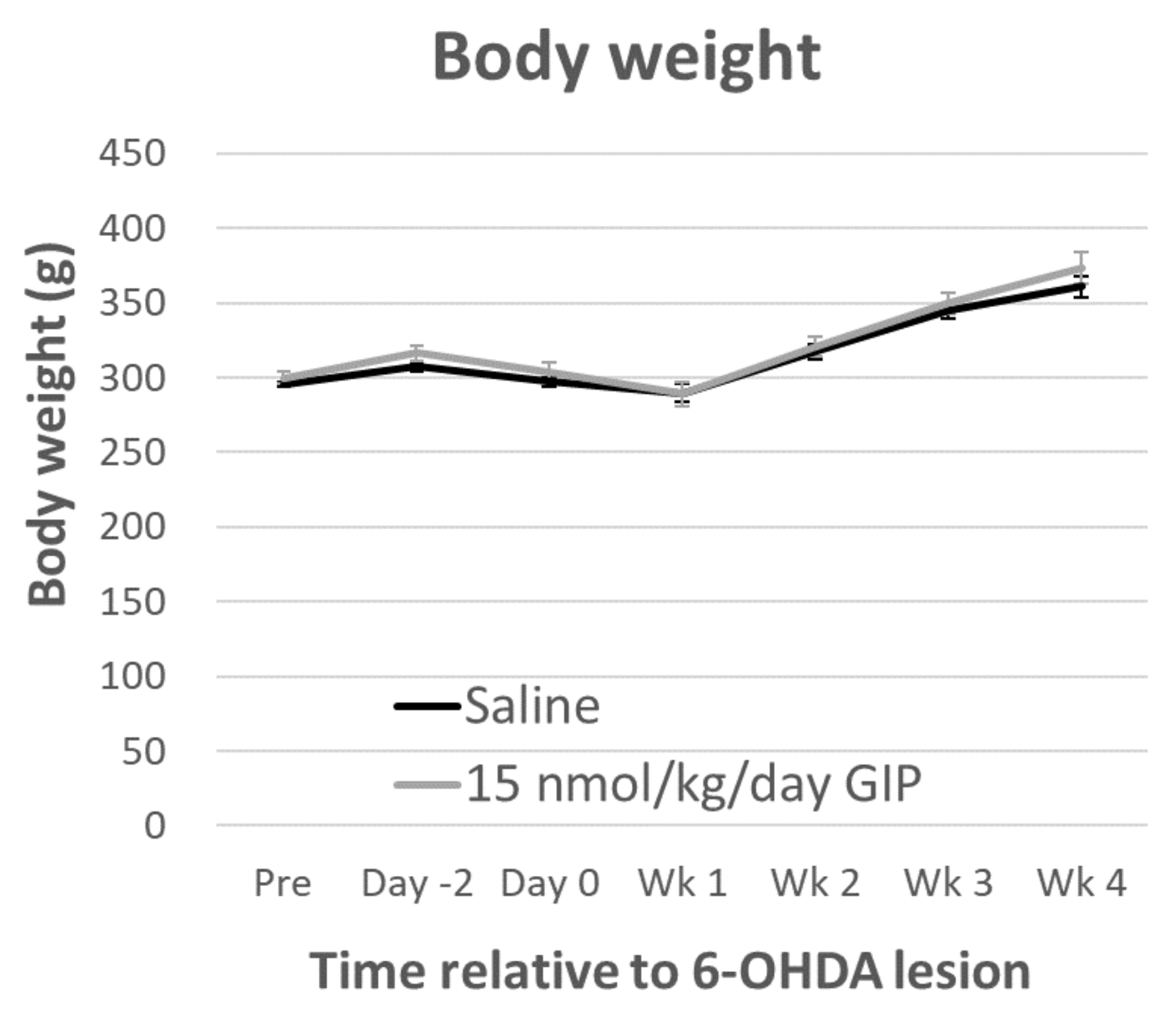
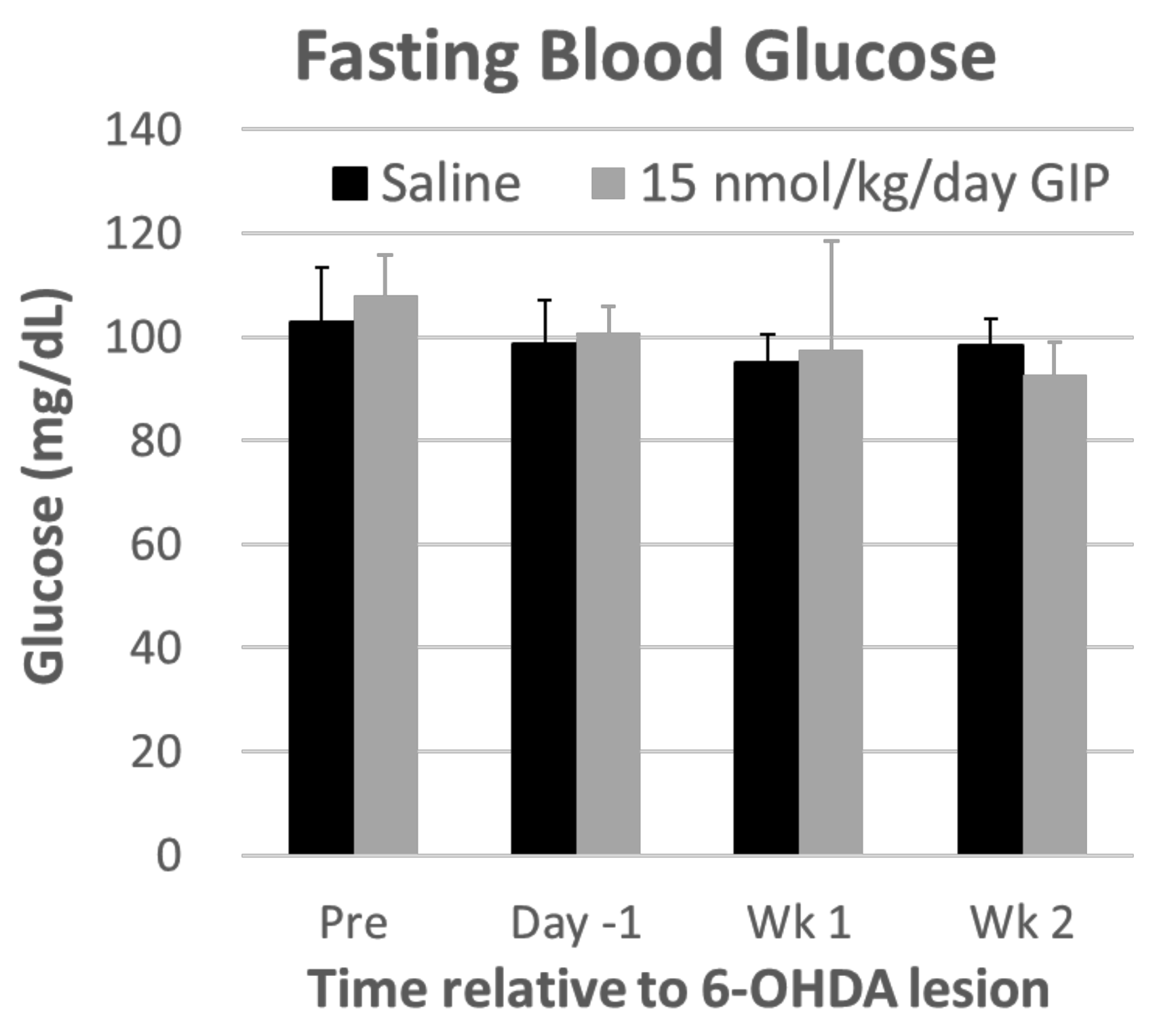
2.1. Body Weight and Fasting Blood Glucose Levels Are Not Affected by GIP Treatment
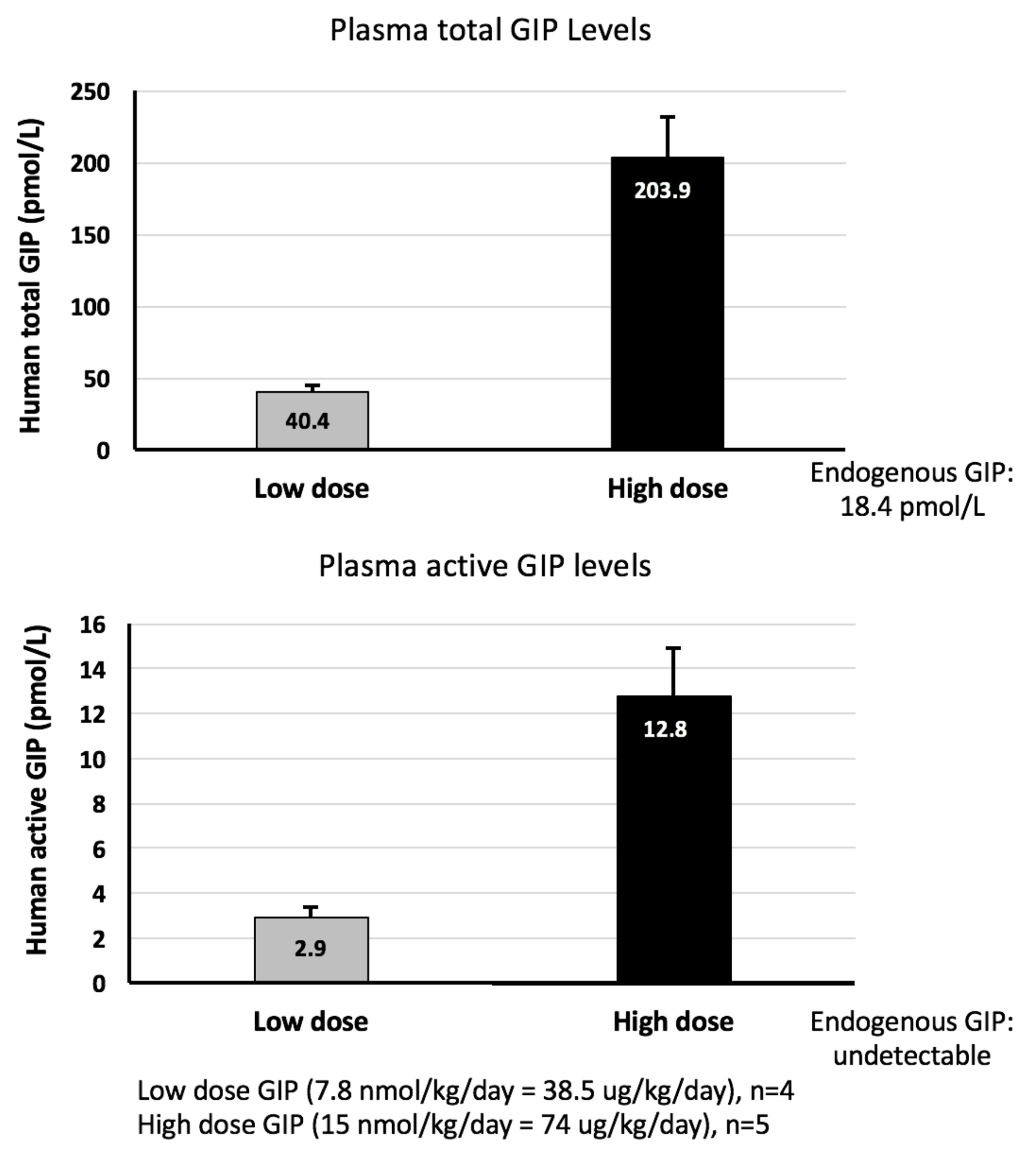
2.2. GIP Plasma Concentrations Are Significantly Elevated Following Steady-State Subcutaneous GIP Administration
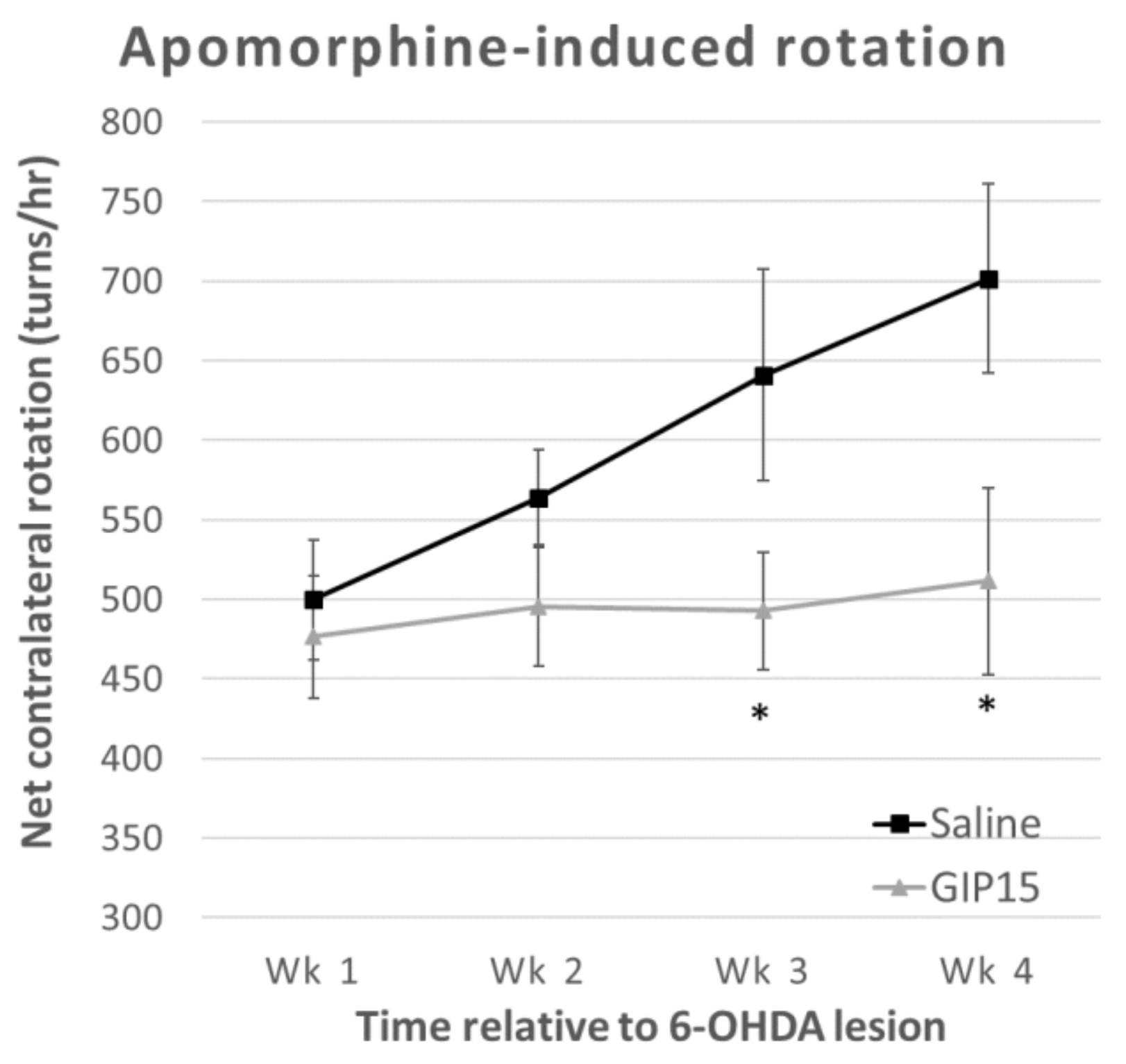
2.3. GIP Reduces the Effects of Progressive Dopaminergic Pathway Dysfunction after 6-OHDA-Lesions
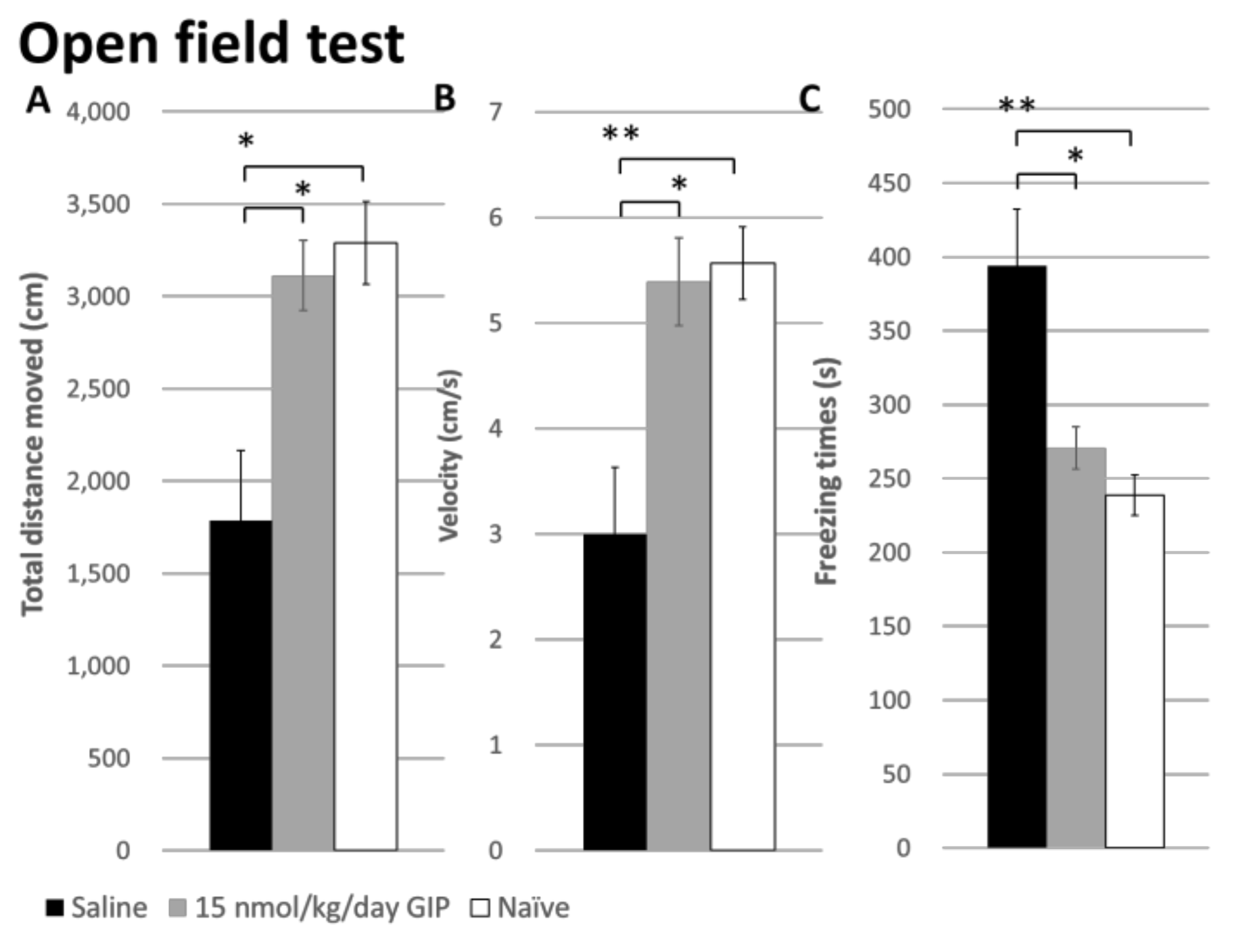
2.4. The Effect of GIP on Changes in Open Field Behavior after a 6-OHDA-Lesion
3. Discussion
4. Materials and Methods
4.1. Animal Handling and Preparation
4.2. Treatment Groups
4.3. Body Weight
4.4. Fasting Blood Glucose
4.5. GIP Plasma Assay
4.6. Apomorphine-Induced Rotation
4.7. Open-Field Tests
4.8. Overview of Experimental Design and Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PD | Parkinson’s Disease |
| 6-OHDA | 6-Hydroxydopamine |
| TH | Tyrosine Hydroxylase |
| DA | Dopaminergic |
| SN | Substantia Nigra |
| MFB | Medial Forebrain Bundle |
References
- Goetz, C.G. The history of Parkinson’s disease: Early clinical descriptions and neurological therapies. Cold Spring Harb. Perspect. Med. 2011, 1, a008862. [Google Scholar] [PubMed]
- Lusis, S.A. Pathophysiology and management of idiopathic Parkinson’s disease. J. Neurosci. Nurs. 1997, 29, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Maetzler, W. Comment: Why do nondopaminergic features in Parkinson disease matter? Neurology 2014, 82, 417. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, I.; Tweedie, D.; Li, Y.; Greig, N.H. Neuroprotective and neurotrophic actions of glucagon-like peptide-1: An emerging opportunity to treat neurodegenerative and cerebrovascular disorders. Br. J. Pharmacol. 2012, 166, 1586–1599. [Google Scholar] [PubMed]
- Greig, N.H.; Tweedie, D.; Rachmany, L.; Li, Y.; Rubovitch, V.; Schreiber, S.; Chiang, Y.H.; Hoffer, B.J.; Miller, J.; Lahiri, D.K.; et al. Incretin mimetics as pharmacologic tools to elucidate and as a new drug strategy to treat traumatic brain injury. Alzheimers Dement. 2014, 10 (Suppl. 1), S62–S75. [Google Scholar] [CrossRef] [PubMed]
- Holscher, C. The incretin hormones glucagonlike peptide 1 and glucose-dependent insulinotropic polypeptide are neuroprotective in mouse models of Alzheimer’s disease. Alzheimers Dement. 2014, 10 (Suppl. 1), S47–S54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Perry, T.; Kindy, M.S.; Harvey, B.K.; Tweedie, D.; Holloway, H.W.; Powers, K.; Shen, H.; Egan, J.M.; Sambamurti, K.; et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. USA 2009, 106, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.; Haughey, N.J.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J. Pharmacol. Exp. Ther. 2002, 302, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.; Lahiri, D.K.; Chen, D.; Zhou, J.; Shaw, K.T.; Egan, J.M.; Greig, N.H. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J. Pharmacol. Exp. Ther. 2002, 300, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.; Lahiri, D.K.; Sambamurti, K.; Chen, D.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Aβ) levels and protects hippocampal neurons from death induced by Aβ and iron. J. Neurosci. Res. 2003, 72, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Bertilsson, G.; Patrone, C.; Zachrisson, O.; Andersson, A.; Dannaeus, K.; Heidrich, J.; Kortesmaa, J.; Mercer, A.; Nielsen, E.; Ronnholm, H.; et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J. Neurosci. Res. 2008, 86, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Harkavyi, A.; Abuirmeileh, A.; Lever, R.; Kingsbury, A.E.; Biggs, C.S.; Whitton, P.S. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J. Neuroinflamm. 2008, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Rachmany, L.; Tweedie, D.; Li, Y.; Rubovitch, V.; Holloway, H.W.; Miller, J.; Hoffer, B.J.; Greig, N.H.; Pick, C.G. Exendin-4 induced glucagon-like peptide-1 receptor activation reverses behavioral impairments of mild traumatic brain injury in mice. Age (Dordr) 2013, 35, 1621–1636. [Google Scholar] [CrossRef] [PubMed]
- Tweedie, D.; Rachmany, L.; Rubovitch, V.; Lehrmann, E.; Zhang, Y.; Becker, K.G.; Perez, E.; Miller, J.; Hoffer, B.J.; Greig, N.H.; et al. Exendin-4, a glucagon-like peptide-1 receptor agonist prevents mTBI-induced changes in hippocampus gene expression and memory deficits in mice. Exp. Neurol. 2013, 239, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Foltynie, T. Protective effects of the GLP-1 mimetic exendin-4 in Parkinson’s disease. Neuropharmacology 2017. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J.; et al. Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1664–1675. [Google Scholar] [CrossRef]
- Campbell, J.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013, 17, 819–837. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Bloom, S. Gut hormones as therapeutic agents in treatment of diabetes and obesity. Curr. Opin. Pharmacol. 2013, 13, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Rayner, C.K.; Horowitz, M. Incretins. Handb. Exp. Pharmacol. 2016, 233, 137–171. [Google Scholar] [PubMed]
- Gault, V.A.; Kerr, B.D.; Irwin, N.; Flatt, P.R. C-terminal mini-PEGylation of glucose-dependent insulinotropic polypeptide exhibits metabolic stability and improved glucose homeostasis in dietary-induced diabetes. Biochem. Pharmacol. 2008, 75, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Paratore, S.; Ciotti, M.T.; Basille, M.; Vaudry, D.; Gentile, A.; Parenti, R.; Calissano, P.; Cavallaro, S. Gastric inhibitory polypeptide and its receptor are expressed in the central nervous system and support neuronal survival. Cent. Nerv. Syst. Agents Med. Chem. 2011, 11, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Kastin, A.J.; Akerstrom, V.; Pan, W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J. Mol. Neurosci. 2002, 18, 7–14. [Google Scholar] [CrossRef]
- Yu, Y.W.; Hsieh, T.H.; Chen, K.Y.; Wu, J.C.; Hoffer, B.J.; Greig, N.H.; Li, Y.; Lai, J.H.; Chang, C.F.; Lin, J.W.; et al. Glucose-dependent insulinotropic polypeptide ameliorates mild traumatic brain injury-induced cognitive and sensorimotor deficits and neuroinflammation in rats. J. Neurotrauma 2016, 33, 2044–2054. [Google Scholar] [CrossRef] [PubMed]
- Herman, G.A.; Bergman, A.; Stevens, C.; Kotey, P.; Yi, B.; Zhao, P.; Dietrich, B.; Golor, G.; Schrodter, A.; Keymeulen, B.; et al. Effect of single oral doses of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on incretin and plasma glucose levels after an oral glucose tolerance test in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 4612–4619. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, T.; Fujita, Y.; Takeda, Y.; Honjo, J.; Sakagami, H.; Kitsunai, H.; Takiyama, Y.; Abiko, A.; Makino, Y.; Kieffer, T.J.; et al. Dipeptidyl peptidase-4 inhibitor treatment induces a greater increase in plasma levels of bioactive GIP than GLP-1 in non-diabetic subjects. Mol. Metab. 2017, 6, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Li, L.; Holscher, C. D-Ala2-GIP-glu-PAL is neuroprotective in a chronic Parkinson’s disease mouse model and increases BNDF expression while reducing neuroinflammation and lipid peroxidation. Eur. J. Pharmacol. 2017, 797, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.K.; Goel, R.; Nandakumar, K.; Nemmani, K.V.S. Effect of D-Ala(2)GIP, a stable GIP receptor agonist on MPTP-induced neuronal impairments in mice. Eur. J. Pharmacol. 2017, 804, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. The incretin effect in healthy individuals and those with type 2 diabetes: physiology, pathophysiology, and response to therapeutic interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Nauck, M.A.; Bartels, E.; Orskov, C.; Ebert, R.; Creutzfeldt, W. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J. Clin. Endocrinol. Metab. 1993, 76, 912–917. [Google Scholar] [PubMed]
- Elliott, R.M.; Morgan, L.M.; Tredger, J.A.; Deacon, S.; Wright, J.; Marks, V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J. Endocrinol. 1993, 138, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Heimesaat, M.M.; Orskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, C.P.; Pamplona, F.A.; Mazzuco, T.L.; Aguiar, A.S., Jr.; Walz, R.; Prediger, R.D. Role of the glucose-dependent insulinotropic polypeptide and its receptor in the central nervous system: therapeutic potential in neurological diseases. Behav. Pharmacol. 2010, 21, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Li, L.; Holscher, C. Neuroprotective effects of a GIP analgue in the MPTP Parkinson’s disease mouse model. Neuropharmacology 2016, 101, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, J.; Anderson, M.F.; Meister, B.; Alborn, A.M.; Strom, A.K.; Brederlau, A.; Illerskog, A.C.; Nilsson, O.; Kieffer, T.J.; Hietala, M.A.; et al. Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J. Neurosci. 2005, 25, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, J.; Jacobsson, C.; Anderson, M.F.; Eriksson, P.S. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. J. Neurosci. Res. 2007, 85, 2099–2119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, L.; Holscher, C. Incretin-based therapy for type 2 diabetes mellitus is promising for treating neurodegenerative diseases. Rev. Neurosci. 2016, 27, 689–711. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.L.; Drucker, D.J. Structure-function of the glucagon receptor family of G protein-coupled receptors: the glucagon, GIP, GLP-1, and GLP-2 receptors. Recept. Channels 2002, 8, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Perlow, M.J.; Freed, W.J.; Hoffer, B.J.; Seiger, A.; Olson, L.; Wyatt, R.J. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science 1979, 204, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.F.; Ungerstedt, U. Supersensitivity to apomorphine following destruction of the ascending dopamine neurons: quantification using the rotational model. Eur. J. Pharmacol. 1977, 41, 361–367. [Google Scholar] [CrossRef]
- Zeiss, C.J.; Allore, H.G.; Beck, A.P. Established patterns of animal study design undermine translation of disease-modifying therapies for Parkinson’s disease. PLoS ONE 2017, 12, e0171790. [Google Scholar] [CrossRef] [PubMed]
- Mohs, R.C.; Greig, N.H. Drug discovery and development: Role of basic biological research. Alzheimers Dement. (NY) 2017, 3, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Asmar, M.; Tangaa, W.; Madsbad, S.; Hare, K.; Astrup, A.; Flint, A.; Bulow, J.; Holst, J.J. On the role of glucose-dependent insulintropic polypeptide in postprandial metabolism in humans. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E614–E621. [Google Scholar] [CrossRef] [PubMed]
- Gault, V.A.; Holscher, C. Protease-resistant glucose-dependent insulinotropic polypeptide agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by β-amyloid. J. Neurophysiol. 2008, 99, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, I.A.; Bader, M.; Li, Y.; Yu, S.J.; Wang, Y.; Talbot, K.; DiMarchi, R.D.; Pick, C.G.; Greig, N.H. Novel GLP-1R/GIPR co-agonist “twincretin” is neuroprotective in cell and rodent models of mild traumatic brain injury. Exp. Neurol. 2017, 288, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Choi, H.I.; Wang, Y.; Luo, Y.; Hoffer, B.J.; Greig, N.H. A New Treatment Strategy for Parkinson’s Disease through the Gut-Brain Axis: The Glucagon-Like Peptide-1 Receptor Pathway. Cell. Transplant. 2017, 26, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.E.; Miller, L.J.; Bataille, D.; Dalle, S.; Goke, B.; Thorens, B.; Drucker, D.J. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 2003, 55, 167–194. [Google Scholar] [CrossRef] [PubMed]
- Schelshorn, D.; Joly, F.; Mutel, S.; Hampe, C.; Breton, B.; Mutel, V.; Lutjens, R. Lateral allosterism in the glucagon receptor family: Glucagon-like peptide 1 induces G-protein-coupled receptor heteromer formation. Mol. Pharmacol. 2012, 81, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Finan, B.; Ma, T.; Ottaway, N.; Muller, T.D.; Habegger, K.M.; Heppner, K.M.; Kirchner, H.; Holland, J.; Hembree, J.; Raver, C.; et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 2013, 5, 209ra151. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Holscher, C.; Xue, G.F.; Li, G.; Li, D. A novel dual-glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide receptor agonist is neuroprotective in transient focal cerebral ischemia in the rat. Neuroreport 2016, 27, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, D.; Feng, P.; Xue, G.; Ji, C.; Li, G.; Holscher, C. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson’s disease. Eur. J. Pharmacol. 2017, 812, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Xue, G.F.; Lijun, C.; Feng, P.; Li, D.; Li, L.; Li, G.; Holscher, C. A novel dual GLP-1 and GIP receptor agonist is neuroprotective in the MPTP mouse model of Parkinson’s disease by increasing expression of BNDF. Brain Res. 2016, 1634, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hudson, J.L.; van Horne, C.G.; Strömberg, I.; Brock, S.; Clayton, J.; Masserano, J.; Hoffer, B.J.; Gerhardt, G.A. Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 1993, 626, 167–174. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Chen, J.J.; Chen, L.H.; Chiang, P.T.; Lee, H.Y. Time-course gait analysis of hemiparkinsonian rats following 6-hydroxydopamine lesion. Behav. Brain Res. 2011, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Broen, M.P.; Narayen, N.E.; Kuijf, M.L.; Dissanayaka, N.N.; Leentjens, A.F. Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2016, 31, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Rutten, S.; van der Ven, P.M.; Weintraub, D.; Pontone, G.M.; Leentjens, A.F.G.; Berendse, H.W.; van der Werf, Y.D.; van den Heuvel, O.A. Predictors of anxiety in early-stage Parkinson’s disease—Results from the first two years of a prospective cohort study. Parkinsonism Relat. Disord. 2017, 43, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.-W.; Hsueh, S.-C.; Lai, J.-H.; Chen, Y.-H.; Kang, S.-J.; Chen, K.-Y.; Hsieh, T.-H.; Hoffer, B.J.; Li, Y.; Greig, N.H.; et al. Glucose-Dependent Insulinotropic Polypeptide Mitigates 6-OHDA-Induced Behavioral Impairments in Parkinsonian Rats. Int. J. Mol. Sci. 2018, 19, 1153. https://doi.org/10.3390/ijms19041153
Yu Y-W, Hsueh S-C, Lai J-H, Chen Y-H, Kang S-J, Chen K-Y, Hsieh T-H, Hoffer BJ, Li Y, Greig NH, et al. Glucose-Dependent Insulinotropic Polypeptide Mitigates 6-OHDA-Induced Behavioral Impairments in Parkinsonian Rats. International Journal of Molecular Sciences. 2018; 19(4):1153. https://doi.org/10.3390/ijms19041153
Chicago/Turabian StyleYu, Yu-Wen, Shih-Chang Hsueh, Jing-Huei Lai, Yen-Hua Chen, Shuo-Jhen Kang, Kai-Yun Chen, Tsung-Hsun Hsieh, Barry J. Hoffer, Yazhou Li, Nigel H. Greig, and et al. 2018. "Glucose-Dependent Insulinotropic Polypeptide Mitigates 6-OHDA-Induced Behavioral Impairments in Parkinsonian Rats" International Journal of Molecular Sciences 19, no. 4: 1153. https://doi.org/10.3390/ijms19041153
APA StyleYu, Y.-W., Hsueh, S.-C., Lai, J.-H., Chen, Y.-H., Kang, S.-J., Chen, K.-Y., Hsieh, T.-H., Hoffer, B. J., Li, Y., Greig, N. H., & Chiang, Y.-H. (2018). Glucose-Dependent Insulinotropic Polypeptide Mitigates 6-OHDA-Induced Behavioral Impairments in Parkinsonian Rats. International Journal of Molecular Sciences, 19(4), 1153. https://doi.org/10.3390/ijms19041153