A Developed NK-92MI Cell Line with Siglec-7neg Phenotype Exhibits High and Sustainable Cytotoxicity against Leukemia Cells
Abstract
1. Introduction
2. Results
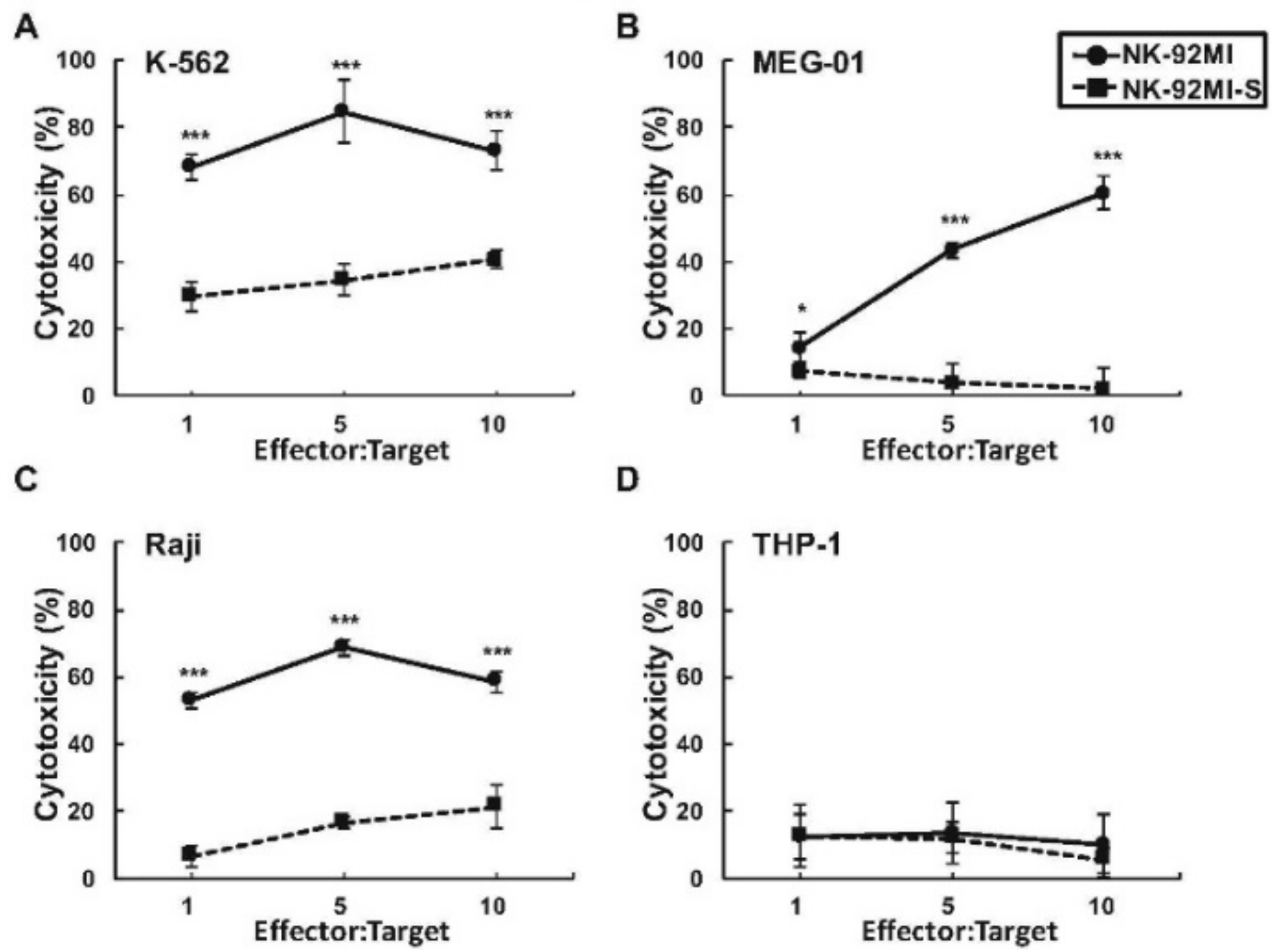
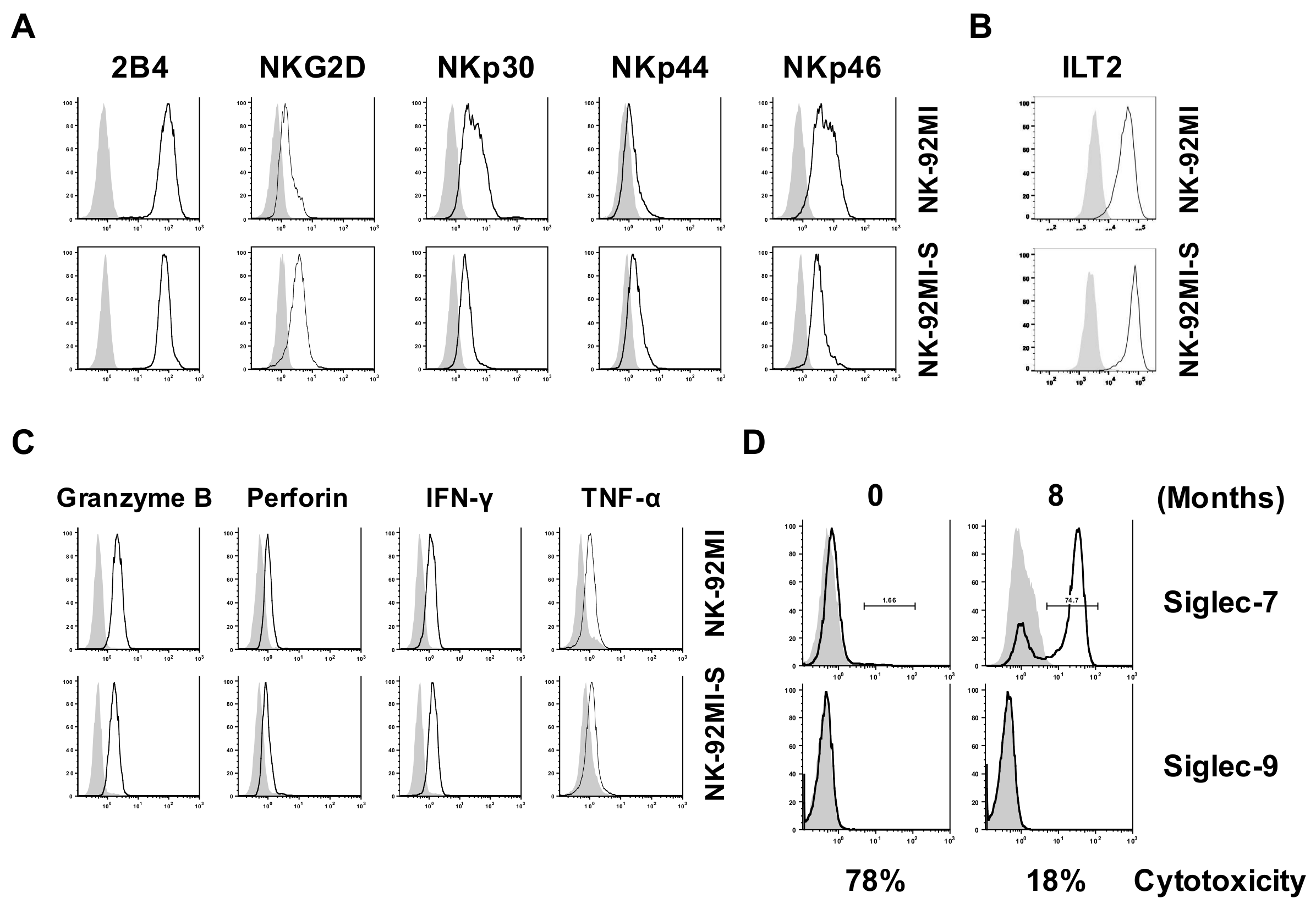
2.1. Presence of Increased Cell Surface Siglec-7 Expression Correlated with Significant Reduction in Cytotoxicity of the NK-92MI Cell
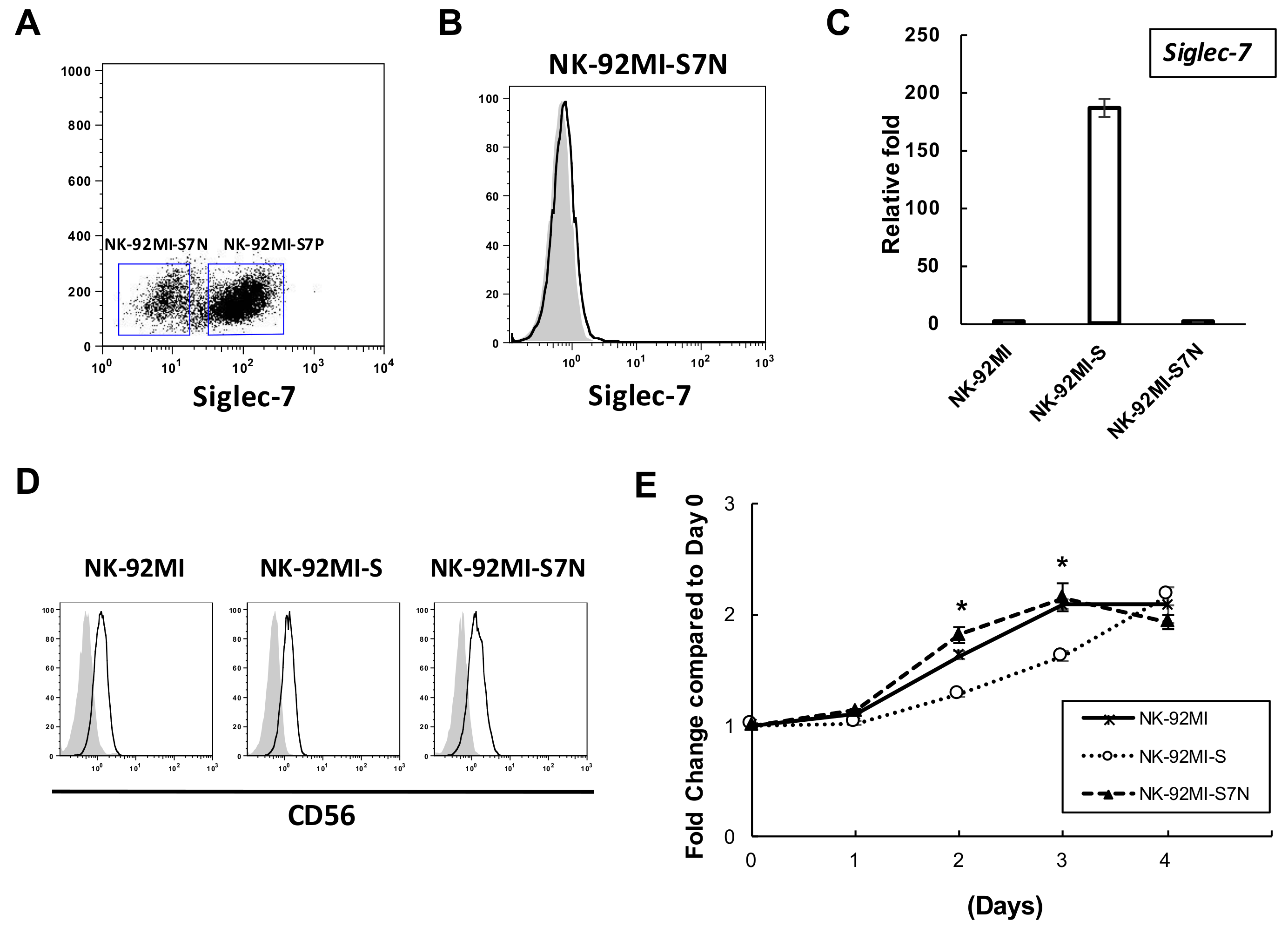
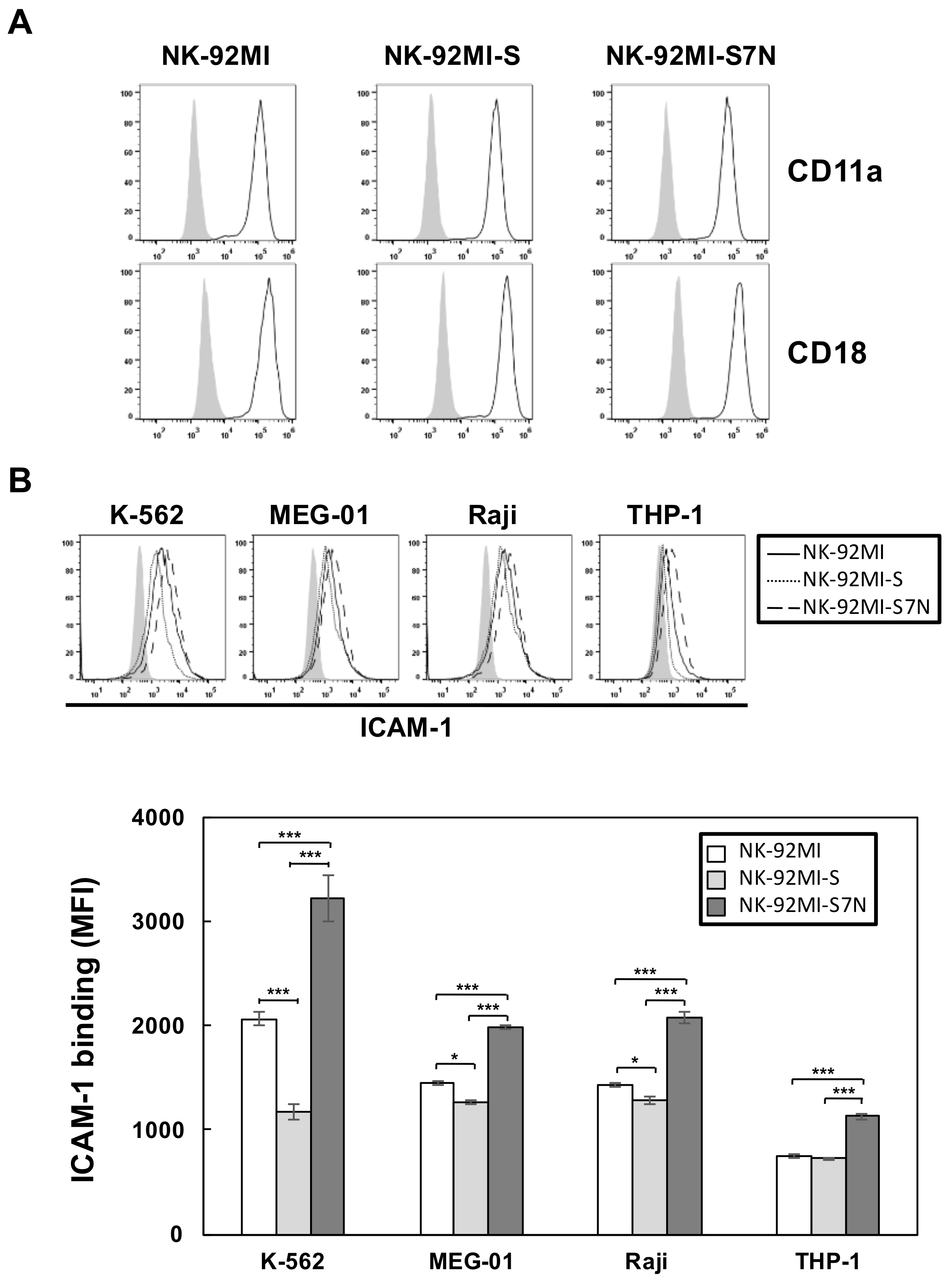
2.2. The Establishment of a Siglec-7neg NK Cell Model
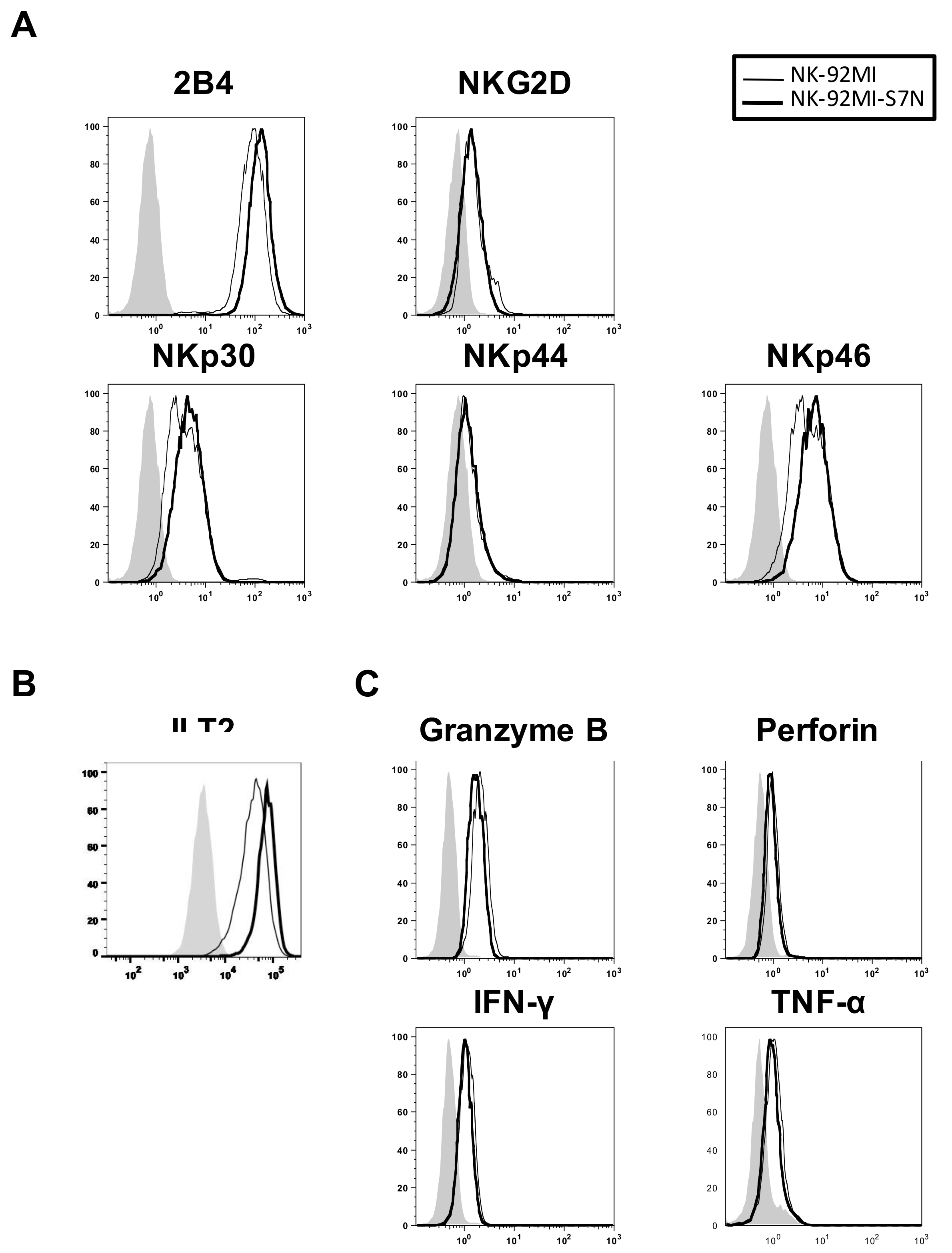
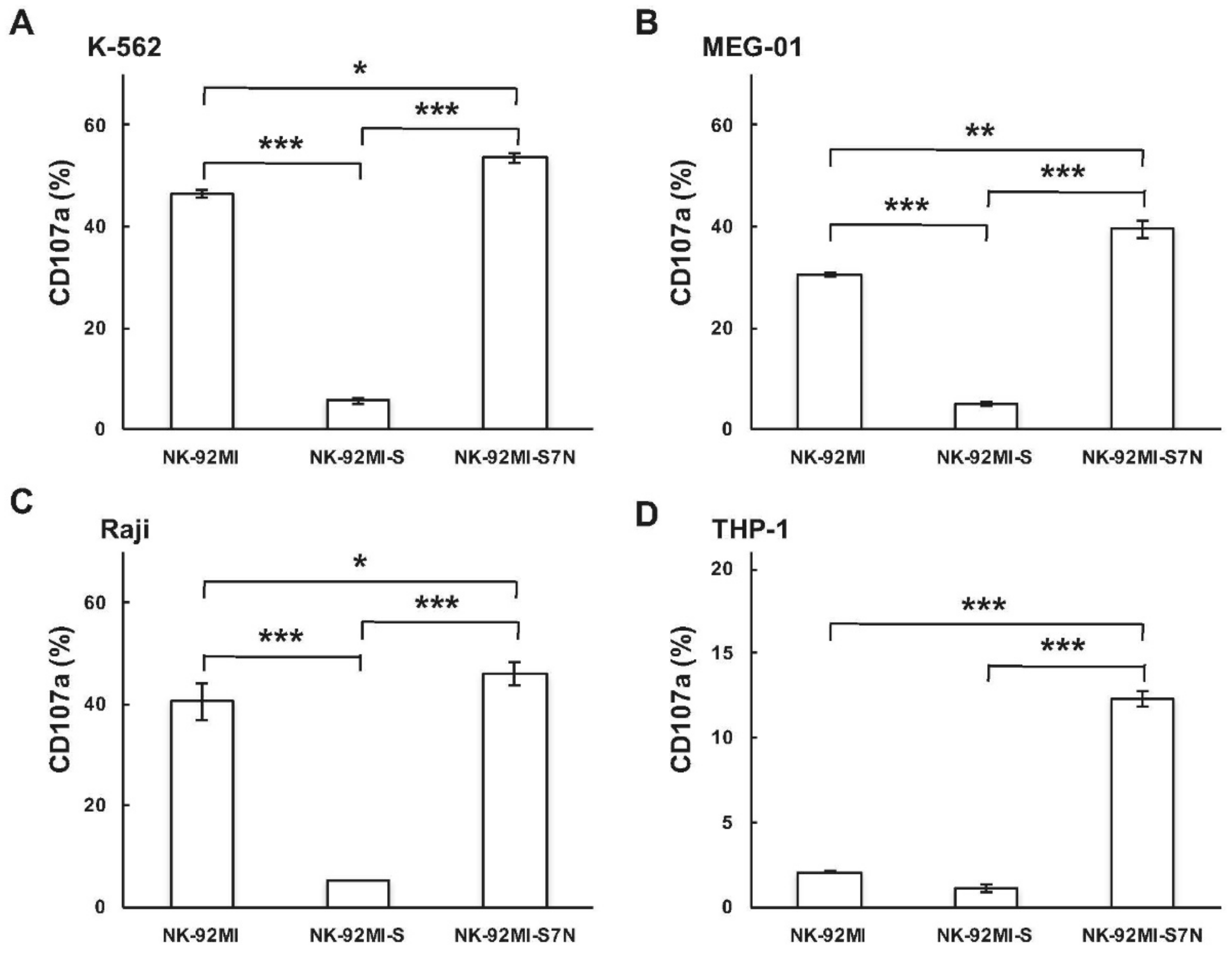
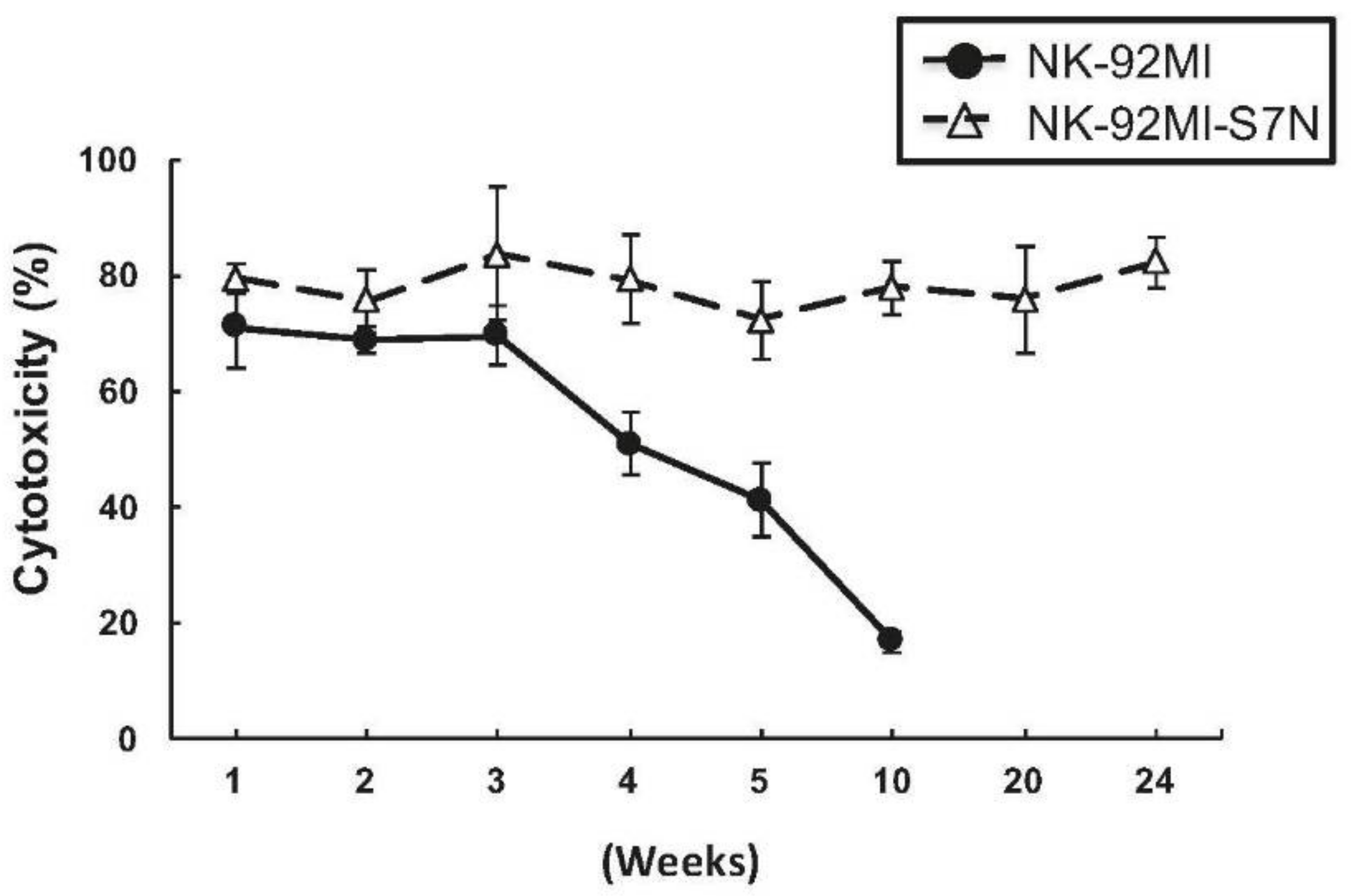
2.3. The Developed NK-92MI-S7N Cell Possessed a High and Sustainable Killing Activity against Leukemia Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Non-Radioactive Cytotoxicity Assay
4.3. Flow Cytometric Analysis
4.4. Establishment of Siglec7neg NK-92MI (NK-92MI-S7N) Cells
4.5. Quantification of Siglec-7 Transcripts
4.6. Proliferation Assay
4.7. NK Degranulation Assay (CD107a Assay)
4.8. Ligand Complex-Based Adhesion Assay (LC-AA)
Supplementary Materials
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Vilches, C.; Parham, P. KIR: Diverse, rapidly evolving receptors of innate and adaptive immunity. Annu. Rev. Immunol. 2002, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Biassoni, R.; Bottino, C.; Mingari, M.C.; Moretta, L. Natural cytotoxicity receptors that trigger human NK-cell-mediated cytolysis. Immunol. Today 2000, 21, 228–234. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Cantoni, C.; Mingari, M.C.; Biassoni, R.; Moretta, L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 2001, 19, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Bottino, C.; Castriconi, R.; Pende, D.; Rivera, P.; Nanni, M.; Carnemolla, B.; Cantoni, C.; Grassi, J.; Marcenaro, S.; Reymond, N.; et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J. Exp. Med. 2003, 198, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 1999, 17, 875–904. [Google Scholar] [CrossRef] [PubMed]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for HLA class-I molecules in human natural killer cells. Annu. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; March, M.E.; Ljunggren, H.G.; Long, E.O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 2006, 214, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Orange, J.S. Formation and function of the lytic NK-cell immunological synapse. Nat. Rev. Immunol. 2008, 8, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Barber, D.F.; Long, E.O. Coexpression of CD58 or CD48 with intercellular adhesion molecule 1 on target cells enhances adhesion of resting NK cells. J. Immunol. 2003, 170, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; March, M.E.; Barber, D.F.; Ljunggren, H.G.; Long, E.O. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 2005, 202, 1001–1012. [Google Scholar] [CrossRef] [PubMed]
- Helander, T.S.; Timonen, T. Adhesion in NK cell function. Curr. Top. Microbiol. Immunol. 1998, 230, 89–99. [Google Scholar] [PubMed]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Boligan, K.F.; Mesa, C.; Fernandez, L.E.; von Gunten, S. Cancer intelligence acquired (CIA): Tumor glycosylation and sialylation codes dismantling antitumor defense. Cell. Mol. Life Sci. 2015, 72, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Croci, D.O. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity 2012, 36, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Samraj, A.N.; Laubli, H.; Varki, N.; Varki, A. Involvement of a non-human sialic Acid in human cancer. Front. Oncol. 2014, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Samraj, A.N.; Pearce, O.M.; Laubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L.; et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. USA 2015, 112, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Jandus, C.; Boligan, K.F.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Demoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.E.; Baerlocher, G.M.; et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Investig. 2014, 124, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Alisson-Silva, F.; Stanczak, M.A.; Siddiqui, S.S.; Deng, L.; Verhagen, A.; Varki, N.; Varki, A. Lectin galactoside-binding soluble 3 binding protein (LGALS3BP) is a tumor-associated immunomodulatory ligand for CD33-related Siglecs. J. Biol. Chem. 2014, 289, 33481–33491. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Pearce, O.M.; Schwarz, F.; Siddiqui, S.S.; Deng, L.; Stanczak, M.A.; Deng, L.; Verhagen, A.; Secrest, P.; Lusk, C.; et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl Acad. Sci. USA 2014, 111, 14211–14216. [Google Scholar] [CrossRef] [PubMed]
- Hudak, J.E.; Canham, S.M.; Bertozzi, C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Angata, T. Siglecs—The major subfamily of I-type lectins. Glycobiology 2006, 16, 1R–27R. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Nycholat, C.M.; Macauley, M.S. Therapeutic Targeting of Siglecs using Antibody- and Glycan-Based Approaches. Trends Pharmacol. Sci. 2015, 36, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Lanier, L.L. Immune inhibitory receptors. Science 2000, 290, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Falco, M.; Biassoni, R.; Bottino, C.; Vitale, M.; Sivori, S.; Augugliaro, R.; Moretta, L.; Moretta, A. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 1999, 190, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, G.; Ni, J.; Liu, D.; Klenerman, P.; Munday, J.; Dubock, S.; Mattei, M.G.; Crocker, P.R. Identification and characterization of a novel siglec, siglec-7, expressed by human natural killer cells and monocytes. J. Biol. Chem. 1999, 274, 34089–34095. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Varki, A. Siglec-7: A sialic acid-binding lectin of the immunoglobulin superfamily. Glycobiology 2000, 10, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, S.; Gibbs, B.F.; Karra, L.; Ben-Zimra, M.; Levi-Schaffer, F. Siglec-7 is an inhibitory receptor on human mast cells and basophils. J. Allergy Clin. Immunol. 2014, 134, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Urmacher, C.; Cordon-Cardo, C.; Houghton, A.N. Tissue distribution of GD3 ganglioside detected by mouse monoclonal antibody R24. Am. J. Dermatopathol. 1989, 11, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Nicoll, G.; Jones, C.; Crocker, P.R. Siglec-9, a novel sialic acid binding member of the immunoglobulin superfamily expressed broadly on human blood leukocytes. J. Biol. Chem. 2000, 275, 22121–22126. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, G.; Avril, T.; Lock, K.; Furukawa, K.; Bovin, N.; Crocker, P.R. Ganglioside GD3 expression on target cells can modulate NK cell cytotoxicity via siglec-7-dependent and -independent mechanisms. Eur. J. Immunol. 2003, 33, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Ahlenstiel, G.; Edlich, B.; Hogdal, L.J.; Rotman, Y.; Noureddin, M.; Feld, J.J.; Holz, L.E.; Titerence, R.H.; Liang, T.J.; Rehermann, B. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology 2011, 141, 1231–1239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oliviero, B.; Mele, D.; Degasperi, E.; Aghemo, A.; Cremonesi, E.; Rumi, M.G.; Tinelli, C.; Varchetta, S.; Mantovani, S.; Colombo, M.; et al. Natural killer cell dynamic profile is associated with treatment outcome in patients with chronic HCV infection. J. Hepatol. 2013, 59, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Tonn, T.; Becker, S.; Esser, R.; Schwabe, D.; Seifried, E. Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J. Hematother Stem. Cell. Res. 2001, 10, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Tonn, T.; Schwabe, D.; Klingemann, H.G.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.G.; Bug, G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Meagher, R.; Swearingen, M.; Myint, H.; Rich, E.; Martinson, J.; Klingemann, H. Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: A phase I trial. Cytotherapy 2008, 10, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Liao, Y.J.; Huang, C.H.; Chang, F.L.; Fan, T.H.; Twu, Y.C. Branched I antigens on leukemia cells enhanced sensitivity against NK cytotoxicity through affecting the target-effector interaction. Transfusion 2017, 57, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Malenfant, J.M.; Altfeld, M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 2004, 294, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Konstandin, M.H.; Sester, U.; Klemke, M.; Weschenfelder, T.; Wabnitz, G.H.; Samstag, Y. A novel flow-cytometry-based assay for quantification of affinity and avidity changes of integrins. J. Immunol. Methods 2006, 310, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Urlaub, D.; Hofer, K.; Muller, M.L.; Watzl, C. LFA-1 Activation in NK Cells and Their Subsets: Influence of Receptors, Maturation, and Cytokine Stimulation. J. Immunol. 2017, 198, 1944–1951. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Drake, C.G.; Sznol, M.; Choueiri, T.K.; Powderly, J.D.; Smith, D.C.; Brahmer, J.R.; Carvajal, R.D.; Hammers, H.J.; Puzanov, I.; et al. Survival, Durable Response, and Long-Term Safety in Patients with Previously Treated Advanced Renal Cell Carcinoma Receiving Nivolumab. J. Clin. Oncol. 2015, 33, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Yamaji, T.; Mitsuki, M.; Teranishi, T.; Hashimoto, Y. Characterization of inhibitory signaling motifs of the natural killer cell receptor Siglec-7: Attenuated recruitment of phosphatases by the receptor is attributed to two amino acids in the motifs. Glycobiology 2005, 15, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, T.; Teranishi, T.; Alphey, M.S.; Crocker, P.R.; Hashimoto, Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 2002, 277, 6324–6332. [Google Scholar] [CrossRef] [PubMed]
- Nara, K.; Watanabe, Y.; Maruyama, K.; Kasahara, K.; Nagai, Y.; Sanai, Y. Expression cloning of a CMP-NeuAc:NeuAc alpha 2-3Gal beta 1-4Glc beta 1-1′Cer alpha 2,8-sialyltransferase (GD3 synthase) from human melanoma cells. Proc. Natl. Acad. Sci. USA 1994, 91, 7952–7956. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Ohmori, K.; Izawa, M.; Koike, T.; Kumamoto, K.; Furukawa, K.; Ando, T.; Kiso, M.; Yamaji, T.; Hashimoto, Y.; et al. Loss of disialyl Lewis(a), the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewis(a) expression on human colon cancers. Cancer Res. 2004, 64, 4498–4505. [Google Scholar] [CrossRef] [PubMed]
- Fraschilla, I.; Pillai, S. Viewing Siglecs through the lens of tumor immunology. Immunol. Rev. 2017, 276, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, H.; Shoji, T.; Iijima, S.; Nishijima, K. Constitutively expressed Siglec-9 inhibits LPS-induced CCR7, but enhances IL-4-induced CD200R expression in human macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, S.; Sutoh, M.; Hatakeyama, S.; Hiraoka, N.; Habuchi, T.; Horikawa, Y.; Hashimoto, Y.; Yoneyama, T.; Mori, K.; Koie, T.; et al. A novel strategy for evasion of NK cell immunity by tumours expressing core2 O-glycans. EMBO J. 2011, 30, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Della Chiesa, M.; Pesce, S.; Muccio, L.; Carlomagno, S.; Sivori, S.; Moretta, A.; Marcenaro, E. Features of Memory-Like and PD-1(+) Human NK Cell Subsets. Front. Immunol. 2016, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.J.; Lee, Y.H.; Chang, F.L.; Ho, H.; Huang, C.H.; Twu, Y.C. The SHP-2-ERK2 signaling pathway regulates branched I antigen formation by controlling the binding of C/EBPa to the IGnTC promoter region during erythroid differentiation. Transfusion 2016, 56, 2691–2702. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Liao, Y.-J.; Fan, T.-H.; Chiou, T.-J.; Lin, Y.-H.; Twu, Y.-C. A Developed NK-92MI Cell Line with Siglec-7neg Phenotype Exhibits High and Sustainable Cytotoxicity against Leukemia Cells. Int. J. Mol. Sci. 2018, 19, 1073. https://doi.org/10.3390/ijms19041073
Huang C-H, Liao Y-J, Fan T-H, Chiou T-J, Lin Y-H, Twu Y-C. A Developed NK-92MI Cell Line with Siglec-7neg Phenotype Exhibits High and Sustainable Cytotoxicity against Leukemia Cells. International Journal of Molecular Sciences. 2018; 19(4):1073. https://doi.org/10.3390/ijms19041073
Chicago/Turabian StyleHuang, Chin-Han, Yi-Jen Liao, Ting-Hsi Fan, Tzeon-Jye Chiou, Yen-Hsi Lin, and Yuh-Ching Twu. 2018. "A Developed NK-92MI Cell Line with Siglec-7neg Phenotype Exhibits High and Sustainable Cytotoxicity against Leukemia Cells" International Journal of Molecular Sciences 19, no. 4: 1073. https://doi.org/10.3390/ijms19041073
APA StyleHuang, C.-H., Liao, Y.-J., Fan, T.-H., Chiou, T.-J., Lin, Y.-H., & Twu, Y.-C. (2018). A Developed NK-92MI Cell Line with Siglec-7neg Phenotype Exhibits High and Sustainable Cytotoxicity against Leukemia Cells. International Journal of Molecular Sciences, 19(4), 1073. https://doi.org/10.3390/ijms19041073




